Abstract
Non-alcoholic fatty liver disease (NAFLD), the hepatic manifestation of the metabolic syndrome, can progress to steatohepatitis (NASH) and advanced liver disease. Mechanisms that underlie this progression remain poorly understood, partly due to lack of good animal models that resemble human NASH. We previously showed that several metabolic syndrome features that develop in LDL receptor-deficient (LDLR−/−) mice fed a diabetogenic diet are worsened by dietary cholesterol. To test whether dietary cholesterol can alter the hepatic phenotype in the metabolic syndrome, we fed LDLR−/− mice a high-fat, high-carbohydrate diabetogenic diet (DD) without or with added cholesterol (DDC). Both groups of mice developed obesity and insulin resistance. Hyperinsulinemia, dyslipidemia, hepatic triglyceride, and alanine aminotransferase (ALT) elevations were greater with DDC. Livers of DD-fed mice showed histological changes resembling NAFLD, including steatosis and modest fibrotic changes; however, DDC-fed animals developed micro- and macrovesicular steatosis, inflammatory cell foci, and fibrosis resembling human NASH. Dietary cholesterol also exacerbated hepatic macrophage infiltration, apoptosis, and oxidative stress. Thus, LDLR−/− mice fed diabetogenic diets may be useful models for studying human NASH. Dietary cholesterol appears to confer a second “hit” that results in a distinct hepatic phenotype characterized by increased inflammation and oxidative stress.
Keywords: fatty liver, metabolic syndrome, oxysterols, apoptosis, oxidative stress, low density lipoprotein, oxidized fatty acids
The obesity epidemic has led to a dramatic increase in the incidence of the metabolic syndrome, insulin resistance, and type 2 diabetes. Nonalcoholic fatty liver disease (NAFLD) is a liver disorder strongly associated with obesity, type 2 diabetes, and insulin resistance (1). The spectrum of changes in the liver in NAFLD ranges from simple, noninflammatory triglyceride accumulation in hepatocytes (“simple” steatosis or fatty liver) to steatosis with inflammation and fibrosis (steatohepatitis), which occasionally progresses to cirrhosis, end-stage liver disease, and hepatocellular carcinoma (2). Thus, NAFLD has emerged as a substantial public health concern and is now considered to be the hepatic manifestation of the metabolic syndrome (3).
Triglyceride accumulation in hepatocytes increases vulnerability of the liver to secondary insults through effects of cytokines or oxidative stress (2). While triglyceride accumulation is believed to occur initially (the “first hit”), it is postulated that progression to more advanced stages of NAFLD, including inflammation, fibrosis (NASH), and cirrhosis, requires a “second hit” superimposed upon hepatic steatosis. This second hit may include genetic susceptibility, dietary factors, or environmental stressors. However, the precise molecular signals that trigger this change have not yet been identified.
Several dietary and genetic mouse models have been used to study the pathogenesis of NAFLD. Of these, the methionine-choline deficient (MCD) diet has been used widely to induce a dietary model of NASH in rodents (4). This diet rapidly leads to intrahepatic lipid accumulation, with cell injury and cell death due to impaired synthesis of phosphotidylcholine, an essential component of lipoprotein phospholipid (4). However, this dietary model results in decreased plasma glucose and insulin levels, improved insulin sensitivity, and weight loss, and therefore, a metabolic phenotype that is very different from that seen in metabolic syndrome in humans. This greatly limits extrapolation of findings in the MCD model to human steatohepatitis. Additional rodent models of NAFLD have involved dietary manipulations in genetically mutant mice, such as leptin-deficient (ob/ob), leptin receptor-deficient (db/db) (5), and apoE2 knock-in mice (6). In these models, high-fat diet feeding induces insulin resistance and triglyceride accumulation in the liver. However, these models lack inflammation and/or fibrosis that are required to fulfill the criteria of NASH (4). Models in which caloric overload by intragastric and enteral feeding are used to induce hepatic steatosis and steatohepatitis (7) show significant variability in the features of NAFLD observed.
Cholesterol feeding can induce several features of the metabolic syndrome, such as dyslipidemia and insulin resistance (8, 9). In animal models, dietary cholesterol appears to be an important risk factor for hepatic steatosis and progression to steatohepatitis (10, 11). However, wide variations in cholesterol content in diets (0.2-2%) and lack of development of steatosis and/or obesity limit extrapolation of these animal models to human NASH (8, 10–12).
The LDL receptor-deficient (LDLR−/−) mouse develops many features of the metabolic syndrome when fed a diet rich in saturated fat and refined carbohydrates (“diabetogenic” diet). These include obesity, insulin resistance, and dyslipidemia, as well as local (adipose tissue), systemic inflammation and atherosclerosis (9, 13). We previously showed that addition of a small amount of added cholesterol (0.15%) to a diet rich in saturated fat and refined carbohydrate increases insulin resistance, adipose tissue inflammation, chronic systemic inflammation, and atherosclerosis in LDLR−/− mice (9). In the present study, we used LDLR−/− mice fed diabetogenic diets without or with added cholesterol to investigate the effects of dietary cholesterol on the hepatic phenotype in the metabolic syndrome. We show that the LDLR−/− mouse is an attractive rodent model to study changes occurring in the liver in NAFLD, and that dietary cholesterol plays an important role in hepatic fat accumulation, inflammation, and fibrosis characteristic of NASH. Importantly, an increase in lipid peroxidation products in the liver suggests that oxidative stress is involved in the pathogenesis of steatohepatitis in this model.
METHODS
Animals and diets
Adult (10-week-old) male LDL receptor-deficient mice on a C57BL/6 background were fed rodent chow, a “diabetogenic” high-fat diet (DD, 35.5% carbohydrate and 36.6% fat, F1850, Bio-serv, Frenchtown, NJ) or a diabetogenic diet with 0.15% added cholesterol (DDC, F4997, Bio-serv). Details of these diets have been published previously (9). Diets were free of added antioxidants and were changed approximately every three days. Diets were stored in a similar fashion to regular chow diet without any added precautions. Both high-fat diets were stored in small plastic containers and stored at −70°C until processing for measurement of oxysterols. Mice were maintained in a temperature- and light-controlled facility and received the diets ad libitum for a total of 24 weeks. Blood was collected after a 4 h fast from the retro-orbital sinus on the day of euthanasia. Livers were rapidly excised after perfusion with 10% phosphate buffered saline and either fixed in 10% formalin for histological examination or snap-frozen in liquid nitrogen and stored at −70°C until further analysis. For tissue oxysterol and lipid peroxide analysis, approximately 200 mg of liver was placed in antioxidant solution [10 μM butylhydroxytoluene (BHT) and diethylene triamine pentaacetic acid in 95% ethanol] and frozen at −70°C until further analysis. This project was approved by the Animal Care and Use Committee of the University of Washington.
