Abstract
Purpose
To investigate the spontaneous germ cell differentiation capacity of VUB hESC lines, develop a protocol for the induction of germ cell differentiation using conditioned medium from Sertoli cells (SCCM) and compare it to existing protocols.
Methods
hESC were allowed to differentiate spontaneously or after the addition of bone morphogenetic proteins (BMPs) and/or SCCM. VASA transcripts were measured by relative quantification real-time RT-PCR to determine the efficiency of germ cell differentiation.
Results
VUB hESC lines can differentiate spontaneously towards the germ cell lineage, however, more consistently in an embryoid body approach than in monolayer cultures. BMPs and SCCM significantly improve VASA expression, but do not have a synergistic effect. Direct contact of differentiating hESC with Sertoli cells does not improve VASA expression.
Conclusions
SCCM contains inductive factors for germ cell differentiation and could represent an element for in-vitro differentiation to germ cells.
Keywords: hESC, Sertoli cells, Germ cells, Differentiation, Embryoid bodies
Introduction
The germ line is responsible for the transmission of genetic information from one generation to the next one. In mammals, germ cell determination takes place at the onset of gastrulation, shortly after embryo implantation. It depends on local extracellular signals from the micro-environment. Studies in the mouse have shown that members of the transforming growth factor-β (TGF-β) super family play a crucial role in the induction of germ cell fate. Germ cells are derived from the proximal epiblast where they become specified in response to signals emanating from the extraembryonic ectoderm, including bone morphogenetic proteins 4 (BMP4) and 8 (BMP8) [1, 2]. The visceral endoderm is also indispensible for germ cell specification, possibly by the secretion of BMP2, another TGF-β family member [3]. Due to obvious biological and ethical constraints, in-vivo studies on human germ cell specification and differentiation are impossible. Therefore, in-vitro derivation of primordial germ cells (PGCs) and gametes could provide a means by which to study germ cell specification and gametogenesis and ultimately represent a new approach to alleviation of infertility.
Embryonic stem cells (ESC) can self-renew indefinitely and differentiate into cells from the three germ layers. They are capable of maintaining pluripotency, both in-vitro and in-vivo, even after extensive in-vitro culture. Recently, it has been shown that both murine and human ESC can differentiate towards the germ cell lineage. Germ cell derivation from murine ESC has been shown to be relatively easily reproducible. The earliest reports of Hubner et al. [4], Toyooka et al. [5] and Geijsen et al. [6] have been repeated by many groups and in 2006, Nayernia et al. even reported the production of live offspring from in-vitro derived male gametes [7].
Recent reports have provided evidence that hESC also have the potential to differentiate in-vitro into cells expressing germ cell markers. Clark et al. [8] were the first to report the detection of germ cell markers, including VASA, BOL, SCP1 and SCP3, in spontaneously differentiated embryoid bodies (EBs) from hESC and their results were confirmed by Chen et al. [9].
Spontaneous differentiation of murine and human ESC leads to low numbers of germ cells. Induced germ cell differentiation protocols can be divided into two main groups. In one group of protocols, specific factors are added to the differentiating cells in order to increase germ cell derivation (BMP4 [5, 10–12], retinoic acid (RA) [7, 13–15], basic fibroblast growth factor (bFGF) [16], testicular cell conditioned medium [14, 17], forskolin [15] and wingless-related MMTV integration site 3A (Wnt3a) [11]). Other protocols focus on the selection and enrichment of the spontaneously derived germ cell population [6, 18, 19].
Because the gene expression profiles of hESC and human PGCs are very similar, it is difficult to distinguish between these two cell types. Undifferentiated hESC express most of the known early germ cell-specific and germ cell-enriched genes, such as POU5F1, GDF3, NANOG, DAZL, STELLAR and cKIT [8, 20, 21]. But genes specific for germ cells during later stages of migration are not expressed by hESC. VASA is one of the earliest differentially expressed germ cell-specific genes. The human VASA gene (also known as DDX4) is a highly conserved, functionally important germ cell gene. It is expressed in male and female germ cells, starting at a gestational age of 7 weeks, at the end of the migratory phase of primordial germ cell development [22], within the PGCs that migrate towards the genital ridge, up till the spermatid stage and oocytes [22]. Even though the use of VASA expression as germ cell marker does not allow the detection of the most primitive germ cell precursors, it is considered as a good marker for germ cell differentiation from hESC.
In this study, we investigated the spontaneous differentiation of germ cells from hESC. In a further step, we introduced mouse Sertoli cells and Sertoli cell-conditioned medium in an attempt to improve germ cell differentiation and compared it to the effect of BMP addition, which has been proven to upregulate VASA expression in differentiating hESC [10]. We selected Sertoli cells for these experiments as they are a key factor in the spermatogonial stem cell niche [23, 24] and we hypothesized that they might therefore act as mediators of germ cell differentiation, by secreting growth factors or by means of cell contacts. Other groups already showed that elements from the adult germ cell niche can positively influence germ cell differentiation from ESC. Lacham-Kaplan et al. introduced conditioned medium from newborn mice testicular cells and reported the differentiation of mESC as EB into ovarian-like structures containing oocyte-like cells [17]. Oocyte-like cells were also derived from mESC by Qing et al. who applied a two-step protocol of spontaneous EB differentiation, followed by co-culture with mouse ovarian granulosa cells [25]. Richards et al. [15] used porcine ovarian fibroblast and conditioned medium of these fibroblasts to induce germ cell differentiation in hESC and recently Aflatoonian et al. [14] also reported germ cell differentiation from hESC using the mouse testicular cell conditioned medium, as described by Lacham-Kaplan et al. [17].
