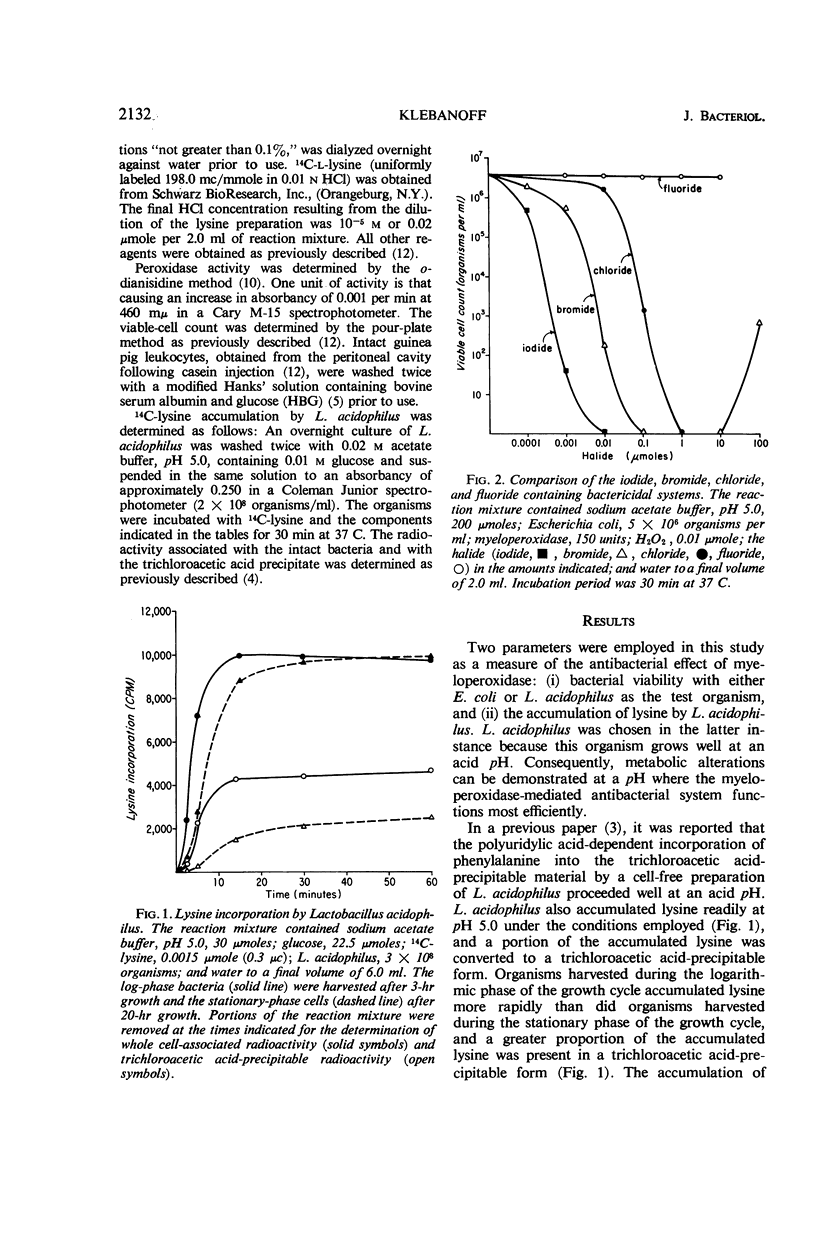
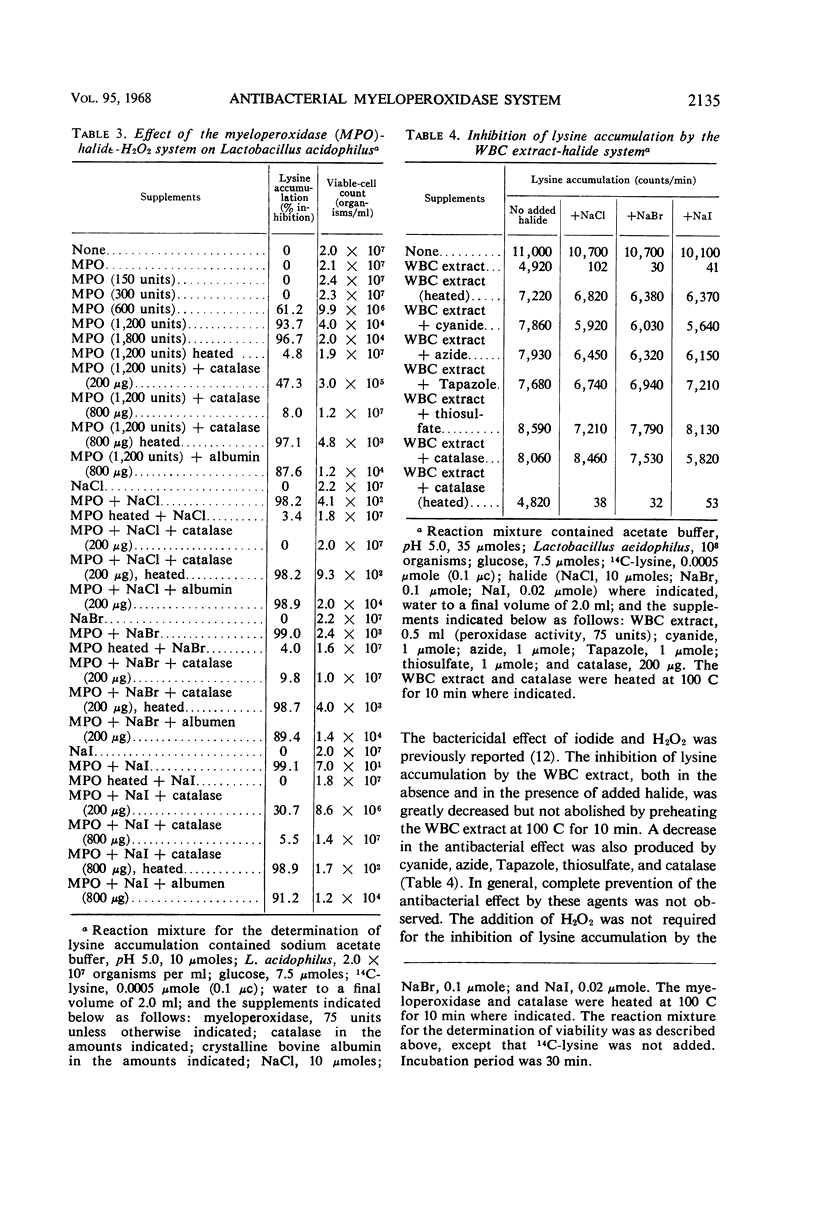
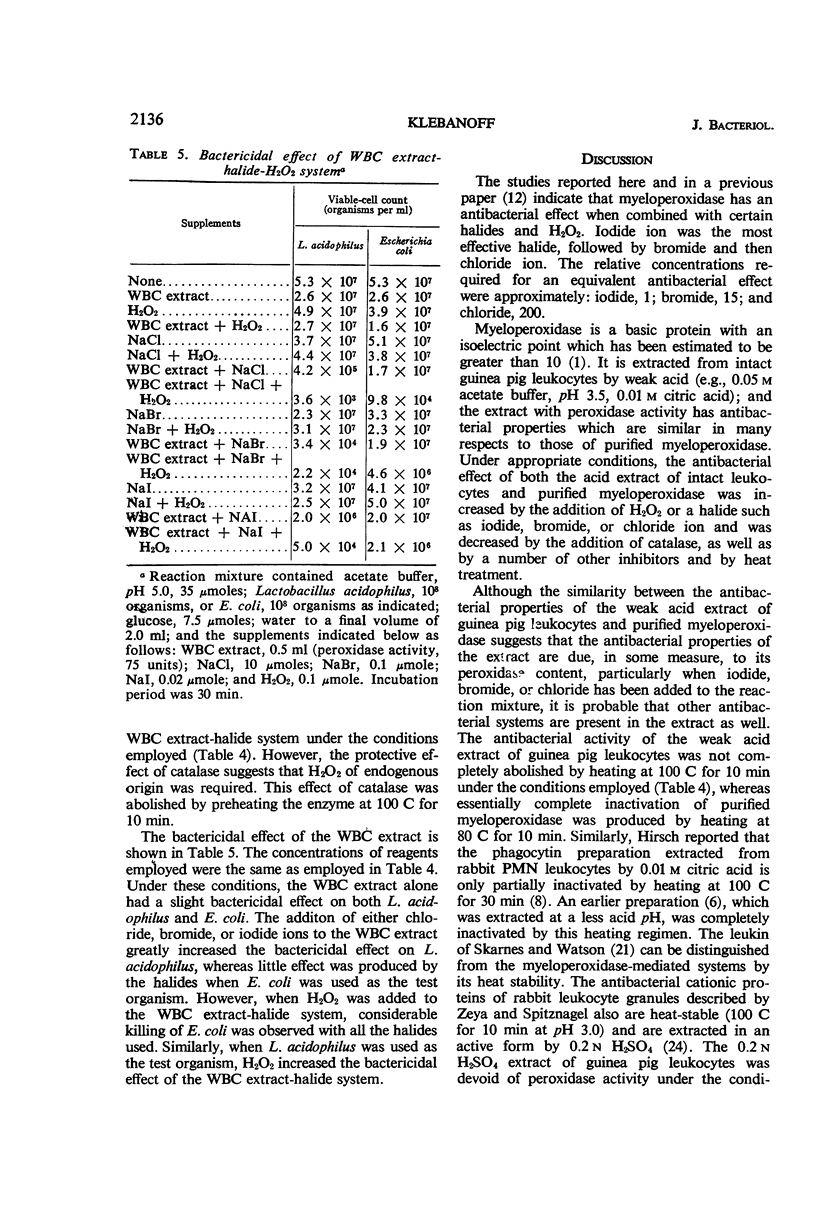
Abstract
An antibacterial effect of myeloperoxidase, a halide, such as iodide, bromide, or chloride ion, and H2O2 on Escherichia coli or Lactobacillus acidophilus is described. When L. acidophilus was employed, the addition of H2O2 was not required; however, the protective effect of catalase suggested that, in this instance, H2O2 was generated by the organisms. The antibacterial effect was largely prevented by preheating the myeloperoxidase at 80 C or greater for 10 min or by the addition of a number of inhibitors; it was most active at the most acid pH employed (5.0). Lactoperoxidase was considerably less effective than was myeloperoxidase when chloride was the halide employed. Myeloperoxidase, at high concentrations, exerted an antibacterial effect on L. acidophilus in the absence of added halide, which also was temperature- and catalase-sensitive. Peroxidase was extracted from intact guinea pig leukocytes by weak acid, and the extract with peroxidase activity had antibacterial properties which were similar, in many respects, to those of the purified preparation of myeloperoxidase. Under appropriate conditions, the antibacterial effect was increased by halides and by H2O2 and was decreased by catalase, as well as by cyanide, azide, Tapazole, and thiosulfate. This suggests that, under the conditions employed, the antibacterial properties of a weak acid extract of guinea pig leukocytes is due, in part, to its peroxidase content, particularly if a halide is present in the reaction mixture. A heat-stable antibacterial agent or agents also appear to be present in the extract.