Analytical procedures
Plasma and liver cholesterol and triglycerides were measured using colorimetric kits. Plasma insulin was measured using an ELISA kit (Millipore, Billerica, MA). Alanine aminotransferase (ALT) was measured using an autoanalyzer through the Nutrition & Obesity Research Center at the University of Washington. Free fatty acid levels were measured colorimetrically (NEFA-C test kit, Wako, Richmond, VA). Liver lipid extraction was performed using a modified Folch technique (14). Hepatic thiobarbituric acid reactive substances (TBARS) concentration was measured in homogenates from 200 mg of liver, as described previously (15).
Liver histology and immunohistochemistry
Formalin-fixed livers embedded in paraffin wax were sectioned and stained with hematoxylin and eosin (H and E) or Masson's trichrome stains for histological analyses. Liver morphology was evaluated by a hepatopathologist (M.M.Y.) in a blinded manner. Macrophages were detected in liver sections immunohistochemically using a rat monoclonal antibody against Mac2 (titer 1:2500, Cedarlane Laboratories, Burlington, NC). Liver fibrosis was quantified in trichrome-stained sections of the liver. Area quantification for MAC2 and fibrosis was performed on digital images of immunostained liver sections using image analysis software (Image-Pro Plus, Media Cybernetics, Bethesda, MD). Liver cell apoptosis was assessed using the terminal deoxynucleotidyl transferase nick-end labeling (TUNEL) assay according to the manufacturer's instructions (ApopTag Peroxidase In Situ Apoptosis Detection Kit, Millipore). To determine the number of apoptotic hepatocytes, liver sections were quantified by counting the number of TUNEL-positive cells in 30 random microscopic fields (20×). Results are expressed as number of TUNEL-positive cells per field magnification.
Real-time quantitative PCR analysis
Total RNA was extracted from 100 mg of liver tissue using TRI reagent (Sigma-Aldrich, St. Louis, MO) according to the manufacturer's protocol. After spectroscopic quantification, 2 µg of RNA was reverse-transcribed, and cDNA thus obtained was analyzed by real-time quantitative PCR by standard protocols using the ABI 7900HT instrument in our laboratory. Primer and FAM probes for individual genes were purchased from Applied Biosystems (Assay-on-Demand, Life Technologies, Carlsbad, CA). Relative quantities of mRNA were calculated with GAPDH used as the reference gene, and the amount of target gene was calculated using the ΔΔCt formula. Levels of the reference gene were not altered in the three animal groups.
LC/ESI/MS/MS analysis of multiple species of lipid peroxidation products
Liver samples (∼50 mg) were homogenized in 500 μl of PBS with 100 µM butylated hydroxytoluene and 1 µM diethylene tetraamino pentaacetic acid (pH 7.0), followed by addition of an internal standard cocktail containing isotopically labeled 9(S)-HODE-d4, 13-(S)HODE-d4, 5-(S)HETE-d8, 12-(S)HETE-d8, 15-(S)HETE-d8, 20-(S)HETE-d6, arachidonic acid-d8, and linoleic acid-d4. The samples were incubated with an excess amount of sodium borohydride for 5 min, subjected to base hydrolysis with 1M KOH in methanol under nitrogen for 1 h at 40°C. The reaction was terminated by the addition of 2 ml of 10% acetic acid, lipids were extracted with chloroform/ethyl acetate (4:1, v/v), and the organic layers were pooled and dried.
Samples were subjected to reverse phase LC for hydroxyoctadecadienoic acid (HODE), hydroxyeicosatetraenoic acid (HETE), and arachidonic acid analysis utilizing an Agilent 1200 LC system (Santa Clara, CA). Mass spectrometric experiments were performed using an Agilent technologies 6410 Triple Quadrupole system equipped with an electrospray source. Quantification of oxidized fatty acids and their precursors were performed by comparing peak areas of the analyte of interest and their corresponding isotopically labeled internal standard. The levels of the oxidized fatty acid (HODEs and HETEs) were normalized to precursor fatty acids (linoleic and arachidonic acids), respectively. 8-iso-prostaglandin-F2α was measured by monitoring transition of the m/z 353 to 193 and m/z 357 to 193 to quantify native 8-iso-prostaglandin-F2α and its isotopically labeled internal standard 8-iso-prostaglandin-F2α-d4. Levels were normalized to arachadonic acid, the precursor fatty acid.
Determination of cholesterol oxidation products (oxysterols) by GC/MS
Liver and diet samples (∼200 mg) were homogenized in a glass Tenbroeck homogenizer in 1 ml PBS and 1 ml aqueous 2% acetic acid (to inhibit lipolytic enzymes). The homogenate was extracted with chloroform/methanol (2:1) containing 100 ppm of BHT as an antioxidant. After centrifugation to separate the phases, the lower (chloroform) layer was removed and evaporated to dryness under nitrogen. Two parts methanol per one part 6M KOH were added to the residue to saponify in the dark overnight. Sterols were extracted three times with diethyl ether and water. The extract was dried under nitrogen and derivatized with N,O-bis(trimethylsilyl) trifluoroacetamide in pyridine and heated at 60°C for 1 h. The sample was dried under nitrogen and taken up in hexane for analysis by GC/MS.
Statistical analyses
Data were analyzed using GraphPad Prism 5 (GraphPad Software Inc., La Jolla, CA) and are presented as means and standard errors. ANOVA (ANOVA) with Bonferroni post hoc testing was used to detect differences among groups. Spearman's correlative quotient was used to calculate associations. P < 0.05 was considered statistically significant.
