Abstract
A strong link between inflammation and metabolism is becoming increasingly evident. A number of recent landmark studies have implicated the activation of the NLRP3 inflammasome, an interleukin-1β family cytokine-activating protein complex, in a variety of metabolic diseases including obesity, atherosclerosis and type 2 diabetes. Here we review these new developments and discuss their implications for better understanding inflammation in metabolic disease and the prospects of targeting the NLRP3 inflammasome for therapeutic intervention.
Inflammation and metabolism: two intertwined pathways
Immune and metabolic responses are fundamental for survival and are highly conserved across species and throughout evolution. Normal cellular homeostasis is dependent on the integration of, and crosstalk between, both immune responses and metabolic regulation. Hence, the balance of these pathways must be tightly regulated to avoid chronic disease states. Since their relatively recent discovery, pattern recognition receptors (PPRs) have provided great insights into host-mediated immune responses to microbial attack. Several families of PRRs have been discovered including the Toll-like receptors (TLRs), Rig-I like receptors (RLRs) and Nod-like receptors (NLRs). The latter PRR family contains proteins that form large multimeric protein complexes, termed inflammasomes [1]. It is now becoming ever more apparent that these same receptors are not only able to recognize microbial signals, but also mediate immune responses to endogenous danger signals, including those arising in metabolic dysfunction. The potent pro-inflammatory cytokine, interleukin-1β (IL-1β), is known to contribute to the inflammatory response in various metabolic diseases [2]. However, the mechanism by which IL-1β is induced in distinctive metabolic dysfunctions have only more recently come to light and they appear to culminate from the activation of a common receptor complex, the NLRP3 (also known as NALP3) inflammasome, by diverse endogenous metabolic danger signals (summarized in Table 1). Activation of the NLRP3 inflammasome has previously been implicated in several metabolic diseases such as gout and pseudogout [3], by monosodium urate and calcium pyrophosphate dehydrate crystals, respectively and also by amyloid-β in Alzheimer’s disease [4]. The recent findings reviewed below further highlight the importance of this receptor complex in metabolic disease and the potential of therapeutic targeting of NLRP3 components or of the IL-1β family members.
Table 1.
Metabolic dangers signals triggering formation of the NLRP3 inflammasome
| Metabolic danger signal | Disease state | References |
|---|---|---|
| Amyloid beta | Alzheimer’s disease | [4] |
| Calcium pyrophosphate dehydrate (CPPD) crystals | Pseudogout | [3] |
| Ceramides | Obesity | [42] |
| Cholesterol crystals | Atherosclerosis | [19] |
| Islet amyloid peptide (IAPP) | T2D progression | [23] |
| Monosodium urate (MSU) crystals | Gout | [3] |
| Palmitate | Early T2D | [46] |
The NLRP3 inflammasome: a platform for IL-1β and IL-18 production
Three members of the NOD-like receptor (NLR) family, NLRP1, NLRP3 and NLRC4 (also termed IPAF) and the PYHIN family protein absence in melanoma 2 (AIM2), have been shown to form high molecular weight signaling platforms, termed ‘inflammasomes’ [1, 5, 6]. The most well characterized inflammasome to date is that formed around NLRP3, which also contains the adaptor molecule, apoptosis-associated speck-like protein containing a CARD (ASC) and pro-caspase-1. Activation induces oligomerization of the NLRP3 inflammasome and leads to the recruitment of ASC through homotypic PYD-PYD interaction. ASC, in turn, forms large speck-like structures and recruits pro-caspase-1 via CARD-CARD contact leading to autocatalytic activation of caspase-1. The produced p10 and p20 caspase-1 subunits assemble to form active caspase-1 hetero-tetramers that are able to convert inactive pro-IL-1β and pro-IL-18 into their bioactive and secreted forms [2, 7]. Following activation of the NLRP3 inflammasome, cells secrete large amounts of pro-inflammatory cytokines and in parallel undergo a highly inflammatory form of caspase-1-induced cell death termed ‘pyroptosis’ [8], the consequence of which is a robust IL-1-mediated immune response.
Due to the drastic outcome for NLRP3 activated cells and the possible damage evoked on surrounding tissues, it is not surprising that a two-step process mediates NLRP3 formation. An initial priming step (signal 1) is required in order for subsequent NLRP3 inflammasome formation by an activating signal (signal 2). The priming step can be mediated through PRRs or cytokine receptors or other factors known to induce activation of NF-κB. Most notably, priming is critical in immune cells, not only to produce a pool of pro-IL-1β substrate, but also to increase NLRP3 to a functional level, as endogenous NLRP3 expression in immune cells is insufficient to permit inflammasome activation and the resulting cleavage of caspase-1 [9] [10]. The NLRP3 inflammasome assembles in response to a variety of diverse exogenous and endogenous activators. Briefly, these include various microbial signals (bacteria, fungi and viruses), pore-forming toxins, crystalline substances, peptide aggregates as well as extracellular ATP released from dying cells. Interestingly, no direct interaction between NLRP3 and any of its diverse activators has been demonstrated as of yet, suggesting that a common upstream event is sensed by the NLRP3 inflammasome [6].