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COHN Z. A., MORSE S. I. Interactions between rabbit polymorphonuclear leucocytes and staphylococci. J Exp Med. 1959 Sep 1;110:419–443. doi: 10.1084/jem.110.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clem W. H., Klebanoff S. J. Inhibitory effect of saliva on glutamic acid accumulation by Lactobacillus acidophilus and the role of the lactoperoxidase-thiocyanate system. J Bacteriol. 1966 May;91(5):1848–1853. doi: 10.1128/jb.91.5.1848-1853.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRSCH J. G. Further studies on preparation and properties of phagocytin. J Exp Med. 1960 Mar 1;111:323–337. doi: 10.1084/jem.111.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRSCH J. G. Phagocytin: a bactericidal substance from polymorphonuclear leucocytes. J Exp Med. 1956 May 1;103(5):589–611. doi: 10.1084/jem.103.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRSCH J. G. Studies of the bactericidal action of phagocytin. J Exp Med. 1956 May 1;103(5):613–621. doi: 10.1084/jem.103.5.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLEBANOFF S. J. INACTIVATION OF ESTROGEN BY RAT UTERINE PREPARATIONS. Endocrinology. 1965 Feb;76:301–311. doi: 10.1210/endo-76-2-301. [DOI] [PubMed] [Google Scholar]