RESULTS
Hepatic triglyceride accumulation increases with the addition of cholesterol to a high-fat, high-carbohydrate (diabetogenic) diet
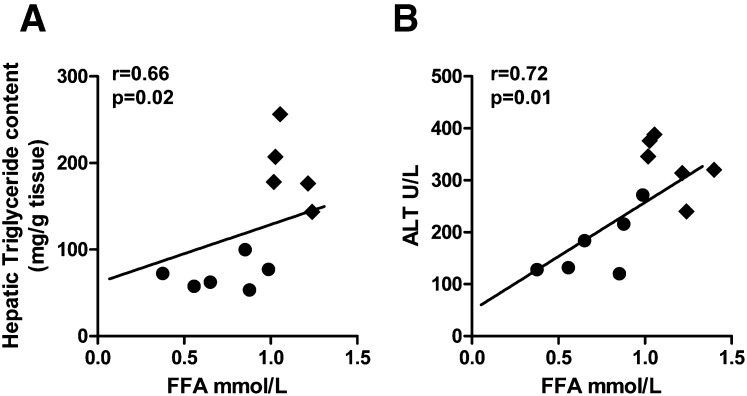
We previously showed that LDLR−/− mice fed a diabetogenic diet (DD) for 24 weeks gained weight, became hyperinsulinemic, and developed dyslipidemia, which was manifested as high triglycerides and cholesterol (9). Addition of dietary cholesterol (DDC) worsened hyperinsulinemia without substantially worsening dyslipidemia. In the present study, we confirmed these findings (Table 1) and found equivalent weight gain in the DD and DDC groups. Liver weights were higher in both DD and DDC groups than in chow-fed animals (Table 1). Hypertriglyceridemia and hypercholesterolemia were not different between the obese LDLR−/− animals on the DD and DDC diets. Circulating free fatty acids were increased in both diabetogenic diet-fed groups but were higher in the DDC group (P < 0.001 versus chow, P < 0.01 versus DD; Table 1). Hepatic triglyceride content was increased in DD animals (P < 0.01 versus chow; Table 1) but was higher in the DDC animals (P < 0.001). Hepatic cholesterol levels were elevated only in the DDC group (Table 1). Circulating FFA levels correlated with hepatic triglyceride levels (r = 0.66, P = 0.02) and plasma ALT levels (r = 0.72, P = 0.01; Fig. 1A, B). Thus, several metabolic alterations that have been implicated in the development of NAFLD were worsened by dietary cholesterol in obese LDLR−/− mice.
TABLE 1.
Metabolic variables in LDLR mice after 24 weeks on the different diets
| Metabolic Variable | Chow | DD | DDC |
| Total body weight at end of study(g) | 30.7 ± 1.5 | 52.7 ± 3.4c | 52.2 ± 1.6c |
| Liver weight (g) | 1.4 ± 0.4 | 2.9 ± 0.5b | 3.2 ± 0.4b |
| Epididymal fat pad weight (g) | 0.2 ± 0.1 | 1.6 ± 0.2c | 1.9 ± 0.2c |
| Plasma insulin(ng/ml) | 0.7 ± 0.2 | 3.9 ± 1.3b | 6.2 ± 1.5b,d |
| Cholesterol (mg/dl) | 284 ± 38 | 821 ± 139b | 875 ± 47c |
| Triglycerides (mg/dl) | 108 ± 16 | 463 ± 166b | 511 ± 139c |
| Free fatty acids (nmol/l) | 0.15 ± 0.1 | 0.8 ± 0.3b | 1.0 ± 0.3b,e |
| Hepatic triglycerides (mg/g) | 58 ± 14 | 72 ± 38 | 135 ± 62b,d |
| Hepatic cholesterol (mg/g) | 16.2 ± 3 | 27 ± 10 | 38 ± 5a |
| ALT (U/l) | 44 ± 11 | 172 ± 50b | 244 ± 106c,d |
Values represent means ± SD (n = 5-8 per group).
P < 0.05 versus chow.
P < 0.01 versus chow.
P < 0.001 versus chow.
P < 0.05 versus DD.
P < 0.01 versus DD.
Fig. 1.
Plasma free fatty acids play a key role in hepatic steatosis in obese mice. Free fatty acid levels (FFA) correlate strongly with (A) hepatic triglyceride content and (B) plasma ALT, a marker of hepatocellular damage. DD, filled circles; DDC, filled diamonds.
Liver morphology is altered in LDLR−/− mice by the addition of cholesterol to a diabetogenic diet
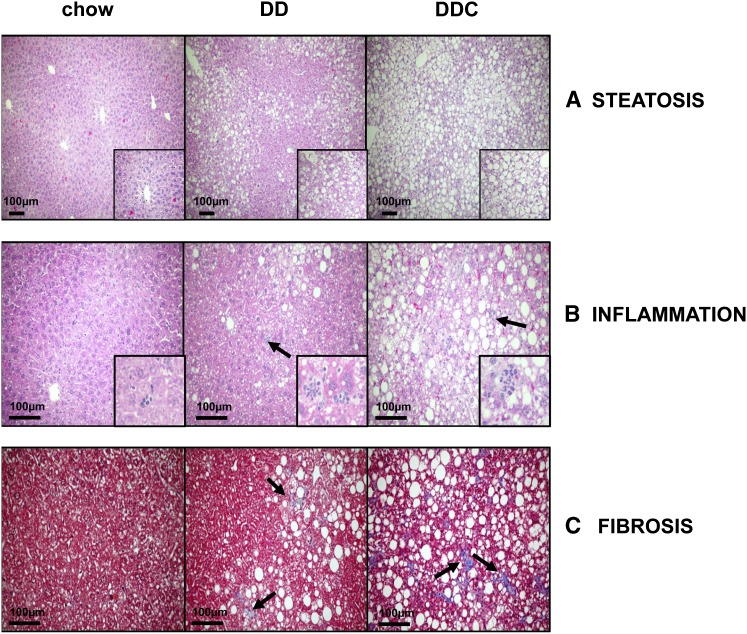
To determine the effect of dietary cholesterol on hepatic morphology, we performed histological examination of the livers. In general, the histopathologic features required for a diagnosis of NASH in humans include macrovesicular steatosis (hepatocyte fat accumulation), lobular inflammation, fibrosis around hepatocytes and hepatic sinusoids, and hepatocyte ballooning (16). Analysis of H and E-stained liver sections from chow-fed animals revealed normal liver histology without lipid accumulation in hepatocytes. In contrast, the DD group demonstrated diffuse macrovesicular steatosis in a nonzonal pattern (Fig. 2A). Inflammation and fibrosis were seen only to a limited extent in the DD group. In the DDC animals, steatosis was both macrovesicular as well as microvesicular, with a predominant centrilobular distribution. Moderate inflammatory cell foci (Fig. 2B) and intrasinusoidal and pericellular fibrosis (Fig. 2C) were seen to a greater extent in the DDC group. Quantification of fibrosis in trichrome-stained sections revealed increased fibrotic areas in the DDC livers (chow 0.05%, DD 0.13%, and DDC 0.21% of total area, P < 0.05 chow versus DDC). Although neither group had hepatocyte ballooning, a feature seen in progressive human NASH, obese LDLR−/− mice showed several features suggestive of human NASH, which were heightened with dietary cholesterol.