To date, a number of potential mechanisms explaining the assembly of the NLRP3 inflammasome have been put forward. According to one hypothesis, NLRP3 recognizes intracellular reactive oxygen species (ROS), which are commonly produced in response to many NLRP3 activators. Initially, ROS derived from NADPH oxidases were proposed to be responsible for NLRP3 activation [11]. However, more recently ROS produced in mitochondria rather than phagosomes have been implicated in NLRP3 activation [12, 13]. A second mechanism involves the disruption of lysosomal membrane integrity by crystalline materials and peptide aggregates [4, 14]. Upon uptake of such substances, lysosomal rupture leads to the leakage of lysosomal proteases, specifically cathepsins B and L, into the cytosol where they could possibly mediate NLRP3 inflammasome activation by an as yet, undefined cleavage event. It is likely that both mechanisms are required for full activation of the NLRP3 inflammasome or that they operate co-dependently. Additionally, cellular potassium efflux has also been shown to be a requirement for inflammasome activation [15]. Given the importance of NLRP3 inflammasome activation for inflammation a complex activation mechanism and elaborate regulatory processes are expected.
The NLRP3 inflammasome and vascular inflammation
Atherosclerosis arises within the walls of large arteries, often progresses to myocardial infarction or stroke, and displays the hallmarks of an inflammatory disease. Indeed, atherosclerosis is characterized by the recruitment of immune cells to artery walls [16]. However, the appearance of disease in germ-free mice suggests that the inflammation associated with atherosclerosis is initiated by endogenous components, rather than by infectious agents [17]. One of the endogenous molecules that has long been associated with atherosclerosis is cholesterol [18]. Elevated blood cholesterol levels are intimately linked to atherosclerotic disease development and dietary administration of increased amounts of cholesterol is sufficient to cause atherosclerosis in a number of animals. Large amount of cholesterol can be found in atherosclerotic lesions in close proximity of activated immune cells either in the form of cholesteryl esters within macrophage foam cells or as crystalline cholesterol inside or outside cells. Cholesterol has very low solubility in aqueous solutions and cholesterol crystals can be detected by standard histology as so-called cholesterol crystal clefts in advanced atherosclerotic lesions. Recently, by using laser reflection microscopy, it was discovered that in addition to the large crystals that leave clefts in tissues an abundance of much smaller cholesterol crystals are present in the extracellular space as well as inside immune cells in atherosclerotic lesions. In vitro experiments showed that primed macrophages secrete large amounts of IL-1β in response to cholesterol crystals in an NLRP3 inflammasome-dependent manner in both mouse and human cells [19, 20]. Notably, when bone marrow from mice deficient in either NLRP3, ASC or IL-1α/β was transferred into irradiated atherosclerotic-prone low density lipoprotein (LDL) receptor-deficient mice and these animals were fed a ‘Western’ high cholesterol diet, they displayed markedly decreased aortic lesion size, as well as reduced levels of circulating IL-18 when compared to mice transplanted with wild-type bone marrow [19]. This study suggested that inflammasome activation in bone marrow derived myeloid cells contributes to murine atherosclerosis. In a recent study using the APOE-deficient murine atherosclerosis model, however, no differences in lesions could be assessed when mice lacked NLRP3 inflammasome components [21]. The different outcome of the atherosclerosis models is not entirely surprising. The speed and extend of development of atherosclerosis may be influenced both by the model and the choice of atherogenic diet. In the study using the APOE-deficient atherosclerosis model [21], an atherogenic diet was used with more than eight-fold higher cholesterol amounts than the diet used in the other described study [19] and by most other investigators. Additionally, the atherogenic diet was supplied for a longer time in the APOE-deficient atherosclerosis model and ApoE-deficient mice represent a more aggressive model for atherosclerosis and are known to yield a strong atherogenic phenotype with exaggerated circulating cholesterol and, in contrast to LDLR-deficient mice, develop atherosclerosis even without a high fat diet. It would therefore not be surprising, and perhaps even expected, that challenging inflammasome-deficient mice with a much higher dose of cholesterol for a longer time would lead to greater disease through an inflammasome-independent pathway. It is well established that several redundant mechanisms contribute to the development of atherosclerosis. In essentially all instances, knockout of inflammatory genes that have been implicated in atherogenesis lead to reduced atherosclerosis but never to a complete absence of disease [22]. Moreover, the observed effects of gene knockouts have typically been found more pronounced in early atherosclerosis and less evident after extensive growth of the atheroma. An alternative approach to study the relevance of cholesterol crystal recognition in atherosclerotic plaques would be to perform experiments aiming at the reduction of cholesterol crystal amounts in atherosclerotic lesions.
