Abstract
During thrombus formation, activated platelets come into close and increasingly stable contact with each other. This produces a microenvironment in which soluble agonists can accumulate and proteins on the surface of adjacent platelets can directly interact with each other, potentially modulating subsequent thrombus growth and stability. In important respects, this microenvironment resembles the synapses that support signal propagation between neurons and the exchange of information between T-cells, B-cells and dendritic cells. Drawing on this analogy, this brief review discusses the role in platelets of Semaphorins and their receptors, two protein families that have previously been defined by their role at cell:cell contacts, in both the developing nervous system and adaptive immunity.
Introduction
Platelets circulate throughout the vasculature ready to respond instantly to damage to the vessel wall. Signaling downstream of collagen receptors, as well as receptors for the secondary mediators ADP and thromboxane A2, activate integrin αIIbβ3 to provide a strong cohesive force between platelets. In addition, αIIbβ3- dependent interactions bring platelets in close proximity to each other, creating a microenvironment in which proteins on the surface of adjacent platelets can directly interact with each other. Adhesion molecules and receptor-ligand pairs have been identified on the platelet surface that can interact in trans across the platelet “synapse”, potentially modulating thrombus growth and stability [1]. As an example, our laboratory has previously characterized contact-dependent signaling in platelets between Eph receptor tyrosine kinases and their ephrin ligands, proteins that are principally known for their involvement in axonal guidance in the developing nervous system [2]. Given the potential similarities between how neurons and platelets communicate across a small space, perhaps it is not surprising that other neuronal proteins may also regulate platelet responses. Along these lines, two members of the large Semaphorin family, Sema4D [3, 4] and Sema7A [5], have recently been identified on platelets. Both of these proteins have previously been shown to participate in information exchange between adjacent neurons and between immune cells by binding to cell surface receptors[6, 7].
Semaphorins and Plexins
Semaphorins were initially identified by their ability to induce growth cone collapse in developing neurons [8] and are defined by a 500 amino acid sema domain [9]. To date, over 25 Semaphorin family members have been described and classified into 8 families. Semaphorins can either be secreted (class 2, 3 and 8) or bound to the cell membrane through a transmembrane domain (class 1, 4, 5 and 6) or a GPI anchor (class 7) [6]. Outside of the nervous system, Semaphorins have been detected in a wide variety of tissues and have been implicated in many biological processes including organogenesis, angiogenesis and immune cell regulation [10].
Many of the biological responses elicited by Semaphorins have been shown to be mediated by members of the Plexin family of cell surface proteins [6, 11]. Plexins, of which 9 vertebrate members in 4 classes are known (denoted A-D), also contain an extracellular sema domain. Several recent structural studies confirm that each sema domain of a Semaphorin homodimer binds to a Plexin sema domain to force Plexin dimerization [12–14].
Semaphorin receptor signaling
Plexins are capable of intracellular signaling via their cytoplasmic domain but may also associate with additional proteins, most often receptor tyrosine kinases, to modulate signaling [15]. Class 3 Semaphorins, with the exception of Sema3E, bind directly to Neuropilins [16, 17] but require Plexin A family members to transduce downstream signaling. All Plexin family members have a large, highly conserved cytoplasmic domain that contains two amino acid stretches that are similar to GTPase-activating proteins (GAPs). The Rho GTPase family member, Rnd1, has been shown to bind to the Plexin cytoplasmic domain and downregulate R-Ras activity [18] leading to an inhibition of β1 integrin-dependent cell migration [19]. Plexins regulate actin dynamics through direct binding to Rac1, thus inhibiting activation of the Rac effector, p21-activated kinase (PAK) [20]. Additionally, Plexin signaling can have opposing effects on Rho activation by binding either RhoGEFs or Rho-GTPases depending on the cellular context and co-receptor association [21]. In the immune system, Semaphorins have also been shown to bind receptors other than Plexins and Neuropilins, including CD72 (Sema4D), TIM-2 (Sema4A) and α1β1 integrin (Sema7A) [7].