Fig. 2.
Dietary cholesterol worsens histological features of NASH in obese LDLR−/− mice. Representative images from chow, DD, and DDC groups showing (A) steatosis (fat accumulation within hepatocytes) in H and E stained sections (inset 40× magnification) and (B) inflammatory foci in H and E sections (arrows indicate inflammatory cell clusters; inset 60× magnification). C: Fibrosis in Masson's trichrome-stained sections for collagen which stains blue (arrows indicate areas of fibrosis).
Dietary cholesterol alters hepatic inflammation in obese, insulin-resistant LDLR−/− mice
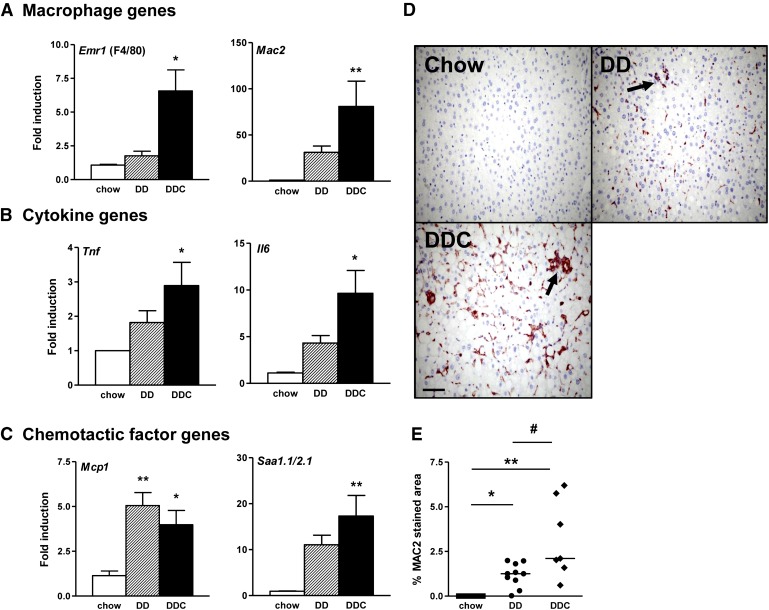
Hepatic inflammation, a key aspect of NASH, is widely believed to be mediated through cytokines released from hepatocytes or resident macrophages otherwise known as Kupffer cells. Since macrophages have been implicated as a critical player in obesity-associated insulin resistance, we investigated the effects of dietary cholesterol on hepatic macrophages. Expression of the macrophage-specific genes F4/80, CD11b (not shown), and Mac2 were increased in the DDC group (Fig. 3A). Expression levels of the pro-inflammatory cytokines TNFα and IL-6 mRNA also were increased in the DDC group (Fig. 3B). Increased expression of MCP-1, a chemotactic factor for monocyte macrophages and hepatic stellate cells, was observed in both groups (Fig. 3C). SAA1.1 and SAA2.1 are homologous isoforms of liver-derived proteins that are elevated in chronic inflammatory states (17) and have chemotactic activity (18). Expression of SAA1.1/SAA2.1 was increased in both groups but was significant only in the DDC group (Fig. 3C). The trend was similar in the DD group but did not achieve statistical significance, likely because of the small number of animals per group. Immunohistochemical analysis of liver sections for the pan-macrophage marker MAC2 showed increased staining in the DD group, but was greater in the DDC group (Fig. 3D, E). Taken together, these findings suggest that the addition of dietary cholesterol induces a greater degree of inflammation in the liver. Moreover, these findings parallel changes that we showed previously in intra-abdominal (visceral) adipose tissue (9), suggesting that hepatic inflammation is an important component of diet-induced obesity in this mouse model.
Fig. 3.
Increased inflammation in livers of obese LDLR−/− mice is magnified by dietary cholesterol. A: Macrophage gene expression Emr1 and Mac2 mRNA. B: Cytokine gene expression for Tnfα and Il6. C: Chemotactic factor genes Mcp1 and Saa1.1/2.1. Chow diet, open bars (n = 8); DD, hatched bars (n = 10); DDC, solid bars (n = 10). D: Representative immunostained photomicrographs of livers stained with macrophage-specific antibody MAC2 (red) (40× magnification; n = 5-8 per group; arrows point to areas of increased staining around portal triad). E: Quantification of MAC2 staining. Chow, filled squares; DD, filled circles; DDC, filled diamonds. *P < 0.05 versus chow; **P < 0.01 versus chow; #P < 0.05 versus DD.
Dietary cholesterol alters expression of hepatic metabolic genes in obese, insulin-resistant LDLR−/− mice
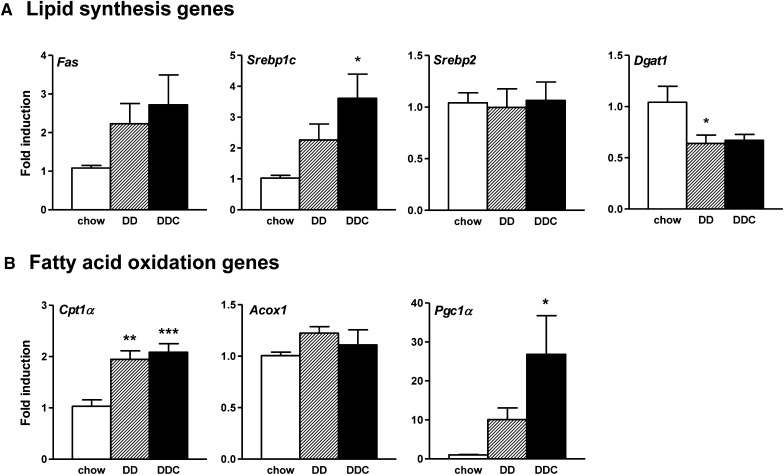
To evaluate the effects of dietary cholesterol on hepatic triglyceride accumulation, we determined the hepatic expression of genes involved in fatty acid metabolism in the liver. No differences were detected in mRNA expression of FAS (Fig. 4A) or ACC (not shown), two genes involved in fatty acid synthesis. Expression of SREBP1c, a transcription factor that activates genes involved in lipogenesis, trended upwards in both obese groups of animals, although it reached statistical significance only in the DDC group, again likely due to the small number of animals (Fig. 4A). Similarly, expression of DGAT1, the enzyme which catalyzes the final step in triglyceride synthesis, was reduced in both groups of obese animals (Fig. 4A). Expression of SREBP2, a transcription factor that regulates cholesterol biosynthesis, was unchanged across the three groups (Fig. 4A). In contrast to several other studies (19, 20), we were not able to show an increase in liver CD36 mRNA expression (data not shown). Disturbed fatty acid β-oxidation has been implicated in the pathogenesis of fatty liver (21). mRNA levels of CPT1α, a rate-limiting fatty acid transporter involved in β-oxidation in the liver, were increased in DD and DDC groups, whereas PGC1α was increased in the DDC group only (Fig. 4B), suggesting upregulation of the hepatic fatty acid oxidation machinery in this model.