- KLEBANOFF S. J., LUEBKE R. G. THE ANTILACTOBACILLUS SYSTEM OF SALIVA. ROLE OF SALIVARY PEROXIDASE. Proc Soc Exp Biol Med. 1965 Feb;118:483–486. doi: 10.3181/00379727-118-29882. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J., Clem W. H., Luebke R. G. The peroxidase-thiocyanate-hydrogen peroxide antimicrobial system. Biochim Biophys Acta. 1966 Mar 28;117(1):63–72. doi: 10.1016/0304-4165(66)90152-8. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J. Iodination of bacteria: a bactericidal mechanism. J Exp Med. 1967 Dec 1;126(6):1063–1078. doi: 10.1084/jem.126.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORRISON M., HULTQUIST D. E. LACTOPEROXIDASE. II. ISOLATION. J Biol Chem. 1963 Aug;238:2843–2849. [PubMed] [Google Scholar]
- McRipley R. J., Sbarra A. J. Role of the phagocyte in host-parasite interactions. XI. Relationship between stimulated oxidative metabolism and hydrogen peroxide formation, and intracellular killing. J Bacteriol. 1967 Nov;94(5):1417–1424. doi: 10.1128/jb.94.5.1417-1424.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRipley R. J., Sbarra A. J. Role of the phagocyte in host-parasite interactions. XII. Hydrogen peroxide-myeloperoxidase bactericidal system in the phagocyte. J Bacteriol. 1967 Nov;94(5):1425–1430. doi: 10.1128/jb.94.5.1425-1430.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul B., Sbarra A. J. The role of the phagocyte in host-parasite interactions. 13. The direct quantitative estimation of H2O2 in phagocytizing cells. Biochim Biophys Acta. 1968 Feb 1;156(1):168–178. doi: 10.1016/0304-4165(68)90116-5. [DOI] [PubMed] [Google Scholar]