LDL is central to progression of atherosclerosis and accumulates within the inner most layer of the artery wall early in the disease process, where it is modified (e.g. oxidized) and promotes recruitment of monocytes and the formation of cholesterol-engorged macrophage foam cells [16]. In an in vivo setting the priming step required for NLRP3 inflammasome activation is likely mediated by a modified form of LDL. Indeed, modified LDL has been shown to increase expression of pro-IL-1β in macrophages [19, 23]. This is mediated by the ability of modified-LDL to activate NF-κB via a receptor complex involving Toll-like receptor (TLR) 4/6 [24], CD14 [25] and various scavenger receptors (e.g., CD36, SR-A) [26, 27] and dependent on signaling through MyD88 [28, 29] and the kinase activity of IRAK-4 [30]. Not surprisingly, mice deficient in any of these components exhibit reduced atherosclerotic lesions. Within the atherosclerotic lesion, oxidized-LDL also provides cells with the activation step of the NLRP3 inflammasome as it facilitates the formation of cholesterol crystals [19, 31]. The role of TLRs during in vivo priming of the inflammasome has been demonstrated in other sterile inflammatory diseases. Previously, TLR9 has been shown to recognize excessively released host DNA, priming subsequent activation of the NLRP3 inflammasome in response to danger signals induced by liver damage [32].
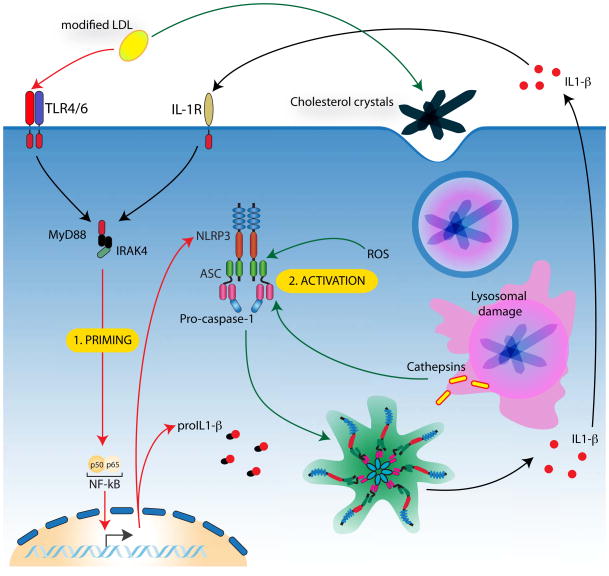
The oxidation of LDL is dependent on ROS produced from macrophages and surrounding epithelial cells. Interestingly, oxidized-LDL itself also induces the production of ROS [33] and causes lysomal damage [34], both of which are implicated in mechanisms of NLRP3 inflammasome activation. Moreover, the reduced secretion of IL-1β observed in cathepsin B and L-deficient mouse macrophages stimulated with cholesterol crystals, suggests phagosomal leakage is required to activate the NLRP3 inflammasome in atherosclerosis [19]. The activation of the inflammasome in early atherosclerosis appears to be quite unique, in that, the same sterile molecule indirectly mediates both signals 1 and 2, albeit by differing modes of action (Figure 1).
Figure 1. NLRP3 inflammasome activation in atherosclerosis.
In order for NLRP3 inflammasome to be activated in atherosclerosis it must first be primed via recognition of modified LDL by TLRs and scavenger receptors on macrophages. This priming step allows upregulation of pro-IL-1β and NLRP3. Subsequently, modified LDL-induced cholesterol crystals that are phagocytosed by macrophages cause lysosomal rupture, allowing release of lysosomal proteases (cathepsins). Potentially in combination with ROS production, cathepsins mediate the activation of the NLRP3 inflammasome resulting in caspase-1 cleavage and the production of mature IL-1 cytokine. Mature IL-1 is then released from cells causing continued upregulation of inflammasome components as well as mediating an inflammatory response that results in an influx of immune cells and the progression of atherosclerotic plaque formation.
Type 2 diabetes progression facilitated by NLRP3
Type 2 diabetes (T2D) is another disease mediated by a sterile inflammatory mechanism, which, until recently was not well understood. Obesity-induced insulin resistance and dysfunction of islet beta cells in the pancreas are characteristic of the disease [35]. An additional feature is pancreatic accumulation of islet amyloid polypeptide (IAPP), which is readily taken up by both dendritic cells (DCs) and macrophages [36]. In a recent study, stimulation of primed bone marrow-derived DCs and macrophages with human IAPP was shown to induce cleavage of caspase-1, formation of ASC specks and production of IL-1β in an NLRP3-dependent manner [23]. In agreement with previous findings, activation of the NLRP3 inflammasome appeared to require activity of the phagosomal protease cathepsin B and ROS production. As mouse IAPP does not form active amyloid aggregates, a transgenic mouse model overexpressing human IAPP was utilized to demonstrate that IAPP induces macrophages to produce IL-1β in pancreatic islets in vivo [23]. The IL-1-induced inflammation produced in the pancreas is likely to result in death of beta cells, T2D disease progression and development of insulin-dependent diabetes.