Fig. 4.
Altered expression of hepatic metabolic genes in obese LDLR−/− fed dietary cholesterol. A: Expression of genes involved in fatty acid synthesis: Fas, Srebp1c, Dgat1, and Srebp2. B: Genes involved in fatty acid oxidation: Cpt1α, Acox1, and Pgc1α. Chow diet, open bars (n = 8); DD, hatched bars (n = 10); DDC, solid bars (n = 10). *P < 0.05 versus chow; **P < 0.01 versus chow; ***P < 0.001 versus chow.
Dietary cholesterol increases apoptosis in livers of obese LDLR−/− mice
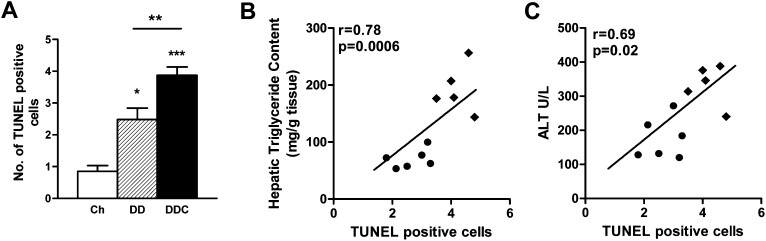
Elevations in ALT levels occur with NAFLD secondary to hepatocellular inflammation and injury. ALT levels were increased in the DD group (P < 0.01 versus chow; Table 1) but more so in the DDC group (P < 0.001 versus chow, P < 0.05 versus DD), suggesting a greater degree of hepatocyte injury with the addition of dietary cholesterol. ALT levels correlated strongly with hepatic triglyceride levels (r = 0.85, P = 0.0008). TUNEL staining revealed a few apoptotic cells in the livers of mice fed DD, which further increased in the DDC group of mice (Fig. 5A). The number of apoptotic cells correlated with hepatic triglycerides (r = 0.78, P = 0.0006; Fig. 5B) and with ALT levels (r = 0.69, P = 0.02; Fig. 5C).
Fig. 5.
Hepatocyte apoptosis in obese LDLR−/− fed dietary cholesterol. A: TUNEL staining of liver sections. Chow diet, open bars (n = 8); DD, hatched bars (n = 10); DDC, solid bars (n = 10). *P < 0.05 versus chow; **P < 0.01 versus DD; ***P < 0.001 versus chow. B: The number of apoptotic cells correlates with hepatic triglyceride content and (C) with ALT levels, a marker of hepatocellular damage. DD, filled circles; DDC, filled diamonds.
Increased hepatic oxidative stress in obese mice fed added dietary cholesterol
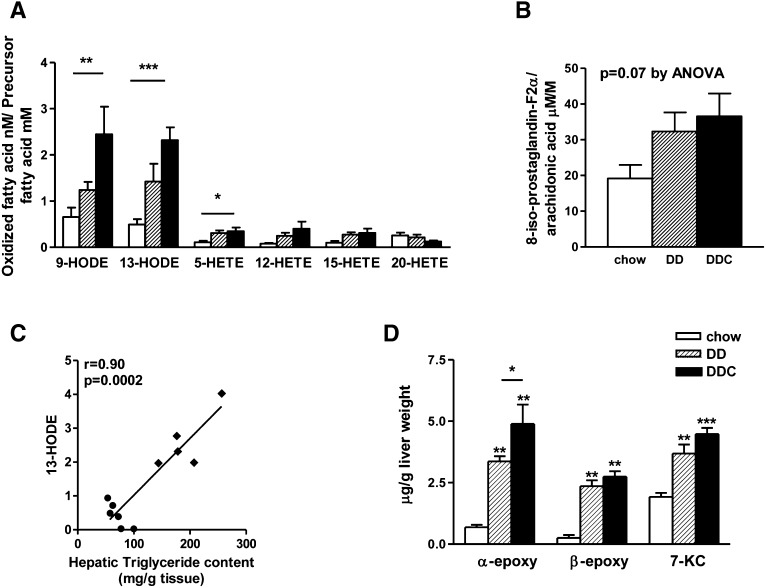
Oxidative stress with increased production of reactive oxygen species has been observed in animal models and human NASH (22, 23). Lipids in the steatotic liver are targets of oxidation, and oxidative metabolites of the major fatty acids in vivo, arachidonic acid and linoleic acid, are potent inflammatory mediators. Total levels of HETE and HODE account for the majority of stable oxidation products of arachidonic acid and linoleic acid, respectively (24). To obtain a comprehensive analysis of lipid peroxidation, we analyzed structurally distinct oxidized fatty acid moieties, including HODEs and HETEs and their precursor fatty acids, from liver. Levels of 9-HODE and 13-HODE, products of linoleic acid oxidation, were significantly increased in the DDC group (Fig. 6A). 13-HODE levels were highly correlated with hepatic triglyceride levels (r = 0.90, P = 0.0002; Fig. 6C). Modest elevations in several arachidonic acid oxidation products were observed in both obese groups. Levels of 8-iso-prostaglandin-F2α, derived from free radical-mediated oxidation of arachidonic acid, trended higher in both DD and DDC groups than in chow-fed animals, but the difference did not reach statistical significance (Fig. 6D). Hepatic TBARS, a crude measure of lipid peroxidation products, also was increased in the DDC group (chow 1.39 ± 0.13, DD 2.90 ± 1.74, DDC 5.98 ± 3.6 nmol/mg protein, P < 0.05 chow versus DDC; data not shown), confirming our findings with mass spectrometry.
