Abstract
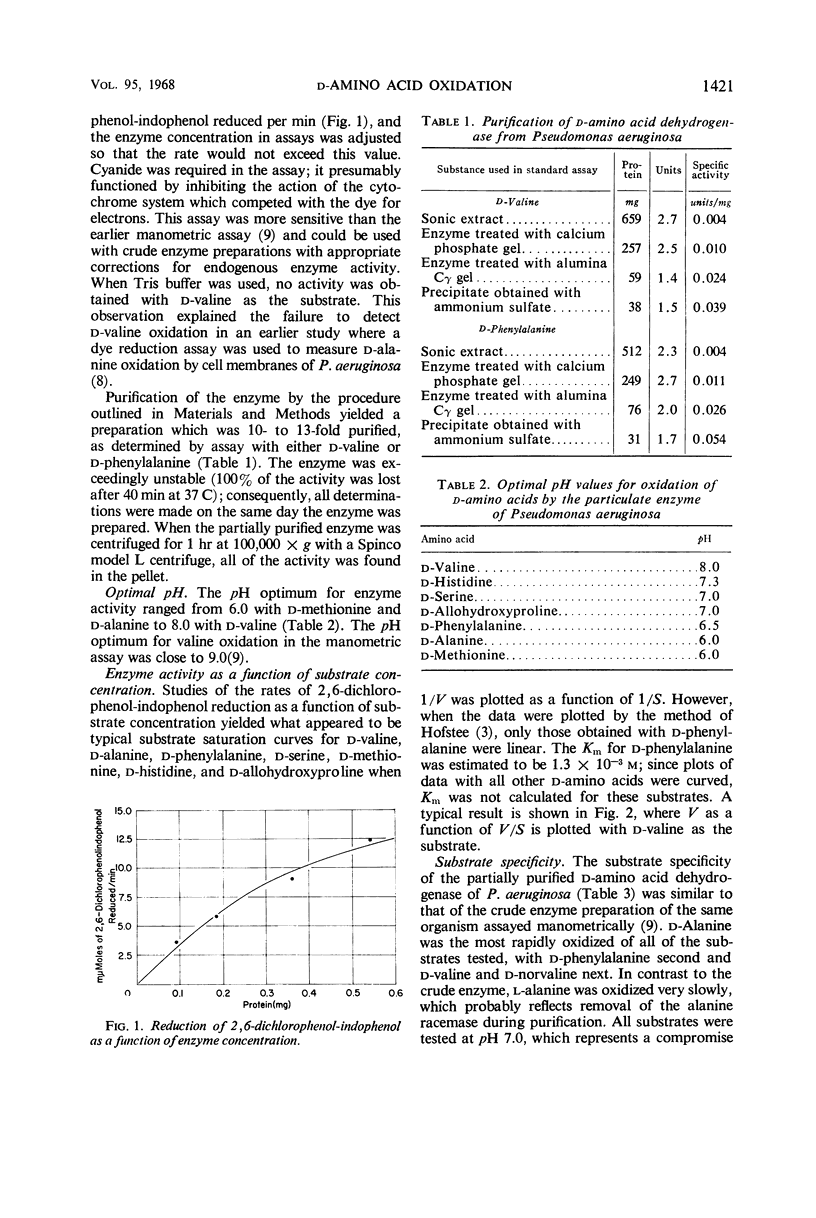
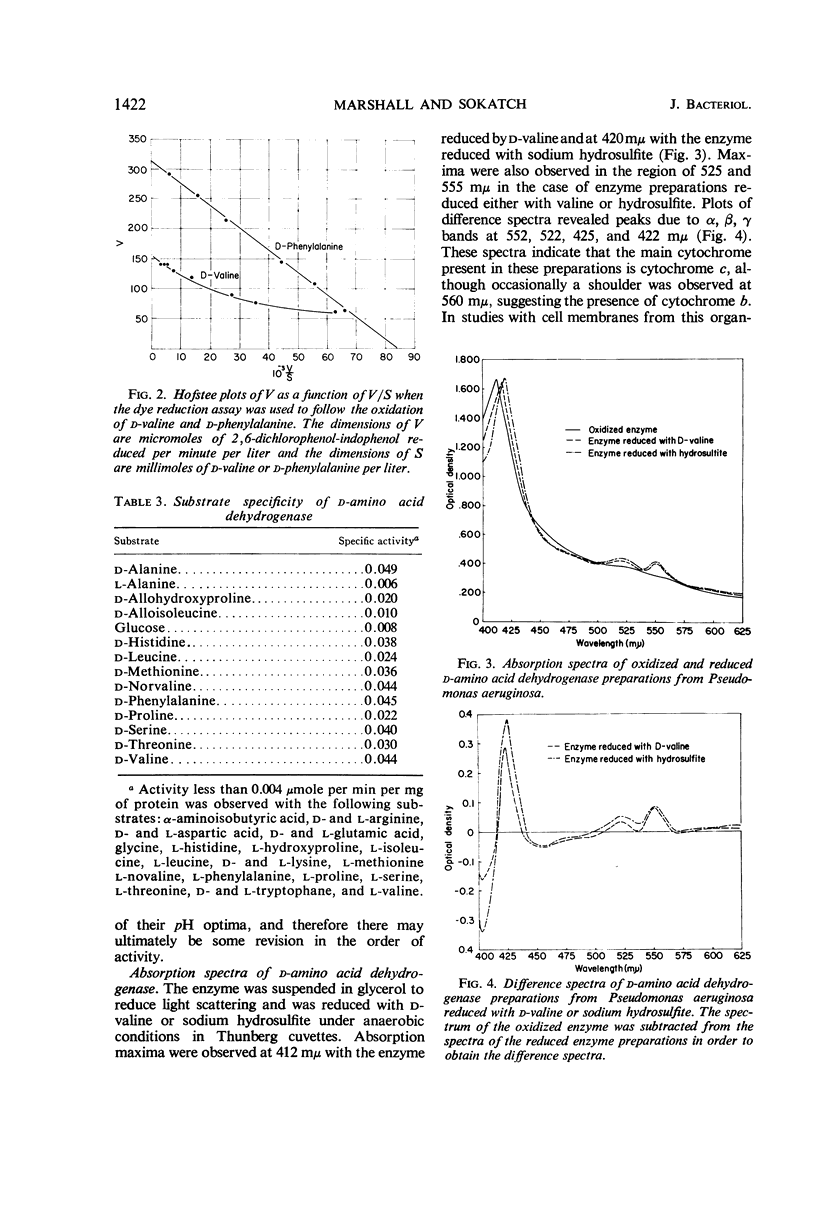
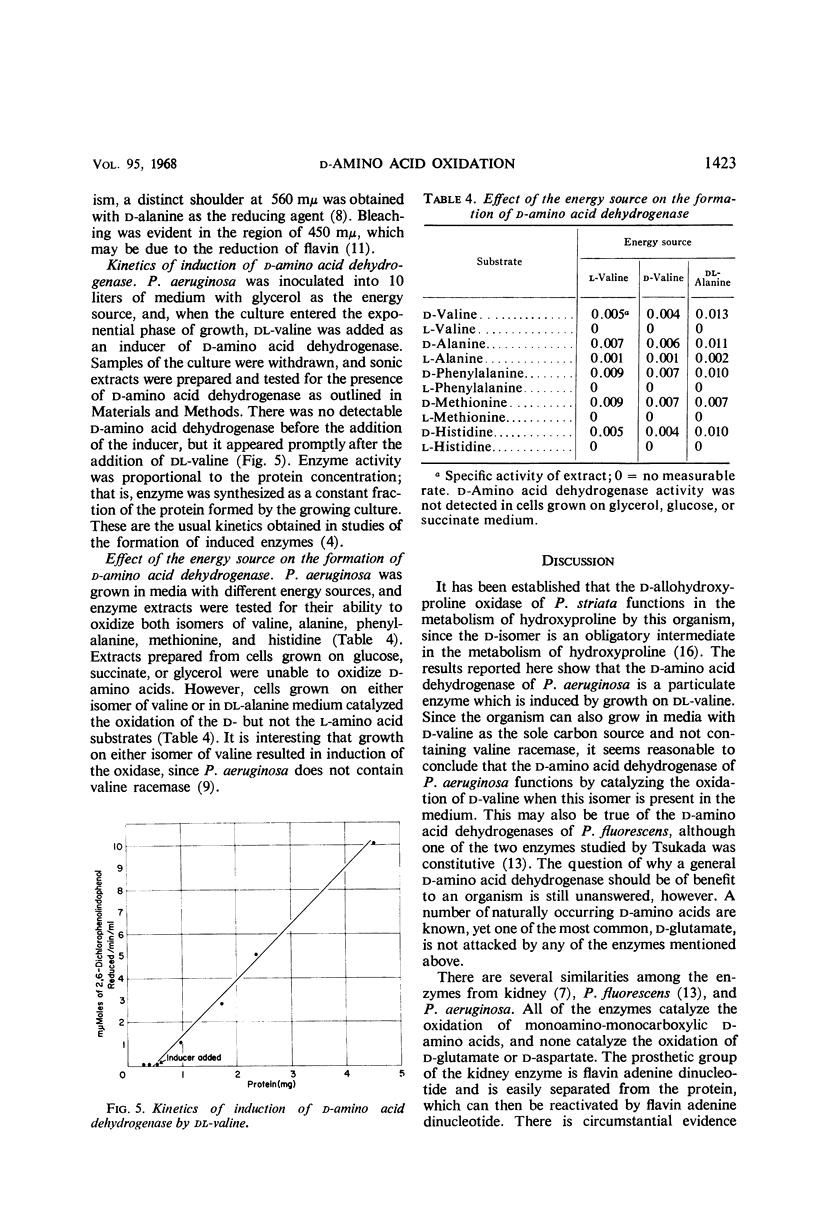
A particulate d-amino acid dehydrogenase has been partially purified from cell free extracts of Pseudomonas aeruginosa grown on dl-valine as the source of carbon and energy. A standard assay was developed which utilized 2,6-dichlorophenol-indophenol as the electron acceptor. The pH optimum for enzyme activity ranged from 6.0 to 8.0, depending on the amino acid assayed. The enzyme was most active with monoamino-monocarboxylic amino acids and histidine. The Michaelis constant for d-phenylalanine was found to be 1.3 × 10-3m d-phenylalanine. Constants could not be calculated for the other amino acids oxidized because anomalous plots of V as a function of V/S were obtained. Spectra of enzyme preparations reduced with d-valine or sodium hydrosulfite exhibited adsorption bands typical of the α, β, and γ bands of cytochromes as well as bleaching in the flavin region of the spectrum. When dl-valine was added to a medium with glycerol as the energy source, d-amino acid dehydrogenase was detected after the addition