We found that mouse Sertoli cell-conditioned medium (SCCM) is able to upregulate VASA expression in hESC differentiating as EB, comparable to the effect of BMP addition, suggesting that one or more inductive factors are secreted into the medium. Direct cell contact of differentiating hESC with Sertoli cells did not result in an extra upregulation of VASA expression.
Materials and methods
Cell culture and differentiation
Four fully characterized hESC lines were used for this study. Three presumed genetically normal lines VUB01 (46, XY), VUB02 (46, XY) and VUB07 (46, XX), were derived from surplus IVF embryos from different couples [26, 27]. The fourth line, VUB09_FSHD (46, XX), was derived from an embryo diagnosed after a preimplantation genetic diagnosis cycle as carrying a mutation for Fascioscapulohumeral muscular dystrophy (FSHD) [27]. All cell lines are registered with the EU hESC registry (www.hescregistry.eu). HESC lines were isolated by immunosurgery and maintained in culture according to standard methods, as described previously [26]. Briefly, the undifferentiated cells were cultured as colonies on mitomycin-inactivated CF1 mice embryonic fibroblasts at 10% CO2 in knock-out Dulbecco’s Modified Eagle Medium (KO-DMEM; Invitrogen) containing 20% knock-out serum replacement (KO-SR; Invitrogen), 2 mmol/l L-glutamine (Invitrogen), 1% non-essential amino acids (NEAA; Invitrogen), 0.1 mmol/l β-mercaptoethanol (Sigma-Aldrich) and 4 ng/ml human recombinant basic fibroblast growth factor (bFGF; Invitrogen).
For differentiation experiments, hESC colonies were collected after 30 min of collagenase type IV treatment (1 mg/ml in KO-DMEM, 37°C), washed and centrifuged for 5 min at 115 g. Cell clumps were re-suspended in differentiation medium and plated in dishes, appropriate for the respective experiments. Basic differentiation medium consisted of KO-DMEM, with 20% fetal calf serum (FCS; Invitrogen), 2 mmol/l L-glutamine, 1% NEAA and 0.1 mmol/l β-mercaptoethanol.
In a first phase, cell clumps were plated in culture dishes, either coated with 0.1% gelatine or in uncoated Petri dishes, to investigate spontaneous differentiation in monolayer or as embryoid bodies (EB) respectively. In both conditions, basic differentiation medium was applied.
For the second phase of the study, all experiments were conducted in Petri dishes with EB differentiation and samples were collected at day 5 of differentiation. To investigate the influence of BMPs on germ cell differentiation, basic differentiation medium was supplemented with different combinations of BMP2 (355-BM, R&D Systems), BMP4 (314-BP, R&D Systems), BMP7 (354-BP, R&D Systems) and/or BMP8b (1073-BP, R&D Systems). In a first set-up, eight different conditions were tested: (1) control differentiation medium without supplementation, (2) +100 ng/ml BMP4, (3) +100 ng/ml BMP7, (4) +100 ng/ml BMP8b, (5) +100 ng/ml BMP4 +50 ng/ml BMP7, (6) +100 ng/ml BMP4 +50 ng/ml BMP8b, (7) +50 ng/ml BMP7 +50 ng/ml BMP8b and (8) +100 ng/ml BMP4 + 50 ng/ml BMP7 +50 ng/ml BMP8b. In a second set-up, we selected the best conditions from the first experiments and additionally investigated the effect of BMP2 in four different conditions: (1) control differentiation medium without supplementation, (2) +100 ng/ml BMP4, (3) +100 ng/ml BMP4 +50 ng/ml BMP7 +50 ng/ml BMP8b, (4) +100 ng/ml BMP4 +50 ng/ml BMP7 +50 ng/ml BMP8b + 100 ng/ml BMP2.
In the third phase of this study, the TM4 line (CRL1715, LGC Standards), a mouse immature Sertoli cell line was used to produce SCCM as an alternative mediator of differentiation by incubating 30 ml of basic differentiation medium in a 175 cm culture flask on a confluent layer of mouse Sertoli cells for 24 h. The medium was filtered (0.22 μm) before further use in differentiation experiments. In a first set-up, spontaneous EB differentiation in basic differentiation medium was compared to EB differentiation in SCCM. In a second set-up, EB differentiation in basic differentiation medium supplemented with 100 ng/ml BMP4 and EB differentiation in SCCM supplemented with 100 ng/ml BMP4 were added as conditions. All samples were collected after 5 days of differentiation.