Previously, thioredoxin-interacting protein (TXNIP) had been implicated in NLRP3 inflammasome activation [37], and additionally, glucose-induced TXNIP has been shown to correlate with activation of caspase-1 and IL-1β production in human and mouse adipose tissue [38]. Conversely, the present study suggests TXNIP is not involved in T2D progression as demonstrated by normal NLRP3-induced IL-1β production in cells from TXNIP-deficient mice. These discrepancies may be explained by differing experimental approaches that may represent induction of NLRP3 by TXNIP only under specific conditions or in specific cells.
In the IAPP transgenic study, the authors also investigated the endogenous priming mechanism of the NLRP3 inflammasome in T2D. Population-based studies have found a correlation between obesity, T2D and increased concentrations of modified LDL [39]. Just as modified LDL is able to prime macrophages for NLRP3 activation in atherosclerosis [19], it can similarly prime cells via TLR4 signaling for IAPP-induced inflammasome activation in T2D [23].
Obesity activates the NLRP3 inflammasome
Obesity predisposes individuals to an array of chronic IL-1β-driven metabolic diseases including atherosclerosis and T2D [40]. Furthermore, an obesity-induced inflammatory state has been linked to the activation of adipose tissue macrophages (ATMs) within fat deposits [41]. Notably, a recent study also found that obesity itself induces the assembly of the NLRP3 inflammasome in ATMs, mediating insulin resistance in early T2D [42]. In free feeding mice on a normal chow diet, increased expression of both NLRP3 and IL-1β in visceral adipose tissue was found to correlate directly with body weight and adiposity when compared to mice feed on a calorie-restricted diet. These observations were somewhat paralleled in humans, where weight loss in obese T2D sufferers was associated with decreased NLRP3 and IL-1β expression in subcutaneous adipose tissue. Direct involvement of NLRP3 in obesity was confirmed with studies that showed gene-deficient mice fed a high fat diet displayed reduced caspase-1 activation and pro-IL-1β expression in adipose tissue and loss of serum IL-18 production compared to their wildtype counterparts. Moreover, NLRP3-deficient and caspase-1-deficient mice are more protected from high fat diet induced insulin resistance [42, 43]. The decrease in insulin sensitivity was found to be a consequence of NLRP3 inflammasome-mediated activation of effector adipose T cells that, through release interferon-gamma, mediate downstream pathways resulting in insulin resistance [42].
A number of sterile danger signals released in fat tissues could activate the tissue resident immune cells such as macrophages or dendritic cells to form a NLRP3 inflammasome. One endogenous danger signal responsible for NLRP3 activation in the case of obesity may possibly be the lipid molecule ceramide, which is composed from sphingoside and fatty acid [44]. During obesity, circulating levels of free fatty acids are increased and are likely to be scavenged by ATMs to generate ceramide [45]. LPS-primed macrophages stimulated with ceramide displayed NLRP3-dependent caspase-1 activation and production of IL-1β albeit at relatively low levels [42]. Likewise, adipose tissue explants from diet-induced obese mice had increased caspase-1 cleavage in response to ceramide. While no evidence has yet been published, it is conceivable that other endogenous triggers, such as material released from dying cells or crystalline substances may activate NLRP3 in this disease setting. More recently another lipid, the saturated fatty acid palmitate, was shown to activate the NLRP3 inflammasome [46]. Palmitate is one the most abundant free fatty acids in plasma and is highly elevated in obesity. Like palmitate, the unstaturated fatty acid oleate, is also highly abundant in plasma, however unlike palmitate, oleate was shown not to activate the NLRP3 inflammasome [46]. Interestingly, in comparison to other described NLRP3 activators, palmitate seems to mediate NLRP3 activation via a unique mechanism involving reduction of AMP-protein kinase (AMPK) activity leading to a defective autophagic process and the subsequent accumulation of mitochondrial ROS. This is consistent with previous findings that mitochondrial ROS is a potential requirement for NLRP3 activation [12, 13]. Results from this study also suggest that the activation of NLRP3, possibly by palmitate, and subsequent IL-1β production mediates insulin resistance both directly, via inhibition of insulin signaling and indirectly via increased production of TNF, a known inducer of insulin resistance [47, 48].
Taken together with the work on IAPP and inflammasome activation, the findings from the studies with ceramide and palmitate suggest that NLRP3 is not only important in the early stages of T2D but also in the chronic progression of the disease.