Semaphorins and Plexins in platelets
Our laboratory has previously reported that platelets express Sema4D [3]. Studies using Sema4D(−/−) mice have shown a positive role for Sema4D in thrombus formation in vivo following FeCl3-induced injury to the carotid as well as injury to cremaster arterioles induced by either rose Bengal or a laser. In vitro, Sema4D(−/−) platelets exhibit decreased aggregation in response to collagen as well as reduced phosphorylation of the tyrosine kinase, Syk, leading to diminished phosphorylation of PLCγ2 and intracellular Ca2+ release [3, 4]. Interestingly, defects in Sema4D(−/−) platelets are only observed when contacts between platelets are encouraged or allowed to occur, suggesting that Sema4D functions in a contact-dependent manner in platelets. Furthermore, we have found that maximal Syk activation requires the formation of platelet:platelet contacts in wild-type platelets and thus propose that Sema4D is an important regulator in the contact-dependent reinforcement of Syk activation. Sema4D-mediated Syk activation requires αIIbβ3 engagement but is distinct from outside-in signaling [4]. We have also reported that platelets express the Sema4D receptors, Plexin-B1 and CD72 [3]. However, it is unclear at this time which, if either, of these proteins act as a receptor for Sema4D in platelets as platelets from single knockout mice do not exhibit any detectable platelet phenotype and functional correlates from human platelets are lacking.
Neuropilin-1 and Plexin A family members (A1–A3) have also been detected in platelets at the protein and mRNA level, respectively [22]. However, Plexin A1–A3 should be confirmed at the protein level. Neuropilin-1 and Plexin A members can form functional receptors for Sema3A, a disulfide-linked homodimer secreted from endothelial cells. The addition of recombinant Sema3A to platelets was shown to inhibit αIIbβ3 activation in response to ADP, U46619, TRAP and convulxin [22]. TRAP-induced platelet spreading on fibrinogen was also inhibited in the presence of Sema3A. Furthermore, Sema3A treatment blunted TRAP-induced Rac1 activation, cofilin phosphorylation and F-actin generation indicating Sema3A-mediated effects on the cytoskeleton [22]. In contrast to these findings, Rac1 activation and cofilin phosphorylation are both increased in neurons following Sema3A binding to Neuropilin-1 [6]. Nevertheless, Kashiwagi et al., has proposed that Sema3A secreted by the endothelium serves to maintain circulating plateletsin a quiescent state or to limit thrombus growth following injury [22]. How such a mechanism would be shut down in response to injury in the context of thrombosis remains to be seen and underscores the need for further study in this area.
Several Plexin and Semaphorin family members have also been identified using proteomics. Lewandrowski et al., have detected Plexin family members A3, A4, B2, B3 and C1 in addition to Neuropilin-2 [23]. Studies from our laboratory also detected Sema4B and Sema7A [5]. The presence of Sema7A was confirmed by immunoblot analysis and flow cytometry and our current studies are focused on elucidating a role for Sema7A in platelets. In the immune system, Sema7A expressed on the surface of activated T-cells has been shown to increase IL-6 production in monocytes through α1β1 integrin [24]. It is therefore tempting to speculate that Sema7A on the surface of platelets may provide a link between injury and inflammation.
Semaphorins shed from the platelet surfacemay serve as bioactive molecules
While Sema4D is bound to the plasma membrane via a transmembrane domain, it has been shown to be proteolytically cleaved from the surface of activated T-cells, both in vitro and in vivo [25]. Moreover, cleaved Sema4D positively influenced antibody production against a subset of antigens [25]. Sema4D can also be shed from the surface of activated platelets and cleavage has been shown to require the metalloproteinase, ADAM17 [3]. Given previous reports showing that soluble Sema4D can induce endothelial cell migration and angiogenesis [26], it is possible that platelet-derived soluble Sema4D generated at sites of vascular injury serves as a regulator of wound healing. Sema7A is also shed from the activated platelet surface, although the biological relevance of this event is still unclear [5].