In the last phase of this study, the same Sertoli cells were also used as feeder layers for culture of hESC in direct contact or in indirect contact. Mitomycin-inactivated Sertoli cells were plated at 4 × 104 cells/cm2 on 0.1% gelatine-coated culture dishes in DMEM, supplemented with 10% FCS, 2 mmol/l L-glutamine and 1% NEAA and left to attach for at least 12 h at 37°C, 5% CO2. For differentiation in direct contact, clumps of hESC were plated directly on the Sertoli cells in basic differentiation medium; for indirect contact, cell clumps were plated in the same medium on cell culture inserts (PICM01250, Millipore) on top of the inactivated Sertoli cells in order to allow the passage of secreted molecules in the medium, but to inhibit direct cell contacts. As control group, EB differentiation in SCCM was applied. Samples were collected at day 5 of differentiation.
Gene expression analysis
RNA was isolated using the RNeasy Micro Kit (Qiagen) and was reverse transcribed using the First-strand cDNA Synthesis Kit (GE Healthcare) with the NotI-d(T)18 primer according to the manufacturer’s protocol. A DNase treatment (RNase-Free DNase Set; Qiagen) was performed in all the samples.
Relative quantification real-time reverse transcription polymerase chain reaction (Rq-RT PCR) was performed on the ABI 7500 real-time thermocycler (Applied Biosystems) with the following cycling parameters: 2 min at 50°C, 10 min at 95°C, 40 cycles of 15 s at 95°C and 1 min at 60°C. PCR reactions were performed in a final reaction volume of 25 μl containing 12.5 μl 2× TaqMan Universal Master Mix (Applied Biosystems) and the appropriate amounts of primers and probes. For VASA, 1.25 μl 20× Assays-on-demand Gene Expression assay mix (Hs00987133_m1, Applied Biosystems) was applied; for GAPDH and Ubiquitin-C, that were used as endogenous controls, 900nM of primer mix and 250nM of probes were added to the mix (for primer- and probe-sequences and amplicon length see Table 1).
Table 1.
primer- and probesequences and amplicon lengths
| Gene | Primers (5’ to 3’) | Probe | Amplicon length |
|---|---|---|---|
| GAPDH | ATG-GAA-ATC-CCA-TCA-CCA-TCT-T | CAG-GAG-CGA-GAT-CC | 57 |
| CGC-CCC-ACT-TGA-TTT-TGG | |||
| Ubiquitin-C | CGC-AGC-CGG-GAT-TTG | TCG-CAG-TTC-TTG-TTT-GTG | 58 |
| TCA-AGT-GAC-GAT-CAC-AGC-GA |
Normalization against two endogenous controls GAPDH and Ubiquitin-C using the ∆∆Ct method of quantification was applied to relatively quantify VASA gene expression between multiple samples. Relative amount of mRNA was calculated as 2-∆∆Ct and quantitative RT-PCR results were statistically analysed by T-test or one-way ANOVA, according to the number of conditions in each experiment.
Immunohistochemistry
To verify the presence of the VASA protein in our experiments, immunofluorescence was applied. Colonies of undifferentiated hESC and EBs at day 5 of differentiation in SCCM were collected, fixed in formaline, dehydrated and embedded in paraffin. Cross sections of 4 μm thickness were used for staining. Sections of human testis tissue with normal spermatogenesis and Sertoli Cell Only Syndrome were used as positive and negative controls respectively. The slides were deparrafinised in xylene (2 × 5 min), followed by a rehydration in 100% (2 × 2 min), 90% (2 min) and 70% (2 min) ethanol respectively. After three washing steps [(tap water, distilled water, phosphate buffered salt solution (PBS)], antigen retrieval was performed by incubation of the slides in preheated 0.01 M citrate buffer (pH 6.0) for 20 min at 95°C. After 20 min of cooling in the same buffer, the slides were washed three times in PBS and blocking for non-specific binding was performed for 30 min at room temperature using PBS with 5% rabbit serum (R9133, Sigma-Aldrich). Excess fluid was removed and a goat polyclonal antibody against VASA [AF2030, R&D Systems; 5 μg/ml in PBS, 1% BSA (A9647, Sigma), 1% Tween20® (P1379, Sigma)] was applied overnight at 4°C. After three washing steps in PBS, the slides were incubated with Alexa Fluor® 488 conjugated F(ab’) rabbit anti-goat IgG (A21222, Invitrogen; 1/250 diluted in PBS, 1% BSA, 1%Tween® 20) for 2 h at room temperature. After washing three times in PBS, the sections were mounted with Prolong® Gold Antifade reagent with DAPI (P36931, Invitrogen) and a cover glass was applied. Confocal scanning microscopy with an argon–krypton laser (488/568) (Fluoview IX70; Olympus, Belgium) was used to record the fluorescent images.
Results
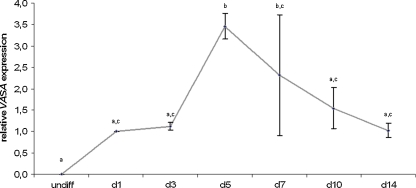
In a first phase, we studied the spontaneous differentiation of VUB hESC lines, both in a monolayer approach as through EB formation. In both methods, we detected VASA expression, however, in the monolayer approach this expression was highly variable whereas VASA transcripts could reproducibly be detected after EB differentiation (data not shown). Therefore, we continued our experiments using the EB differentiation approach. Over time, VASA expression within spontaneously differentiating EBs increased until day 5, followed by a rapid decrease (see Fig. 1). In this regard, we chose to collect samples for the following experiments on day 5 of differentiation.