Fig. 6.
Evidence of increased oxidative stress in LDLR−/− mice is amplified by dietary cholesterol. A: Lipid peroxidation as measured by mass spectrometry. Levels of HODEs and 5-HETE were increased in the DDC animals. B: 8-isoprostaglandin-F2α levels in livers of lean and obese mice. C: 13-HODE levels strongly correlate with hepatic triglyceride content. DD, filled circles; DDC, filled diamonds. D: Cholesterol oxidative products (oxysterol) levels in liver were increased in obese mice. Chow diet, open bars (n = 8); DD, hatched bars (n = 10); DDC, solid bars (n = 10). *P < 0.05 versus chow; **P < 0.01 versus chow; ***P < 0.001 versus chow; †P < 0.01 versus DD. 7KC, 7-ketocholesterol; α epoxy, 5,6,α-epoxy cholesterol; β-epoxy, 5,6,β-epoxy cholesterol.
Cholesterol oxidative products (oxysterols) generated by auto-oxidation or enzymatic/nonenzymatic peroxidation of sterols also have been implicated in apoptosis and liver injury. As dietary cholesterol can be modified by oxidation into oxysterols, we measured liver and dietary oxysterols such as 5,6,α-epoxy cholesterol, 5,6,β-epoxy cholesterol, and 7-ketocholesterol. Levels of these oxysterols were undetectable in the diet (data not shown). However, hepatic levels of α- and β-epoxy-cholesterol and 7-ketocholesterol were increased in the livers of mice on both diabetogenic diets, with α-epoxy-cholesterol being higher in the DDC group (Fig. 6B). Taken together, these results reveal evidence of increased degree of oxidative stress in the obese LDLR−/− mice that received the diabetogenic diet with added cholesterol.
DISCUSSION
We previously showed that the addition of cholesterol to a diet rich in saturated fat and refined carbohydrate (diabetogenic diet) worsens adipose tissue inflammation, insulin resistance, and systemic inflammation in LDLR−/− mice (9). Thus, this is a good model to study features of the metabolic syndrome. In the present study, we used the LDLR−/− mouse to evaluate the effect of these diets on the hepatic phenotype of the metabolic syndrome. Mice fed the diabetogenic diet without added cholesterol developed histological changes that closely resembled key features seen in human NAFLD, such as inflammation and fibrosis. Addition of dietary cholesterol resulted in worsened hepatic inflammation and fibrosis. Thus our data implicates dietary cholesterol as an important determinant of the severity of phenotypic changes that occur with progression of NAFLD.
Features of NAFLD in mouse models often do not fully mimic those seen in humans (4). Although the methionine-choline-deficient diet commonly used to study NAFLD in mice results in hepatic fibrosis and inflammation, this model does not replicate human NASH effectively, as mice on this diet lose weight and become more insulin sensitive (25), in contrast to the phenotype that occurs in the metabolic syndrome. Caloric excess by high-fat and intragastric feeding seems to have many features of NASH, but technical challenges and failure to demonstrate reproducible results limit its widespread use (4). LDLR−/− mice develop obesity, dyslipidemia, and insulin resistance similar to that seen in the metabolic syndrome in humans. We now show that inflammatory changes also occur in the liver, similar to what we previously showed in adipose tissue (9). In line with a recent report (26), we found several histopathologic features reminiscent of human NAFLD in LDLR−/− mice fed a diabetogenic diet, with exacerbation by the addition of dietary cholesterol. Thus, LDLR−/− mice that develop obesity on a diet enriched in refined carbohydrate and saturated fat with added cholesterol have histological features that mimic NASH, together with a metabolic phenotype similar to that seen in obese humans. This model is, therefore, particularly attractive for studying the pathogenesis of liver disease in the metabolic syndrome, especially the role of dietary cholesterol.
A key feature in obesity associated with insulin resistance is adipose tissue inflammation, with accumulation of macrophages in fat depots in mice and humans (27, 28). We previously showed that macrophages accumulated in the visceral adipose tissue of LDLR−/− mice fed these diabetogenic diets and that the extent of macrophage accumulation and adipose tissue inflammatory gene expression were increased by cholesterol supplementation of the diabetogenic diet (9). Here, we demonstrate that parallel changes occur in the livers of obese LDLR−/− mice, raising the possibility that similar mechanisms leading to inflammation and insulin resistance might be operative in both adipose tissue and liver. In this study, obese mice showed significant inflammatory changes in the liver, including increased expression of macrophage-related genes and an increase in the number of MAC2-positive cells on immunostaining. While these effects were modest in mice that received the diabetogenic diet, they were amplified by addition of cholesterol to the diet. We also observed increased expression of MCP-1, a macrophage chemotactic factor, although expression levels were increased equally in both DD and DDC groups, suggesting that other chemotactic factors may play a role in liver monocyte-macrophage accumulation. Similarly, macrophage accumulation was not decreased in livers of MCP-1-deficient mice, although these mice were not obese nor did they have fatty livers or insulin resistance (29).
Traditionally, the two-hit hypothesis has been proposed for progression of simple steatosis to NASH (30). Triglyceride accumulation in hepatocytes is widely accepted as an initial prerequisite for the development of NAFLD, which predisposes to cellular injury (31). Dietary cholesterol has been proposed as a second hit (8, 10). Other second insults that have been implicated in inflammatory liver damage and fibrogenesis seen in NASH include oxidative stress, mitochondrial dysfunction, and upregulation of pro-inflammatory cytokines (5). In this study, we have begun to address several of these potential contributors. Circulating FFA levels are increased in the metabolic syndrome and NAFLD in humans, and plasma levels correlate with disease severity (32). In our study, plasma FFAs correlated with hepatic triglyceride levels, suggesting that circulating FFAs are an important source of hepatic triglycerides. Studies in rodents and humans have established fatty acid transporter CD36 as an important contributor to the pathogenesis of insulin resistance and NASH (19, 20). However, we were unable to confirm these findings in our study. One of the most consistent findings in the present study is that changes in the liver were exacerbated by the addition of cholesterol to the diet. Dietary cholesterol has been shown to induce hepatic inflammation in several studies. While other groups have evaluated the role of dietary cholesterol in NASH, several important differences exist between our studies and those of others. For example, short-term feeding studies in mice using 2% dietary cholesterol showed that hepatic inflammation developed without steatosis (10). A hamster model of diet-induced obesity showed steatosis and insulin resistance without significant weight gain (8). Sprague-Dawley rats fed a high-fat diet with 2% cholesterol showed increased steatosis, inflammation, and mitochondrial changes (33). Large doses of cholesterol in the diet prevented weight gain and were toxic to the liver, especially in conjunction with cholate (12). We used the LDLR−/− mouse model fed a diabetogenic diet without or with 0.15% cholesterol, an amount that appeared to be well tolerated and did not blunt weight gain. Our use of lower, nontoxic doses of cholesterol allowed us to gain additional insight into mechanisms that might be more relevant to the metabolic syndrome in humans. Genome-based studies have shown that cholesterol feeding induced a wide range of alterations in hepatic metabolic and inflammatory genes, dependent upon the amount of cholesterol in the diet (34, 35).