Obesity: taking the handbrake off the NLRP3 inflammasome
Obesity is now prevalent in approximately 30% of the adult population [49], and is associated with an underlying chronic low-level inflammatory state [50]. Indeed, coinciding with rises in obesity are increased incidences of various inflammatory driven metabolic diseases, including atherosclerosis and T2D [51]. Alarmingly, the World Heath Organization predicts the current worldwide obesity trend will continue to rise with around 2.3 billion overweight adults, of which 700 million will be obese by 2015. The findings reviewed above have demonstrated NLRP3 is a common receptor activated in response to various metabolic danger factors. What is also becoming clear is that obesity itself can provide the priming signals required for NLRP3 activation in multiple metabolic diseases, thereby removing the safety switch from NLRP3 signaling (Figure 2). This would help to explain the vulnerability of obese individuals, not only to metabolic diseases such as atherosclerosis, T2D and gout, but also to NLRP3-dependent infectious and environmentally driven diseases.
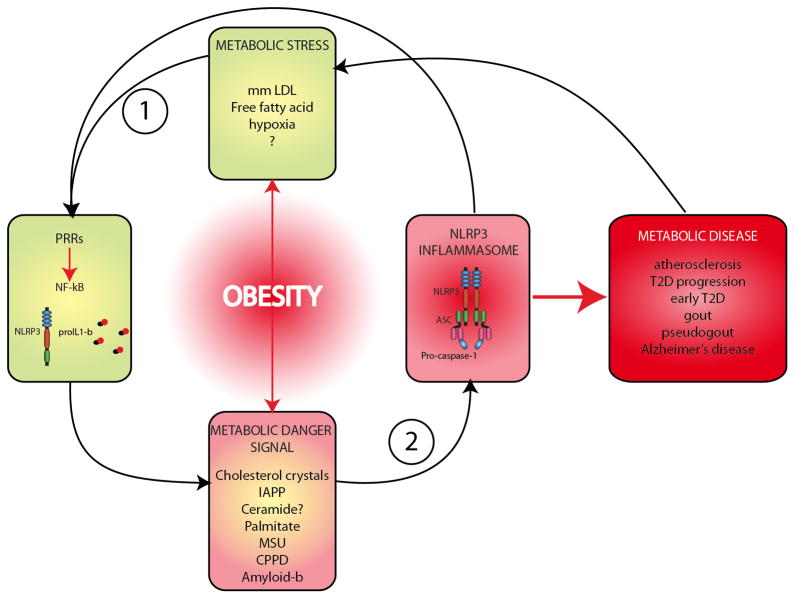
Figure 2. Obesity is a key facilitator of NLRP3 inflammasome induction in metabolic disease.
Obesity often supplies the initial signals required to prime the NLRP3 inflammasome that can include modified LDL and free fatty acids. Many of the metabolic danger signals subsequently sensed by the NLRP3 inflammasome are also a direct/indirect result of obesity. Once the inflammasome has been engaged by a specific danger signal the resulting pro-inflammatory induced state often leads to progression of a particular metabolic disease.
During obesity, endogenous molecules that have been found to prime the NLRP3 inflammasome have also been shown to increase and accumulate at local tissue sites. Modified LDL is one such molecule that is likely to prime macrophages in vivo for subsequent NLRP3 activation in both atherosclerosis and T2D. Circulation of free fatty acids is also increased in obesity and can accumulate in adipose tissues during T2D progression [45, 52]. Like modified LDL, free fatty acids can induce NF-κB signaling via TLR4 [52] and could therefore act as an alternative priming signal for the NLRP3 inflammasome in T2D. Expression of TLR2 and TLR4 in adipose tissue increases with obesity [53] and deficiency of TLR4 in mice has been shown to be protective against fat-induced inflammation and insulin resistance [54–57]. It is probable that many more as yet unidentified NLRP3 priming molecules exist in obesity and their identification will provide greater insights into metabolic diseases. The inflammation in obesity can therefore prime cells for NLRP3 inflammasome activation making the inflammasome an attractive target for drug therapy.
Treating metabolic disease by targeting the NLRP3 inflammasome
To date no therapeutics directly targeting the NLRP3 inflammasome have been developed. Instead, available therapies that neutralize the major downstream product of inflammasome activation, IL-1β, have been trailed in a number of metabolic diseases. Clinical trials with anakinra, a recombinant human IL-1 receptor antagonist, have shown positive results in treatment of gout, pseudogout and T2D [58–61]. Moreover, chronic gouty arthritis has also been reported to be suppressed using rilonacept (often referred to as IL-1 trap), an IL-1α/β neutralizer [62]. It would also be of great interest to observe the effects of such treatments on other inflammasome-mediated metabolic diseases, such as atherosclerosis and Alzheimer’s disease.