Fig. 1.
Spontaneous differentiation. This figure shows the average relative VASA expression in 3 hESC lines (VUB01, VUB02 and VUB07) over time, during spontaneous differentiation as EBs, respectively in undifferentiated (undiff) cells and after 1, 3, 5, 7, 10 and 14 days of differentiation. Time points marked with different letters were significantly different after one-way ANOVA (undiff vs. d5: P < 0.001; undiff vs. d7, d1 vs. d5, d3 vs. d5, d5 vs. d14: p < 0.01; d5 vs. d10: p < 0.05). (hESC human embryonic stem cell, EB embryoid body)
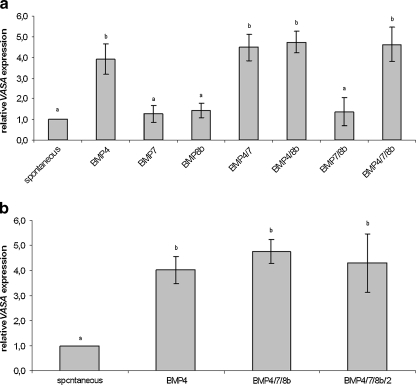
In the second phase of the study, BMPs were added to the standard differentiation medium in an attempt to upregulate germ cell differentiation. As shown in Fig. 2a, the addition of BMP4 was a determining factor, responsible for a significant increase in VASA expression. The addition of BMP7 and/or BMP8b alone did not lead to a higher VASA expression when compared to spontaneously differentiated EBs, neither did these factors seem to have an additive effect on BMP4 action. Moreover, BMP2 addition could not further increase VASA expression when compared to differentiation under influence of BMP4 or a combination of BMP4, BMP7 and BMP8b (see Fig. 2b).
Fig. 2.
Effect of BMP addition. This figure shows the average relative VASA expression in three hESC lines after the addition of different combinations of a BMP4, BMP7 and BMP8b in VUB01, VUB02 and VUB07 and b BMP4, BMP7, BMP8b and BMP2 in VUB01, VUB02 and VUB09_FSHD. Conditions marked with different letters were significantly different after one-way ANOVA (For a: spont vs. BMP4, spont vs. BMP4/7, spont vs. BMP4/8b, spont vs. BMP4/7/8b, BMP4 vs. BMP7, BMP7 vs. BMP4/7, BMP7 vs. BMP4/8b, BMP7 vs. BMP4/7/8b, BMP8b vs. BMP4/7, BMP8b vs. BMP4/8b, BMP8b vs. BMP4/7/8b, BMP4/7 vs. BMP7/8b, BMP4/8b vs. BMP7/8b, BMP7/8b vs. BMP4/7/8b: P < 0.001; BMP4 vs. BMP8b, BMP4 vs. BMP7/8b: P < 0.01. For b all P < 0.01). (hESC human embryonic stem cell, BMP bone morphogenetic protein)
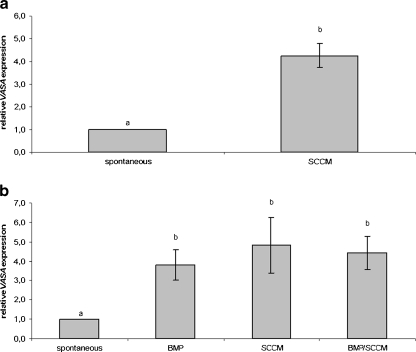
In the third phase of this study, hESC were allowed to differentiate as EBs in SCCM. This medium significantly upregulated VASA expression when compared to spontaneously differentiated EBs (see Fig. 3a). Moreover, the effect of SCCM was comparable to the effect of BMP addition but the combination of these two factors did not result in an additional increase in VASA transcripts (see Fig. 3b).
Fig. 3.
Effect of SCCM on VASA expression. This figure shows the average relative VASA expression of VUB01, VUB02 and VUB09_FSHD after a the use of SCCM for differentiation as EBs (SCCM) compared to spontaneous EB differentiation (spontaneous) or after b spontaneous differentiation as EBs (spontaneous) compared to differentiation induced by the addition of BMP4, BMP7 and BMP8b to the standard differentiation medium (BMP), differentiation induced by SCCM (SCCM) or differentiation by the addition of BMP4, BMP7 and BMP8b to the SCCM (SCCM/BMP). Conditions marked with different letters were significantly different after T-test (aP < 0.05) or one-way ANOVA (b spont vs. BMP: P < 0.05; spont vs. SCCM, spont vs. SCCM/BMP: P < 0.01). (SCCM Sertoli cell-conditioned medium, EB embryoid body, BMP bone morphogenetic protein)
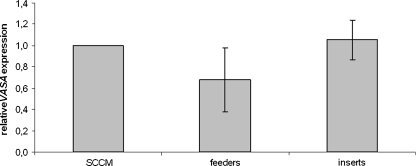
In the last phase of this study, we wanted to check if direct contact with the Sertoli cells could additionally increase germ cell differentiation when compared to SCCM or indirect contact with medium in which Sertoli cells are present. We found that VASA expression was comparable in cells that were growing as aggregates in SCCM or in inserts with indirect contact with a Sertoli cell feeder layer. Both conditions seemed to give better results than when the hESC were plated directly on the Sertoli cell feeder layer, but this difference was not significant (see Fig. 4).