The exact mechanisms by which dietary cholesterol induces hepatic inflammation and oxidative stress in our study are unclear and require further investigation. Cytotoxicity of free cholesterol is well established. A recent study showed that an important mechanism for cholesterol-mediated liver injury was the sensitization of hepatocyte mitochondria to cytokine-mediated injury (36). Other potential mechanisms include endoplasmic reticular stress-mediated apoptosis (37). Apoptotic cell death is a central feature of lipotoxic liver injury (38), and hepatocyte apoptosis correlates with disease severity in NASH (39). In our model, although apoptosis occurred in livers of mice on both diabetogenic diets, dietary cholesterol increased apoptosis. Dietary cholesterol also increased circulating FFAs, which can trigger a pro-inflammatory response and induce lipoapoptosis (40).
Oxidative stress is a well-recognized mechanism contributing to disease progression in NAFLD (41–43). We detected a significant increase in certain subsets of lipid peroxidative products, such as 9-HODE, 13-HODE, and 5-HETE, in mice fed dietary cholesterol. Generation of such lipid peroxides, either enzymatic or free radical-mediated, are established markers of oxidative stress (44) and reflect the balance between pro-oxidant and antioxidant mechanisms within the tissue. As in our mouse model, increased levels of free-radical-mediated linoleic acid oxidation products, namely, 9- and 13-HODEs, have been detected in human NASH (44). In vitro evidence suggests that HODEs can stimulate apoptosis in monocytes (45). We also found increased 5-HETE levels and a statistically nonsignificant trend in 8-iso-prostaglandin-F2α derived from free-radical lipid peroxidation. The strong correlation between hepatic 13-HODE and triglyceride levels provides evidence for a possible role for enzymatic pathways involving 15-lipoxygenase. Thus, these findings are consistent with the notion that both free-radical and enzymatic oxidative pathways contribute to the formation of oxidized fatty acids in this model. In addition to these fatty acid by-products, cholesterol can undergo auto-oxidation to oxysterols. Early studies indicated that substantial amounts of oxysterols can be present in the diet (46); however, oxysterols can also be formed endogenously by enzymatic conversion of cholesterol to a variety of cholesterol oxidation products (47). In our model, we found increased levels of several oxysterols in liver from both groups of mice fed the diabetogenic diets, although we were unable to detect oxysterols in the diet. Oxysterols have been shown to be cytotoxic (48), and accumulation of oxysterols in the liver may have contributed to the increased hepatocyte apoptosis seen in our model. In vitro evidence suggests that certain oxysterols can trigger pro-inflammatory and profibrotic changes in hepatocytes (49). Hypercholesterolemic apoE−/− mice have high hepatic oxysterol levels and increased susceptibility to liver injury and fibrosis (49). Thus, dietary cholesterol may have contributed to the fibrosis seen in these mice by conversion to oxysterols in vivo.
In conclusion, the LDLR−/− mouse has many characteristics seen in human NAFLD/NASH and, therefore, may serve as a model for studying the early and late changes that occur in the liver in obesity. We also showed that dietary cholesterol exacerbated the progression from simple steatosis to steatohepatitis by worsening hepatic inflammation. While the two-hit hypothesis finds wide acceptance in this process, in our model, dietary cholesterol appears to play a role in both triglyceride accumulation (first hit) and cytotoxicity, which may be a prelude to macrophage accumulation and hepatic inflammation, as changes in the liver mirror those seen in adipose tissue. Progression from simple steatosis to steatohepatitis might be the result of a complex interplay of multiple pathogenic factors, such as hyperlipidemia, circulating FFAs, increased oxidized fatty acids, and oxysterols, all of which contribute to inflammatory changes and cytotoxicity in the liver.