of valine and was produced at a rate directly proportional to the synthesis of total protein. The enzyme was formed when d-valine, l-valine, or dl-alanine was the source of carbon and energy, but not when glucose, glycerol, or succinate was the energy source.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- HOFSTEE B. H. J. On the evaluation of the constants Vm and KM in enzyme reactions. Science. 1952 Sep 26;116(3013):329–331. doi: 10.1126/science.116.3013.329. [DOI] [PubMed] [Google Scholar]
- JACOB F., MONOD J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 1961 Jun;3:318–356. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- NORTON J. E., BULMER G. S., SOKATCH J. R. THE OXIDATION OF D-ALANINE BY CELL MEMBRANES OF PSEUDOMONAS AERUGINOSA. Biochim Biophys Acta. 1963 Oct 8;78:136–147. doi: 10.1016/0006-3002(63)91619-6. [DOI] [PubMed] [Google Scholar]
- Norton J. E., Sokath J. R. Oxidation of D- and L-valine by enzymes of Pseudomonas aeruginosa. J Bacteriol. 1966 Jul;92(1):116–120. doi: 10.1128/jb.92.1.116-120.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REDFIELD R. R. Two-dimensional paper chromatographic systems with high resolving power for amino acids. Biochim Biophys Acta. 1953 Feb;10(2):344–345. doi: 10.1016/0006-3002(53)90260-1. [DOI] [PubMed] [Google Scholar]
- STANIER R. Y., GUNSALUS I. C., GUNSALUS C. F. The enzymatic conversion of mandelic acid to benzoic acid. II. Properties of the particulate fractions. J Bacteriol. 1953 Nov;66(5):543–547. doi: 10.1128/jb.66.5.543-547.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEYN-PARVE E. P., BEINERT H. On the mechanism of dehydrogenation of fatty acyl derivatives of coenzyme A. VI. Isolation and properties of stable enzyme-substrate complexes. J Biol Chem. 1958 Oct;233(4):843–852. [PubMed] [Google Scholar]
- Tsukada K. D-amino acid dehydrogenases of Pseudomonas fluorescens. J Biol Chem. 1966 Oct 10;241(19):4522–4528. [PubMed] [Google Scholar]
- WADDELL W. J. A simple ultraviolet spectrophotometric method for the determination of protein. J Lab Clin Med. 1956 Aug;48(2):311–314. [PubMed] [Google Scholar]
- YONEYA T., ADAMS E. Hydroxyproline metabolism. V. Inducible allohydroxy-D-proline oxidase of Pseudomonas. J Biol Chem. 1961 Dec;236:3272–3279. [PubMed] [Google Scholar]