Fig. 4.
(In)direct effect. Comparison of the average relative VASA expression of VUB01, VUB02 and VUB09_FSHD after differentiation as EBs in SCCM (SCCM) or in well inserts in indirect contact with a Sertoli cell feeder layer (inserts), compared to differentiation as monolayers on Sertoli cell feeder layers (feeders). No significant differences were observed. (SCCM Sertoli cell-conditioned medium, EB embryoid body)
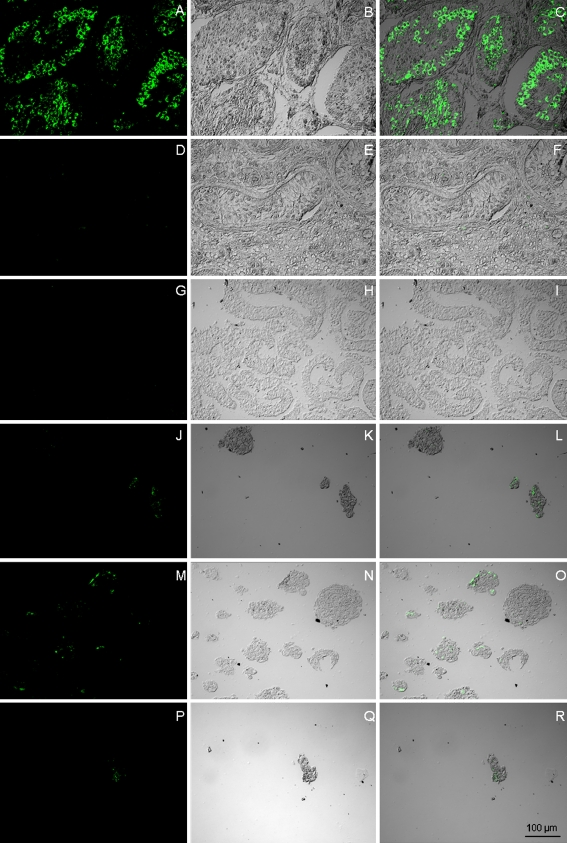
In the last part of this study, we checked whether VASA could also be detected at the protein level in EBs differentiated in SCCM. No expression of VASA was detected in undifferentiated hESC. For the three hESC lines differentiating using SCCM, we detected expression of VASA in most (but not all) EBs. Yet, for those EBs in which staining for VASA was positive, this marker could only be detected in a few cells (see Fig. 5).
Fig. 5.
VASA protein expression. Immunostaining for VASA in (a,b,c) human testis with normal spermatogenesis (positive control), (d,e,f) human testis with Sertoli-cell-only-syndrome (negative control), (g,h,i) undifferentiated hESC (VUB07), (j,k,l) VUB01 after differentiation as EB in SCCM, (m,n,o) VUB02 after differentiation as EB in SCCM, (p,q,r) VUB09_FSHD after differentiation as EB in SCCM. (hESC human embryonic stem cell, EB embryoid body, SCCM Sertoli cell-conditioned medium)
Discussion
hESC are a powerful tool in developmental and stem cell biology. Even though often considered as culture artifacts, they are believed to represent undifferentiated cells that have the capacity to differentiate in-vitro into derivatives of the three germ layers, through pathways that are used during in-vivo embryonic differentiation. Therefore, they could represent an in-vitro alternative for the study of early developmental processes in the human that, due to obvious ethical restrictions, cannot be conducted in-vivo. An important and still only superficially known event during this early human development is germ cell allocation and differentiation. In recent years, in-vitro germ cell differentiation from mESC and hESC has been more and more under investigation as it could be used as a tool for studying the processes of germ cell allocation, imprint erasure, epigenetic reprogramming and gamete formation. Alternatively, in-vitro derivation of germ cell could contribute to other areas of interest, in particular fertility restoration, as it could serve as an unlimited source of germ cell precursors or even gametes. For example, in-vitro derived spermatogonial stem cells could theoretically be used for intratesticular stem cell transplantation in order to restore fertility in the male or in-vitro derived gametes could be used as donor cells for artificial reproductive techniques such as in-vitro fertilization or intracytoplasmic sperm injection.