Recently, the effectiveness of the high affinity monoclonal antibody to IL-1β, XOMA 052, has been tested in mice in the context of atherosclerosis with promising results [63]. Specifically, over a range of doses XOMA 052 inhibited the formation of atherosclerotic lesions in ApoAE-deficient mice. This antibody is also currently in Phase IIb clinical trials in T2D patients. However, in March 2011 the manufactures of the antibody, XOMA Ltd., announced that over a six-month period the trials had been unsuccessful in reducing hyperglycemia in T2D patients. Promisingly the antibody still appears to have potential as a treatment in cardiovascular disease, with large decreases in biomarkers for heart disease observed and significant increases in levels of high-density lipoprotein in treated patients. The mixed clinical trial results with XOMA 052 may be indicative of the role of NLRP3 in the diseases themselves. It may be that NLRP3 and therefore IL-1β plays a more prominent role in certain diseases such as gout and cardiovascular disease than in obesity and T2D and this is somewhat evident in the results presented in this review. Hence, targeting IL-1β may only be effective in some metabolic diseases involving NLRP3.
Given the recent insights into NLRP3 related metabolic diseases with clear links to obesity, perhaps a more preventative therapeutic approach needs to be taken rather than one aimed at blocking the outcomes of inflammasome activation. In this regard therapeutics directly targeting components of the NLRP3 inflammasome are of interest. Indeed, a recent study found that direct inhibition of caspase-1 was beneficial in reducing obesity and improving insulin sensitivity in mice [43]. Furthermore, as obesity is clearly a common precursor for NLRP3-mediated metabolic diseases, therapeutics and lifestyle changes that promote weight loss could also be beneficial in this regard.
Alternatively, as obesity seems to prime the inflammasome, at least in part, via the activity of TLR-induced NF-κB, drugs limiting TLR activation could be valuable. However, given the importance of TLRs in protection from infection by pathogens and in wound repair [64, 65], great caution would have to be taken to ensure such processes where maintained.
Concluding remarks
The findings reviewed here highlight the huge potential for directly targeting components of the NLRP3 inflammasome in combating various metabolic conditions. Although a number of key features of the NLRP3 inflammasome remain elusive, the ever-expanding body of work focused on NLRP3 suggests that it is only a matter of time before new concepts and knowledge of the molecular intricacies of its activation are revealed that will hopefully provide new targets to be actively pursued in drug therapies. The NLRP3 inflammasome has evolved to recognize unwanted entry of microbes, such as funguses, certain bacteria or viruses into the cells’ interior and it therefore represents a cell-autonomous microbe detection system. If triggered, the effector molecules can govern a profound immune response that culminate in the recruitment of a host of immune cells to the affected site leading to antimicrobial resistance and to the induction of reparative processes. NLRP3 inflammasome activation, however, can also induce seemingly spurious inflammatory responses, as seen in the various tissues pathologies induced during the ‘syndrome X’. In these situations, sterile molecules that are prone to form aggregates and crystals, or excessive amounts of certain lipids, are sensed and the NLRP3-mediated immune response can cause collateral damage, in particular if the inciting stimulus is not removed in a timely manner. This latter sterile inflammatory threat only surfaces when certain molecules are present in excess or accumulate locally. Recognition of the transition of molecules from soluble to solid, or sensing of local accumulation of certain substances, does appear to make ‘immunological sense’ as by this mechanisms the immune system can respond to metabolic disturbances or local tissue derangements. In addition, IL-1β and - to a lower extent - other pro-inflammatory cytokines have long been shown to induce behavioral changes leading to reduced food consumption and an anorectic phenotype [66–68]. These changes have likely evolved to ensure a behavioral modification during immunological stress situations [69] and paradoxically could also aim to counterbalance calorie intake after ‘metabolic stress’. The clear correlation of NLRP3-driven pathologies with the emergence of contemporary western lifestyles of calorie overload and physical inactivity is intriguing. At the same time this inappropriate immune response towards metabolic cues is in large parts preventable and gives hope to those willing to achieve a fraction of the cardiorespiratory fitness that was so important for the survival of our common ancestors, the hunter-gatherers.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Martinon F, et al. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 2.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 3.Martinon F, et al. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 4.Halle A, et al. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol. 2008;9:857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ting JP, et al. The NLR gene family: a standard nomenclature. Immunity. 2008;28:285–287. doi: 10.1016/j.immuni.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stutz A, et al. Inflammasomes: too big to miss. J Clin Invest. 2009;119:3502–3511. doi: 10.1172/JCI40599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson KP, et al. Structure and mechanism of interleukin-1 beta converting enzyme. Nature. 1994;370:270–275. doi: 10.1038/370270a0. [DOI] [PubMed] [Google Scholar]