Semaphorins and Atherosclerosis
In addition to their primary role in hemostasis, platelets are also intimately involved in the progression and consequences of atherosclerosis. In dyslipidemic states, platelets become hyper-reactive to agonists [27]. As Sema4D has been found to be a positive regulator of platelet function [3], we examined the contributions of Sema4D toward platelet hyper-reactivity in settings of dyslipidemia and observed that genetic deletion of Sema4D significantly reduces platelet hyper-reactivity otherwise found in dyslipidemia. In addition, the absence of Sema4D was found to confer protection against the development of atherosclerosis [28]. However, it should be noted that the latter finding may, in part, reflect the deletion of Sema4D on cells other than platelets as Sema4D and its receptors are expressed on T-cells, B-cells, monocytes and endothelial cells, all of which affect the development of atherosclerosis. A recent report suggests that proteolytically cleaved Sema4D from infiltrating T-cells accelerates plaque growth by promoting intimal neovascularization [29].
Conclusions
To date, 3 Semaphorins (4B, 4D, 7A) and 8 Plexins (A1-4, B1-3, C1) have been detected at either the mRNA or protein level in platelets by various methods. The expression of numerous members of a ligand-receptor family suggests that Semaphorins and their receptors have more than just a casual role in platelets. Studies on Sema4D(−/−) mice show an essential role for Sema4D in maximal responses to collagen in vitro and vascular injury in vivo [3, 4]. The absence of gross bleeding in Sema4D(−/−) mice suggests that Sema4D could be a promising drug target to prevent unwanted platelet activation in pathological states without disrupting normal hemostasis. Further studies on Sema4D, as well as other Semaphorins and Plexins that are expressed in platelets could lead to a new class of antithrombotic agents.
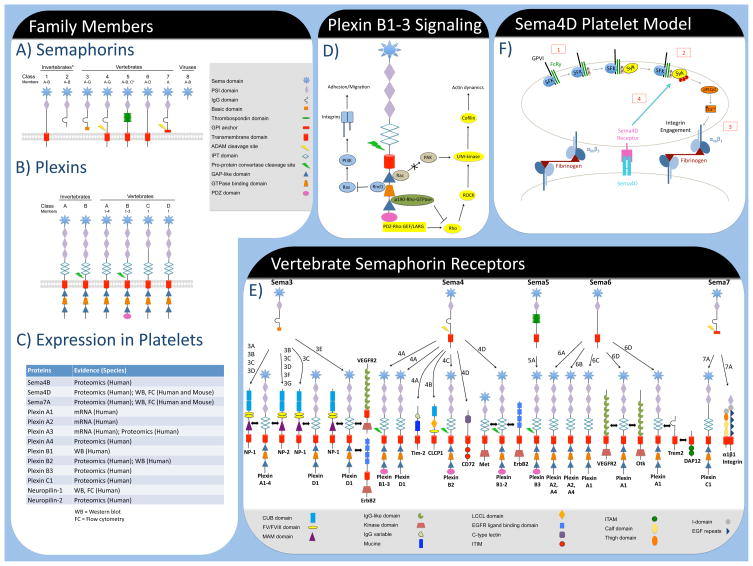
Figure 1.
Schematic of Semaphorin (A) and Plexin (B) family members. (C) Several Semaphorins and Plexin family members have been reported to be expressed in platelets using a variety of techniques. (D) Plexins can regulate cellular actin dynamics by sequestering active Rac, thereby preventing activation of PAK and subsequent downstream effectors. Alternatively, Plexins can bind p190-Rho-GTPase to inhibit Rho activation. Conversely, Plexin B members can increase Rho activation through PDZ-RHO-GEF/LARG. In addition, Plexins can inactivate R-Ras through direct binding of the GTPase Rnd1 leading to effects on integrin-mediated cell adhesion and migration. (E) Schematic of vertebrate Semaphorin (Class 3–7) receptors. (F) Proposed model of Sema4D contributions to GPVI signaling [4]. Clustering of GPVI results in Src family kinase (SFK)-mediated phosphorylation of FcRγ(1) leading to the recruitment and phosphorylation of the tyrosine kinase, Syk (2). Subsequent signaling leads to PLCγ2-dependent intracellular Ca2+ release and integrin activation (3) leading to the formation of stable contacts between platelets which allows Sema4D to binds its receptor on an adjacent platelet and provide positive feedback to enhance Syk phosphorylation (4).