As reported previously, we show that hESC can differentiate spontaneously towards the germ cell lineage [8, 9]. Moreover, we were able to confirm the positive effect of BMPs, and more in particular BMP4, on germ cell differentiation in hESC [10, 12]. In mice, BMPs, produced by the extraembryonic ectoderm, have been shown to be crucial for germ cell differentiation from the epiblast cells in-vivo [1, 2]. In-vitro studies on mouse epiblast cells and mouse ESC have also shown the inductive effect of these BMPs on germ cell differentiation [5, 28, 29]. Therefore, it has been hypothesized that BMPs might also influence germ cell differentiation in the human. As in the study of Kee et al. [10], we found a significant increase in VASA expression in differentiating EBs from hESC after adding BMP4 to the differentiation medium. However, in contrast to their study, we could not detect an additive effect of BMP7 and/or BMP8b. Ying et al. [2] suggested that, in the mouse, BMP4 and BMP8b could both act as homodimers and heterodimers in PGC specification. Although they assumed that both factors are required, BMP4 seemed to have a more important impact on germ cell differentiation when compared to BMP8b [28]. This hypothesis was confirmed by Toyooka et al. [5] who found that co-culture of mESC with BMP4-producing cells could significantly increase the expression of mouse vasa homolog (Mvh) in their experiments, whereas BMP8b had only little effect on PGC induction. Our results can support this model in the human, suggesting that, in a high concentration of BMP4, this factor on its own is sufficient to trigger germ cell differentiation and the addition of BMP8b has no further effect whereas a possible additive effect of BMP8b and/or BMP7 action on lower concentrations of BMP4 is still possible. As for BMP7 and BMP8b, the addition of BMP2, which has also proven to be required for in-vivo germ cell differentiation in the mouse [3], did not lead to an increase in germ cell differentiation in our experiments. To our knowledge, no other reports on the effect of BMP2 on germ cell differentiation from mESC or hESC have been published. More research will therefore be necessary to further analyse a possible effect of this factor in higher concentrations.
In a second part of this study, we found that mouse SCCM can increase the expression of the germ cell marker VASA, comparable to the effect of BMP addition. A thorough analysis of the components of this SCCM in order to identify the factor(s) responsible for the increase in VASA expression could give new insights in the molecular mechanisms controlling germ cell differentiation in-vivo. Observing the comparable impact on VASA expression of the SCCM and BMP addition and the absence of an additive effect when combining these two factors, BMP4 itself is a potential candidate, responsible for the observed increase in VASA expression in our experiments. It has been reported that immature Sertoli cells express BMP4 [24, 30]. The TM4 line that was used in this study was derived from 11–13 days old BALB/c mice [31]. In a study with several inbred mouse strains, the earliest signs of puberty were reported at day 20.8 [32]. Therefore, we can hypothesize that this TM4 line probably produces BMP4. Moreover, as human and mouse BMP4 share 98% amino acid sequence identity (datasheet 314-BP, R&D Systems), it is plausible that the murine BMP4, secreted by Sertoli cells is able to bind and activate the human BMP4-receptor.
Gene expression analysis is associated with a number of caveats. The detection of the expression of a certain gene does not necessarily imply the translation of the mRNA into a protein, nor can it give information on the number of cells expressing this protein and the location of these cells. Moreover, it does not allow to discriminate whether the protein is in the right location within the cell to fulfill its biological function or the expression is ectopic and biologically unrelated due to culture artifacts. To tackle some of the previous problems, we applied immunofluorescent stainings on paraffin coupes of cultured EBs using antibody against the VASA protein. Immunofluorescence for the VASA protein revealed that, using our protocol, only a limited number of cells within the EBs differentiated towards the germ cell lineage. Even though the final aim of an in-vitro germ cell differentiation protocol would be to obtain a highly enriched or even pure population of differentiated germ cells, this result is consistent with the in-vivo situation, in which only a small population of epiblast cells are primed for germ cell fate [33, 34]. Our primary experiments on spontaneous germ cell differentiation comparing EB formation and monolayer approach as well as our further experiments in which indirect contact of EBs with Sertoli cells was compared to direct contact by monolayer culture, seemed to indicate a more consistent VASA expression in the 3D structure of the EB. Whereas monolayer approaches are usually an attempt to provide a more defined differentiation environment [35], it has been hypothesized that the EB structure might be recapitulating more accurately the complex assembly of cell adhesion and intracellular signalling within the early embryo, mimicking the in-vivo niche for germ cell differentiation. Geijsen et al. [6] showed a spatial arrangement of germ cell-like and haematopoietic-like cells within differentiating EBs, similarly as in the early developing mouse embryo, while Wei et al. [11] reported a more effective imprinting erasure in germ-cell like cells in an EB approach than in attachment culture.
Germ cell differentiation from hESC or other human pluripotent stem cells could significantly improve our knowledge of the pathways involved in developmental processes such as germ cell allocation, differentiation and even maturation. Moreover, it could have an important impact on fertility restoration in a possible clinical setting. This manuscript provides a simple, but reproducible protocol to induce germ cell differentiation in EBs from hESC, using conditioned medium derived from a murine Sertoli cell line. Its efficiency is comparable to earlier published germ cell differentiation protocols using human recombinant BMP proteins. As with our protocol, only a limited number of cells are triggered towards the germ cell differentiation pathway it would be worthwhile to try to improve its efficiency if a future clinical use for in-vitro derived germ cells would be considered. On the other hand, if the study of the pathways involved in germ cell differentiation is intended, this low number of germ cell precursors might be an advantage, since it is more consistent with the in-vivo situation. However, with both goals in mind, we can state that more research in the area of in-vitro germ cell differentiation from pluripotent stem cells is absolutely necessary.
Acknowledgements
Our research is supported by grants from the Research Foundation—Flanders (FWO-Vlaanderen) and the research council of the Brussels Free University (OZR).
Footnotes
Capsule
Sertoli cell-conditioned medium contains (an) inductive factor(s) for germ cell differentiation from human embryonic stem cells, possibly representing an element for in-vitro germ cell differentiation.