- 8.Bergsbaken T, et al. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009;7:99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bauernfeind FG, et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183:787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franchi L, et al. Cutting edge: TNF-alpha mediates sensitization to ATP and silica via the NLRP3 inflammasome in the absence of microbial stimulation. J Immunol. 2009;183:792–796. doi: 10.4049/jimmunol.0900173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dostert C, et al. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou R, et al. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 13.Nakahira K, et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2011;12:222–230. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hornung V, et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petrilli V, et al. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 2007;14:1583–1589. doi: 10.1038/sj.cdd.4402195. [DOI] [PubMed] [Google Scholar]
- 16.Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wright SD, et al. Infectious agents are not necessary for murine atherogenesis. J Exp Med. 2000;191:1437–1442. doi: 10.1084/jem.191.8.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldstein JL, Brown MS. Lipoprotein receptors, cholesterol metabolism, and atherosclerosis. Arch Pathol. 1975;99:181–184. [PubMed] [Google Scholar]
- 19.Duewell P, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rajamaki K, et al. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS One. 2010;5:e11765. doi: 10.1371/journal.pone.0011765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Menu P, et al. Atherosclerosis in ApoE-deficient mice progresses independently of the NLRP3 inflammasome. Cell Death Dis. 2011;2:e137. doi: 10.1038/cddis.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kleemann R, et al. Cytokines and atherosclerosis: a comprehensive review of studies in mice. Cardiovasc Res. 2008;79:360–376. doi: 10.1093/cvr/cvn120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Masters SL, et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1beta in type 2 diabetes. Nat Immunol. 2010;11:897–904. doi: 10.1038/ni.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stewart CR, et al. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2010;11:155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller YI, et al. Minimally modified LDL binds to CD14, induces macrophage spreading via TLR4/MD-2, and inhibits phagocytosis of apoptotic cells. J Biol Chem. 2003;278:1561–1568. doi: 10.1074/jbc.M209634200. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki H, et al. A role for macrophage scavenger receptors in atherosclerosis and susceptibility to infection. Nature. 1997;386:292–296. doi: 10.1038/386292a0. [DOI] [PubMed] [Google Scholar]
- 27.Febbraio M, et al. Targeted disruption of the class B scavenger receptor CD36 protects against atherosclerotic lesion development in mice. J Clin Invest. 2000;105:1049–1056. doi: 10.1172/JCI9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bjorkbacka H, et al. Reduced atherosclerosis in MyD88-null mice links elevated serum cholesterol levels to activation of innate immunity signaling pathways. Nat Med. 2004;10:416–421. doi: 10.1038/nm1008. [DOI] [PubMed] [Google Scholar]
- 29.Michelsen KS, et al. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci U S A. 2004;101:10679–10684. doi: 10.1073/pnas.0403249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim TW, et al. The Critical Role of IL-1 Receptor-Associated Kinase 4-Mediated NF-{kappa}B Activation in Modified Low-Density Lipoprotein-Induced Inflammatory Gene Expression and Atherosclerosis. J Immunol. 2011;186:2871–2880. doi: 10.4049/jimmunol.1002242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klinkner AM, et al. Evidence of foam cell and cholesterol crystal formation in macrophages incubated with oxidized LDL by fluorescence and electron microscopy. J Histochem Cytochem. 1995;43:1071–1078. doi: 10.1177/43.10.7560885. [DOI] [PubMed] [Google Scholar]
- 32.Imaeda AB, et al. Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. J Clin Invest. 2009;119:305–314. doi: 10.1172/JCI35958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Napoli C, et al. Multiple role of reactive oxygen species in the arterial wall. J Cell Biochem. 2001;82:674–682. doi: 10.1002/jcb.1198. [DOI] [PubMed] [Google Scholar]
- 34.Yuan XM, et al. The toxicity to macrophages of oxidized low-density lipoprotein is mediated through lysosomal damage. Atherosclerosis. 1997;133:153–161. doi: 10.1016/s0021-9150(97)00094-4. [DOI] [PubMed] [Google Scholar]
- 35.Kahn SE, et al. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 36.de Koning EJ, et al. Macrophages and pancreatic islet amyloidosis. Amyloid. 1998;5:247–254. doi: 10.3109/13506129809007297. [DOI] [PubMed] [Google Scholar]
- 37.Zhou R, et al. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11:136–140. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]