References
- 1.Brass LF, Zhu L, Stalker TJ. Minding the gaps to promote thrombus growth and stability. J Clin Invest. 2005;115:3385–3392. doi: 10.1172/JCI26869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prevost N, Woulfe D, Tanaka T, Brass LF. Interactions between Eph kinases and ephrins provide a mechanism to support platelet aggregation once cell-to-cell contact has occurred. Proc Natl Acad Sci U S A. 2002;99:9219–9224. doi: 10.1073/pnas.142053899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu L, Bergmeier W, Wu J, Jiang H, Stalker TJ, Cieslak M, Fan R, Boumsell L, Kumanogoh A, Kikutani H, et al. Regulated surface expression and shedding support a dual role for semaphorin 4D in platelet responses to vascular injury. Proc Natl Acad Sci U S A. 2007;104:1621–1626. doi: 10.1073/pnas.0606344104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wannemacher KM, Zhu L, Jiang H, Fong KP, Stalker TJ, Lee D, Tran AN, Neeves KB, Maloney S, Kumanogoh A, et al. Diminished contact-dependent reinforcement of Syk activation underlies impaired thrombus growth in mice lacking Semaphorin 4D. Blood. 2010;116:5707–5715. doi: 10.1182/blood-2010-04-279943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fong KP, Barry C, Tran AN, Traxler EA, Wannemacher KM, Tang HY, Speicher KD, Blair IA, Speicher DW, Grosser T, et al. Deciphering the human platelet sheddome. Blood. 2011;117:e15–26. doi: 10.1182/blood-2010-05-283838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Negishi M, Oinuma I, Katoh H. Plexins: axon guidance and signal transduction. Cell Mol Life Sci. 2005;62:1363–1371. doi: 10.1007/s00018-005-5018-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takamatsu H, Okuno T, Kumanogoh A. Regulation of immune cell responses by semaphorins and their receptors. Cell Mol Immunol. 2010;7:83–88. doi: 10.1038/cmi.2009.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luo Y, Raible D, Raper JA. Collapsin: a protein in brain that induces the collapse and paralysis of neuronal growth cones. Cell. 1993;75:217–227. doi: 10.1016/0092-8674(93)80064-l. [DOI] [PubMed] [Google Scholar]
- 9.Kolodkin AL, Matthes DJ, Goodman CS. The semaphorin genes encode a family of transmembrane and secreted growth cone guidance molecules. Cell. 1993;75:1389–1399. doi: 10.1016/0092-8674(93)90625-z. [DOI] [PubMed] [Google Scholar]
- 10.Roth L, Koncina E, Satkauskas S, Cremel G, Aunis D, Bagnard D. The many faces of semaphorins: from development to pathology. Cell Mol Life Sci. 2009;66:649–666. doi: 10.1007/s00018-008-8518-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tamagnone L, Artigiani S, Chen H, He Z, Ming GI, Song H, Chedotal A, Winberg ML, Goodman CS, Poo M, et al. Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell. 1999;99:71–80. doi: 10.1016/s0092-8674(00)80063-x. [DOI] [PubMed] [Google Scholar]
- 12.Nogi T, Yasui N, Mihara E, Matsunaga Y, Noda M, Yamashita N, Toyofuku T, Uchiyama S, Goshima Y, Kumanogoh A, et al. Structural basis for semaphorin signalling through the plexin receptor. Nature. 2010;467:1123–1127. doi: 10.1038/nature09473. [DOI] [PubMed] [Google Scholar]
- 13.Liu H, Juo ZS, Shim AH, Focia PJ, Chen X, Garcia KC, He X. Structural basis of semaphorin-plexin recognition and viral mimicry from Sema7A and A39R complexes with PlexinC1. Cell. 2010;142:749–761. doi: 10.1016/j.cell.2010.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janssen BJ, Robinson RA, Perez-Branguli F, Bell CH, Mitchell KJ, Siebold C, Jones EY. Structural basis of semaphorin-plexin signalling. Nature. 2010;467:1118–1122. doi: 10.1038/nature09468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franco M, Tamagnone L. Tyrosine phosphorylation in semaphorin signalling: shifting into overdrive. EMBO Rep. 2008;9:865–871. doi: 10.1038/embor.2008.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He Z, Tessier-Lavigne M. Neuropilin is a receptor for the axonal chemorepellent Semaphorin III. Cell. 1997;90:739–751. doi: 10.1016/s0092-8674(00)80534-6. [DOI] [PubMed] [Google Scholar]