References
- 1.Lawson KA, Dunn NR, Roelen BA, Zeinstra LM, Davis AM, Wright CV, et al. Bmp4 is required for the generation of primordial germ cells in the mouse embryo. Genes Dev. 1999;13:424–436. doi: 10.1101/gad.13.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ying Y, Liu XM, Marble A, Lawson KA, Zhao GQ. Requirement of Bmp8b for the generation of primordial germ cells in the mouse. Mol Endocrinol. 2000;14:1053–1063. doi: 10.1210/me.14.7.1053. [DOI] [PubMed] [Google Scholar]
- 3.Sousa Lopes SM, Roelen BA, Monteiro RM, Emmens R, Lin HY, Li E, et al. BMP signaling mediated by ALK2 in the visceral endoderm is necessary for the generation of primordial germ cells in the mouse embryo. Genes Dev. 2004;18:1838–1849. doi: 10.1101/gad.294004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hübner K, Fuhrmann G, Christenson LK, Kehler J, Reinbold R, Fuente R, et al. Derivation of oocytes from mouse embryonic stem cells. Science. 2003;300:1251–1256. doi: 10.1126/science.1083452. [DOI] [PubMed] [Google Scholar]
- 5.Toyooka Y, Tsunekawa N, Akasu R, Noce T. Embryonic stem cells can form germ cells in vitro. Proc Natl Acad Sci USA. 2003;100:457–462. doi: 10.1073/pnas.1932826100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geijsen N, Horoschak M, Kim K, Gribnau J, Eggan K, Daley GQ. Derivation of embryonic germ cells and male gametes from embryonic stem cells. Nature. 2004;427:148–154. doi: 10.1038/nature02247. [DOI] [PubMed] [Google Scholar]
- 7.Nayernia K, Nolte J, Michelmann HW, Lee JH, Rathsack K, Drusenheimer N, et al. In vitro-differentiated embryonic stem cells give rise to male gametes that can generate offspring mice. Dev Cell. 2006;11:125–132. doi: 10.1016/j.devcel.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 8.Clark AT, Bodnar MS, Fox M, Rodriquez RT, Abeyta MJ, Firpo MT, et al. Spontaneous differentiation of germ cells from human embryonic stem cells in vitro. Hum Mol Genet. 2004;13:727–739. doi: 10.1093/hmg/ddh088. [DOI] [PubMed] [Google Scholar]
- 9.Chen HF, Kuo HC, Chien CL, Shun CT, Yao YL, Ip PL, et al. Derivation, characterization and differentiation of human embryonic stem cells: comparing serum-containing versus serum-free media and evidence of germ cell differentiation. Hum Reprod. 2007;22:567–577. doi: 10.1093/humrep/del412. [DOI] [PubMed] [Google Scholar]
- 10.Kee K, Gonsalves JM, Clark AT, Pera RA. Bone morphogenetic proteins induce germ cell differentiation from human embryonic stem cells. Stem Cells Dev. 2006;15:831–837. doi: 10.1089/scd.2006.15.831. [DOI] [PubMed] [Google Scholar]
- 11.Wei W, Qing T, Ye X, Liu H, Zhang D, Yang W, et al. Primordial germ cell specification from embryonic stem cells. PLoS ONE. 2008;3:e4013. doi: 10.1371/journal.pone.0004013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.West FD, Roche-Rios MI, Abraham S, Rao RR, Natrajan MS, Bacanamwo M, et al. KIT ligand and bone morphogenetic protein signaling enhances human embryonic stem cell to germ-like cell differentiation. Hum Reprod. 2010;25:168–178. doi: 10.1093/humrep/dep338. [DOI] [PubMed] [Google Scholar]
- 13.Kerkis A, Fonseca SA, Serafim RC, Lavagnolli TM, Abdelmassih S, Abdelmassih R, et al. In vitro differentiation of male mouse embryonic stem cells into both presumptive sperm cells and oocytes. Cloning Stem Cells. 2007;9:535–548. doi: 10.1089/clo.2007.0031. [DOI] [PubMed] [Google Scholar]
- 14.Aflatoonian B, Ruban L, Jones M, Aflatoonian R, Fazeli A, Moore HD. In vitro post-meiotic germ cell development from human embryonic stem cells. Hum Reprod. 2009;24:3150–3159. doi: 10.1093/humrep/dep334. [DOI] [PubMed] [Google Scholar]
- 15.Richards M, Fong CY, Bongso A. Comparative evaluation of different in vitro systems that stimulate germ cell differentiation in human embryonic stem cells. Fertil Steril. 2010;93:986–994. doi: 10.1016/j.fertnstert.2008.10.030. [DOI] [PubMed] [Google Scholar]
- 16.West FD, Machacek DW, Boyd NL, Pandiyan K, Robbins KR, Stice SL. Enrichment and differentiation of human germ-like cells mediated by feeder cells and basic fibroblast growth factor signalling. Stem Cells. 2008;26:2768–2776. doi: 10.1634/stemcells.2008-0124. [DOI] [PubMed] [Google Scholar]