- RECHCIGL M., Jr, EVANS W. H. ROLE OF CATALASE AND PEROXIDASE IN THE METABOLISM OF LEUCOCYTES. Nature. 1963 Sep 7;199:1001–1002. doi: 10.1038/1991001b0. [DOI] [PubMed] [Google Scholar]
- SIEGEL E., SACHS B. A. IN VITRO LEUKOCYTE UPTAKE OF 131-I LABELED IODIDE, THYROXINE AND TRIIODOTHYRONINE, AND ITS RELATION TO THYROID FUNCTION. J Clin Endocrinol Metab. 1964 Apr;24:313–318. doi: 10.1210/jcem-24-4-313. [DOI] [PubMed] [Google Scholar]
- SKARNES R. C., WATSON D. W. Characterization of leukin: an antibacterial factor from leucocytes active against gram-positive pathogens. J Exp Med. 1956 Dec 1;104(6):829–845. doi: 10.1084/jem.104.6.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILSON D. L., MANERY J. F. The permeability of rabbit leucocytes to sodium, potassium and chloride. J Cell Physiol. 1949 Dec;34(3):493–519. doi: 10.1002/jcp.1030340312. [DOI] [PubMed] [Google Scholar]
- Zeya H. I., Spitznagel J. K. Cationic proteins of polymorphonuclear leukocyte lysosomes. I. Resolution of antibacterial and enzymatic activities. J Bacteriol. 1966 Feb;91(2):750–754. doi: 10.1128/jb.91.2.750-754.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeya H. I., Spitznagel J. K. Cationic proteins of polymorphonuclear leukocyte lysosomes. II. Composition, properties, and mechanism of antibacterial action. J Bacteriol. 1966 Feb;91(2):755–762. doi: 10.1128/jb.91.2.755-762.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]