- 17.Kolodkin AL, Levengood DV, Rowe EG, Tai YT, Giger RJ, Ginty DD. Neuropilin is a semaphorin III receptor. Cell. 1997;90:753–762. doi: 10.1016/s0092-8674(00)80535-8. [DOI] [PubMed] [Google Scholar]
- 18.Oinuma I, Ishikawa Y, Katoh H, Negishi M. The Semaphorin 4D receptor Plexin-B1 is a GTPase activating protein for R-Ras. Science. 2004;305:862–865. doi: 10.1126/science.1097545. [DOI] [PubMed] [Google Scholar]
- 19.Oinuma I, Katoh H, Negishi M. Semaphorin 4D/Plexin-B1-mediated R-Ras GAP activity inhibits cell migration by regulating beta(1) integrin activity. J Cell Biol. 2006;173:601–613. doi: 10.1083/jcb.200508204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vikis HG, Li W, Guan KL. The plexin-B1/Rac interaction inhibits PAK activation and enhances Sema4D ligand binding. Genes Dev. 2002;16:836–845. doi: 10.1101/gad.966402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swiercz JM, Worzfeld T, Offermanns S. ErbB-2 and met reciprocally regulate cellular signaling via plexin-B1. J Biol Chem. 2008;283:1893–1901. doi: 10.1074/jbc.M706822200. [DOI] [PubMed] [Google Scholar]
- 22.Kashiwagi H, Shiraga M, Kato H, Kamae T, Yamamoto N, Tadokoro S, Kurata Y, Tomiyama Y, Kanakura Y. Negative regulation of platelet function by a secreted cell repulsive protein, semaphorin 3A. Blood. 2005;106:913–921. doi: 10.1182/blood-2004-10-4092. [DOI] [PubMed] [Google Scholar]
- 23.Lewandrowski U, Wortelkamp S, Lohrig K, Zahedi RP, Wolters DA, Walter U, Sickmann A. Platelet membrane proteomics: a novel repository for functional research. Blood. 2009;114:e10–19. doi: 10.1182/blood-2009-02-203828. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki K, Okuno T, Yamamoto M, Pasterkamp RJ, Takegahara N, Takamatsu H, Kitao T, Takagi J, Rennert PD, Kolodkin AL, et al. Semaphorin 7A initiates T-cell-mediated inflammatory responses through alpha1beta1 integrin. Nature. 2007;446:680–684. doi: 10.1038/nature05652. [DOI] [PubMed] [Google Scholar]
- 25.Wang X, Kumanogoh A, Watanabe C, Shi W, Yoshida K, Kikutani H. Functional soluble CD100/Sema4D released from activated lymphocytes: possible role in normal and pathologic immune responses. Blood. 2001;97:3498–3504. doi: 10.1182/blood.v97.11.3498. [DOI] [PubMed] [Google Scholar]
- 26.Basile JR, Barac A, Zhu T, Guan KL, Gutkind JS. Class IV semaphorins promote angiogenesis by stimulating Rho-initiated pathways through plexin-B. Cancer Res. 2004;64:5212–5224. doi: 10.1158/0008-5472.CAN-04-0126. [DOI] [PubMed] [Google Scholar]
- 27.Akkerman JW. From low-density lipoprotein to platelet activation. Int J Biochem Cell Biol. 2008;40:2374–2378. doi: 10.1016/j.biocel.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 28.Zhu L, Stalker TJ, Fong KP, Jiang H, Tran A, Crichton I, Lee EK, Neeves KB, Maloney SF, Kikutani H, et al. Disruption of SEMA4D ameliorates platelet hypersensitivity in dyslipidemia and confers protection against the development of atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:1039–1045. doi: 10.1161/ATVBAHA.109.185405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yukawa K, Tanaka T, Kishino M, Yoshida K, Takeuchi N, Ito T, Takamatsu H, Kikutani H, Kumanogoh A. Deletion of Sema4D gene reduces intimal neovascularization and plaque growth in apolipoprotein E-deficient mice. Int J Mol Med. 2010;26:39–44. doi: 10.3892/ijmm_00000432. [DOI] [PubMed] [Google Scholar]