- 17.Lacham-Kaplan O, Chy H, Trounson A. Testicular cell conditioned medium supports differentiation of embryonic stem cells into ovarian structures containing oocytes. Stem Cells. 2006;24:266–273. doi: 10.1634/stemcells.2005-0204. [DOI] [PubMed] [Google Scholar]
- 18.Tilgner K, Atkinson SP, Golebiewska A, Stojkovic M, Lako M, Armstrong L. Isolation of primordial germ cells from differentiating human embryonic stem cells. Stem Cells. 2008;26:3075–3085. doi: 10.1634/stemcells.2008-0289. [DOI] [PubMed] [Google Scholar]
- 19.Saiti D, Lacham-Kaplan O. Densitiy gradients for the isolation of germ cells from embryoid bodies. RBM Online. 2008;16:730–740. doi: 10.1016/s1472-6483(10)60489-0. [DOI] [PubMed] [Google Scholar]
- 20.Zwaka TP, Thomson JA. A germ cell origin of embryonic stem cells? Development. 2005;132:227–233. doi: 10.1242/dev.01586. [DOI] [PubMed] [Google Scholar]
- 21.Hua J, Sidhu K. Recent advances in the derivation of germ cells from the embryonic stem cells. Stem Cells Dev. 2008;17:399–411. doi: 10.1089/scd.2007.0225. [DOI] [PubMed] [Google Scholar]
- 22.Castrillon DH, Quade BJ, Wang TY, Quigley C, Crum CP. The human VASA gene is specifically expressed in the germ cell lineage. Proc Natl Acad Sci USA. 2000;97:9585–9590. doi: 10.1073/pnas.160274797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rooij DG, Repping S, Pelt AM. Role for adhesion molecules in the spermatogonial stem cell niche. Cell Stem Cell. 2008;3:467–468. doi: 10.1016/j.stem.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 24.Rooij DG. The spermatogonial stem cell niche. Microsc Res Tech. 2009;72:580–585. doi: 10.1002/jemt.20699. [DOI] [PubMed] [Google Scholar]
- 25.Qing T, Shi Y, Qin H, Ye X, Wei W, Liu H, et al. Induction of oocyte-like cells from mouse embryonic stem cells by co-culture with ovarian granulosa cells. Differentiation. 2007;75:902–911. doi: 10.1111/j.1432-0436.2007.00181.x. [DOI] [PubMed] [Google Scholar]
- 26.Mateizel I, Temmerman N, Ullmann U, Cauffman G, Sermon K, Velde H, et al. Derivation of human embryonic stem cell lines from embryos obtained after IVF and after PGD for monogenic disorders. Hum Reprod. 2006;21:503–511. doi: 10.1093/humrep/dei345. [DOI] [PubMed] [Google Scholar]
- 27.Mateizel I, Spits C, Rycke M, Liebaers I, Sermon K. Derivation, culture and characterization of VUB hESC lines. In Vitro Cell Dev Biol Anim. 2010;46:300–308. doi: 10.1007/s11626-010-9284-4. [DOI] [PubMed] [Google Scholar]
- 28.Ying Y, Qi X, Zhao GQ. Induction of primordial germ cells from murine epiblasts by synergistic action of BMP4 and BMP8B signaling pathways. Proc Natl Acad Sci USA. 2001;98:7858–7862. doi: 10.1073/pnas.151242798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoshimizu T, Obinata M, Matsui Y. Stage-specific tissue and cell interactions play key roles in mouse germ cell specification. Development. 2001;128:481–490. doi: 10.1242/dev.128.4.481. [DOI] [PubMed] [Google Scholar]
- 30.Pellegrini M, Grimaldi P, Rossi P, Geremia R, Dolci S. Developmental expression of BMP4/ALK3/SMAD5 signaling pathway in the mouse testis: a potential role of BMP4 in spermatogonia differentiation. J Cell Sci. 2003;116:3363–3372. doi: 10.1242/jcs.00650. [DOI] [PubMed] [Google Scholar]
- 31.Mather JP. Establishment and characterization of two distinct mouse testicular epithelial cell lines. Biol Reprod. 1980;23:243–252. doi: 10.1095/biolreprod23.1.243. [DOI] [PubMed] [Google Scholar]
- 32.Pinter O, Beda Z, Csaba Z, Gerendai I. Differences in the onset of puberty in selected inbred mouse strains. Endocr Abstr. 2007;14:P617. [Google Scholar]
- 33.Ohinata Y, Payer B, O’Carroll D, Ancelin K, Ono Y, Sano M, et al. Blimp1 is a critical determinant of the germ cell lineage in mice. Nature. 2005;436:207–213. doi: 10.1038/nature03813. [DOI] [PubMed] [Google Scholar]
- 34.Felici M. Primordial germ cell biology at the beginning of the XXI century. Int J Dev Biol. 2009;53:891–894. doi: 10.1387/ijdb.082815mf. [DOI] [PubMed] [Google Scholar]
- 35.Bratt-Leal AM, Carpenedo RL, McDevitt TC. Engineering the embryoid body microenvironment to direct embryonic stem cell differentiation. Biotechnol Prog. 2009;25:43–51. doi: 10.1002/btpr.139. [DOI] [PMC free article] [PubMed] [Google Scholar]