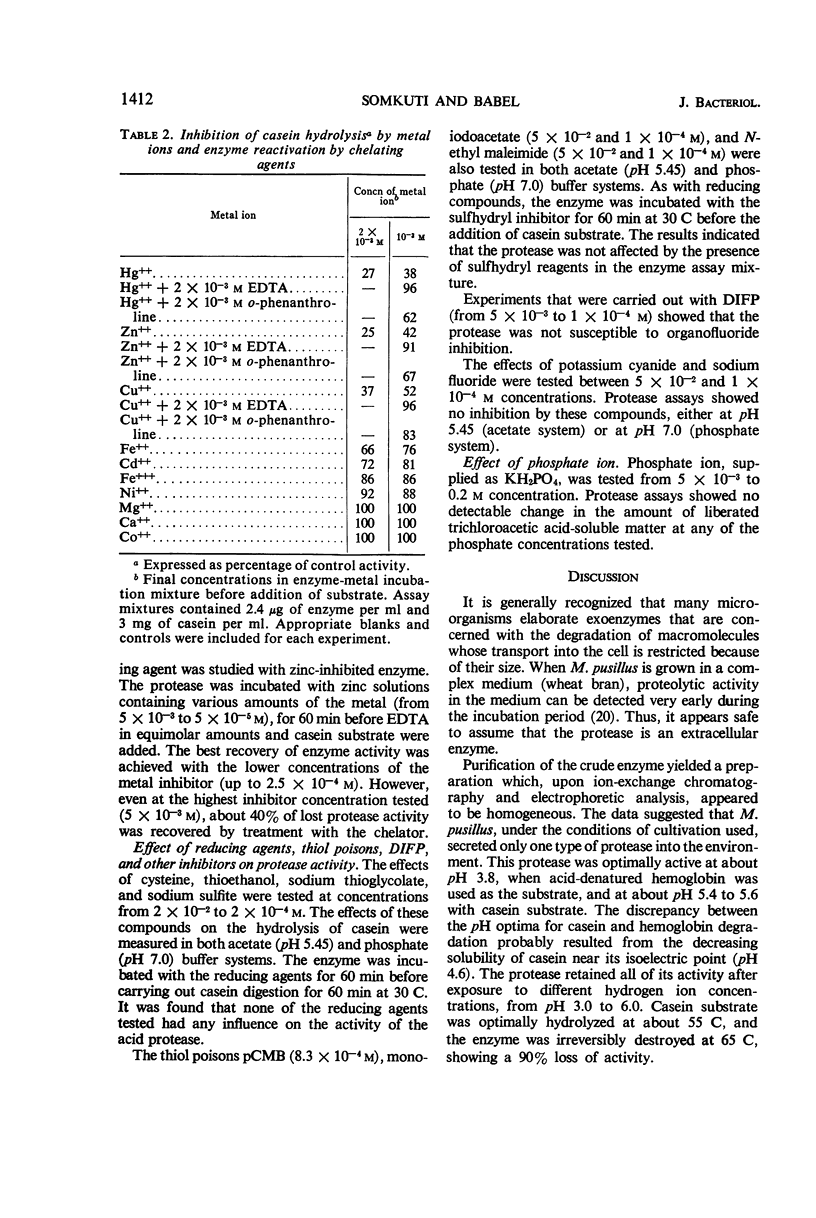
Abstract
The protease produced by Mucor pusillus was recovered from a wheat bran medium by treatment with ammonium sulfate, ethyl alcohol, gel filtration and ion-exchange chromatography. The yield of the enzyme was 55%. The overall increase in the specific activity of the protease was 34-fold. The purified protease was most active at pH 3.8 and 5.6 against hemoglobin and casein, respectively. Optimal hydrolysis of casein was observed at 55 C. The enzyme was stable from pH 3.0 to 6.0. Enzyme inactivated by metal ions was reactivated by ethylenediaminetetraacetate and o-phenanthroline. Reducing agents and thiol poisons had no effect on the protease, suggesting that free sulfhydryl groups were not required for enzyme activity. Diisopropyl fluorophosphate did not inhibit the protease, indicating the probable absence of serine in the active center. The Michaelis-Menten constant for casein was 0.357%. Electrophoretic analysis of active protein recovered by ion-exchange chromatography showed that the protease preparation was homogeneous.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- HOFMANN T., SHAW R. PROTEOLYTIC ENZYMES OF PENICILLIUM JANTHINELLUM. I. PURIFICATION AND PROPERTIES OF A TRYPSINOGEN-ACTIVATING ENZYME (PEPTIDASE A). Biochim Biophys Acta. 1964 Dec 23;92:543–557. [PubMed] [Google Scholar]
- ICHISHIMA E., YOSHIDA F. CHROMATOGRAPHIC PURIFICATION AND PHYSICAL HOMOGENEITY OF ACID PROTEINASE OF ASPERGILLUS SAITOI. Biochim Biophys Acta. 1965 May 18;99:360–366. doi: 10.1016/s0926-6593(65)80133-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MCDONALD C. E., CHEN L. L. THE LOWRY MODIFICATION OF THE FOLIN REAGENT FOR DETERMINATION OF PROTEINASE ACTIVITY. Anal Biochem. 1965 Jan;10:175–177. doi: 10.1016/0003-2697(65)90255-1. [DOI] [PubMed] [Google Scholar]
- SCHLAMOWITZ M., PETERSON L. U. Studies on the optimum pH for the action of pepsin on "native" and denatured bovine serum albumin and bovine hemoglobin. J Biol Chem. 1959 Dec;234:3137–3145. [PubMed] [Google Scholar]
- Sardinas J. L. Rennin enzyme of Endothia parasitica. Appl Microbiol. 1968 Feb;16(2):248–255. doi: 10.1128/am.16.2.248-255.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somkuti G. A., Babel F. J. Conditions influencing the synthesis of acid protease by Mucor pusillus Lindt. Appl Microbiol. 1967 Nov;15(6):1309–1312. doi: 10.1128/am.15.6.1309-1312.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. L. Release of proteinase from mycelium of Mucor hiemalis. J Bacteriol. 1967 Jun;93(6):1794–1799. doi: 10.1128/jb.93.6.1794-1799.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]