- 38.Koenen TB, et al. Hyperglycemia activates caspase-1 and TXNIP-mediated IL-1beta transcription in human adipose tissue. Diabetes. 2011;60:517–524. doi: 10.2337/db10-0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Njajou OT, et al. Association between oxidized LDL, obesity and type 2 diabetes in a population-based cohort, the Health, Aging and Body Composition Study. Diabetes Metab Res Rev. 2009;25:733–739. doi: 10.1002/dmrr.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ouchi N, et al. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Odegaard JI, Chawla A. Mechanisms of macrophage activation in obesity-induced insulin resistance. Nat Clin Pract Endocrinol Metab. 2008;4:619–626. doi: 10.1038/ncpendmet0976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vandanmagsar B, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17:179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stienstra R, et al. The inflammasome-mediated caspase-1 activation controls adipocyte differentiation and insulin sensitivity. Cell Metab. 2010;12:593–605. doi: 10.1016/j.cmet.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boden G. Ceramide: a contributor to insulin resistance or an innocent bystander? Diabetologia. 2008;51:1095–1096. doi: 10.1007/s00125-008-1015-y. [DOI] [PubMed] [Google Scholar]
- 45.Shah C, et al. Protection from high fat diet-induced increase in ceramide in mice lacking plasminogen activator inhibitor 1. J Biol Chem. 2008;283:13538–13548. doi: 10.1074/jbc.M709950200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wen H, et al. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol. 2011;12:408–415. doi: 10.1038/ni.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hotamisligil GS, et al. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science. 1996;271:665–668. doi: 10.1126/science.271.5249.665. [DOI] [PubMed] [Google Scholar]
- 48.Uysal KT, et al. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature. 1997;389:610–614. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- 49.Flegal KM, et al. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 50.Shoelson SE, et al. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 52.Shi H, et al. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vitseva OI, et al. Inducible Toll-like receptor and NF-kappaB regulatory pathway expression in human adipose tissue. Obesity (Silver Spring) 2008;16:932–937. doi: 10.1038/oby.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Davis JE, et al. Tlr-4 deficiency selectively protects against obesity induced by diets high in saturated fat. Obesity (Silver Spring) 2008;16:1248–1255. doi: 10.1038/oby.2008.210. [DOI] [PubMed] [Google Scholar]
- 55.Suganami T, et al. Attenuation of obesity-induced adipose tissue inflammation in C3H/HeJ mice carrying a Toll-like receptor 4 mutation. Biochem Biophys Res Commun. 2007;354:45–49. doi: 10.1016/j.bbrc.2006.12.190. [DOI] [PubMed] [Google Scholar]
- 56.Suganami T, et al. Role of the Toll-like receptor 4/NF-kappaB pathway in saturated fatty acid-induced inflammatory changes in the interaction between adipocytes and macrophages. Arterioscler Thromb Vasc Biol. 2007;27:84–91. doi: 10.1161/01.ATV.0000251608.09329.9a. [DOI] [PubMed] [Google Scholar]
- 57.Song MJ, et al. Activation of Toll-like receptor 4 is associated with insulin resistance in adipocytes. Biochem Biophys Res Commun. 2006;346:739–745. doi: 10.1016/j.bbrc.2006.05.170. [DOI] [PubMed] [Google Scholar]
- 58.McGonagle D, et al. Successful treatment of resistant pseudogout with anakinra. Arthritis Rheum. 2008;58:631–633. doi: 10.1002/art.23119. [DOI] [PubMed] [Google Scholar]
- 59.So A, et al. A pilot study of IL-1 inhibition by anakinra in acute gout. Arthritis Res Ther. 2007;9:R28. doi: 10.1186/ar2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Larsen CM, et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356:1517–1526. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- 61.Osborn O, et al. Treatment with an Interleukin 1 beta antibody improves glycemic control in diet-induced obesity. Cytokine. 2008;44:141–148. doi: 10.1016/j.cyto.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Terkeltaub R, et al. The interleukin 1 inhibitor rilonacept in treatment of chronic gouty arthritis: results of a placebo-controlled, monosequence crossover, non-randomised, single-blind pilot study. Ann Rheum Dis. 2009;68:1613–1617. doi: 10.1136/ard.2009.108936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bhaskar V, et al. Monoclonal antibodies targeting IL-1 beta reduce biomarkers of atherosclerosis in vitro and inhibit atherosclerotic plaque formation in Apolipoprotein E-deficient mice. Atherosclerosis. 2011 doi: 10.1016/j.atherosclerosis.2011.02.026. [DOI] [PubMed] [Google Scholar]
- 64.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 65.Martin P, Leibovich SJ. Inflammatory cells during wound repair: the good, the bad and the ugly. Trends Cell Biol. 2005;15:599–607. doi: 10.1016/j.tcb.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 66.Plata-Salaman CR, et al. Anorexia induced by chronic central administration of cytokines at estimated pathophysiological concentrations. Physiol Behav. 1996;60:867–875. [PubMed] [Google Scholar]
- 67.Sonti G, et al. Anorexia induced by cytokine interactions at pathophysiological concentrations. Am J Physiol. 1996;270:R1394–1402. doi: 10.1152/ajpregu.1996.270.6.R1394. [DOI] [PubMed] [Google Scholar]
- 68.Ling PR, et al. Metabolic changes in rats during a continuous infusion of recombinant interleukin-1. Am J Physiol. 1996;270:E305–312. doi: 10.1152/ajpendo.1996.270.2.E305. [DOI] [PubMed] [Google Scholar]
- 69.Kent S, et al. Mechanisms of sickness-induced decreases in food-motivated behavior. Neurosci Biobehav Rev. 1996;20:171–175. doi: 10.1016/0149-7634(95)00037-f. [DOI] [PubMed] [Google Scholar]