Footnotes
Abbreviations:
- ACC
- acetyl CoA carboxylase
- ACOX
- acyl CoA oxidase
- ALT
- alanine aminotransferase
- CPT1α
- carnitine palmitoyl transferase-1α
- DD
- diabetogenic diet
- DDC
- diabetogenic diet with cholesterol
- DGAT1
- diacylglcerol:acyl transferase-1
- H and E
- hematoxylin and eosin
- HETE
- hydroxyeicosatetraenoic acid
- HODE
- hydroxyoctadecadienoic acid
- IL-6
- interleukin-6
- LDLR
- LDL receptor
- MCD
- methionine-choline-deficient
- NAFLD
- non-alcoholic fatty liver disease
- NASH
- non-alcoholic steatohepatitis
- PGC1α
- PPAR-γ coactivator 1α
- SAA1.1/2.1
- serum amyloid A1.1/2.1
- SREBP
- sterol regulatory binding protein
- TBARS
- thiobarbituric acid reactive substance
- TNFα
- tumor necrosis factor-α
- TUNEL
- terminal deoxynucleotidyl transferase nick-end labeling
This work was supported by National Institutes of Health Grants P30 DK-035816 (A.C. and S.S.), P01 HL-092969 (A.C.), DK-082841 (S.P.), DK-089503 (S.P.), and a mentor-based American Diabetes Association postdoctoral fellowship award (S.S.). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Marchesini G., Moscatiello S., Di Domizio S., Forlani G. 2008. Obesity-associated liver disease. J. Clin. Endocrinol. Metab. 93: S74–S80. [DOI] [PubMed] [Google Scholar]
- 2.Farrell G. C., Larter C. Z. 2006. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 43: S99–S112. [DOI] [PubMed] [Google Scholar]
- 3.Marchesini G., Brizi M., Bianchi G., Tomassetti S., Bugianesi E., Lenzi M., McCullough A. J., Natale S., Forlani G., Melchionda N. 2001. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 50: 1844–1850. [DOI] [PubMed] [Google Scholar]
- 4.Larter C. Z., Yeh M. M. 2008. Animal models of NASH: getting both pathology and metabolic context right. J. Gastroenterol. Hepatol. 23: 1635–1648. [DOI] [PubMed] [Google Scholar]
- 5.Browning J. D., Horton J. D. 2004. Molecular mediators of hepatic steatosis and liver injury. J. Clin. Invest. 114: 147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shiri-Sverdlov R., Wouters K., van Gorp P. J., Gijbels M. J., Noel B., Buffat L., Staels B., Maeda N., van Bilsen M., Hofker M. H. 2006. Early diet-induced non-alcoholic steatohepatitis in APOE2 knock-in mice and its prevention by fibrates. J. Hepatol. 44: 732–741. [DOI] [PubMed] [Google Scholar]
- 7.Schattenberg J. M., Galle P. R. 2010. Animal models of non-alcoholic steatohepatitis: of mice and man. Dig. Dis. 28: 247–254. [DOI] [PubMed] [Google Scholar]
- 8.Basciano H., Miller A. E., Naples M., Baker C., Kohen R., Xu E., Su Q., Allister E. M., Wheeler M. B., Adeli K. 2009. Metabolic effects of dietary cholesterol in an animal model of insulin resistance and hepatic steatosis. Am. J. Physiol. Endocrinol. Metab. 297: E462–E473. [DOI] [PubMed] [Google Scholar]
- 9.Subramanian S., Han C. Y., Chiba T., McMillen T. S., Wang S. A., Haw A., 3rd, Kirk E. A., O'Brien K. D., Chait A. 2008. Dietary cholesterol worsens adipose tissue macrophage accumulation and atherosclerosis in obese LDL receptor-deficient mice. Arterioscler. Thromb. Vasc. Biol. 28: 685–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wouters K., van Gorp P. J., Bieghs V., Gijbels M. J., Duimel H., Lutjohann D., Kerksiek A., van Kruchten R., Maeda N., Staels B., et al. 2008. Dietary cholesterol, rather than liver steatosis, leads to hepatic inflammation in hyperlipidemic mouse models of nonalcoholic steatohepatitis. Hepatology. 48: 474–486. [DOI] [PubMed] [Google Scholar]
- 11.Kainuma M., Fujimoto M., Sekiya N., Tsuneyama K., Cheng C., Takano Y., Terasawa K., Shimada Y. 2006. Cholesterol-fed rabbit as a unique model of nonalcoholic, nonobese, non-insulin-resistant fatty liver disease with characteristic fibrosis. J. Gastroenterol. 41: 971–980. [DOI] [PubMed] [Google Scholar]
- 12.Matsuzawa N., Takamura T., Kurita S., Misu H., Ota T., Ando H., Yokoyama M., Honda M., Zen Y., Nakanuma Y., et al. 2007. Lipid-induced oxidative stress causes steatohepatitis in mice fed an atherogenic diet. Hepatology. 46: 1392–1403. [DOI] [PubMed] [Google Scholar]
- 13.Schreyer S. A., Vick C., Lystig T. C., Mystkowski P., LeBoeuf R. C. 2002. LDL receptor but not apolipoprotein E deficiency increases diet-induced obesity and diabetes in mice. Am. J. Physiol. Endocrinol. Metab. 282: E207–E214. [DOI] [PubMed] [Google Scholar]
- 14.Folch J., Lees M., Sloane Stanley G. H. 1957. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226: 497–509. [PubMed] [Google Scholar]
- 15.Ohkawa H., Ohishi N., Yagi K. 1979. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 95: 351–358. [DOI] [PubMed] [Google Scholar]
- 16.Kleiner D. E., Brunt E. M., Van Natta M., Behling C., Contos M. J., Cummings O. W., Ferrell L. D., Liu Y. C., Torbenson M. S., Unalp-Arida A., et al. 2005. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 41: 1313–1321. [DOI] [PubMed] [Google Scholar]
- 17.Chait A., Han C. Y., Oram J. F., Heinecke J. W. 2005. Thematic review series: The immune system and atherogenesis. Lipoprotein-associated inflammatory proteins: markers or mediators of cardiovascular disease? J. Lipid Res. 46: 389–403. [DOI] [PubMed] [Google Scholar]
- 18.Uhlar C. M., Whitehead A. S. 1999. Serum amyloid A, the major vertebrate acute-phase reactant. Eur. J. Biochem. 265: 501–523. [DOI] [PubMed] [Google Scholar]
- 19.Greco D., Kotronen A., Westerbacka J., Puig O., Arkkila P., Kiviluoto T., Laitinen S., Kolak M., Fisher R. M., Hamsten A., et al. 2008. Gene expression in human NAFLD. Am. J. Physiol. Gastrointest. Liver Physiol. 294: G1281–G1287. [DOI] [PubMed] [Google Scholar]
- 20.Koonen D. P., Jacobs R. L., Febbraio M., Young M. E., Soltys C. L., Ong H., Vance D. E., Dyck J. R. 2007. Increased hepatic CD36 expression contributes to dyslipidemia associated with diet-induced obesity. Diabetes. 56: 2863–2871. [DOI] [PubMed] [Google Scholar]
- 21.Reddy J. K., Rao M. S. 2006. Lipid metabolism and liver inflammation. II. Fatty liver disease and fatty acid oxidation. Am. J. Physiol. Gastrointest. Liver Physiol. 290: G852–G858. [DOI] [PubMed] [Google Scholar]
- 22.Seki S., Kitada T., Yamada T., Sakaguchi H., Nakatani K., Wakasa K. 2002. In situ detection of lipid peroxidation and oxidative DNA damage in non-alcoholic fatty liver diseases. J. Hepatol. 37: 56–62. [DOI] [PubMed] [Google Scholar]
- 23.Yang S., Zhu H., Li Y., Lin H., Gabrielson K., Trush M. A., Diehl A. M. 2000. Mitochondrial adaptations to obesity-related oxidant stress. Arch. Biochem. Biophys. 378: 259–268. [DOI] [PubMed] [Google Scholar]
- 24.Yoshida Y., Hayakawa M., Niki E. 2005. Total hydroxyoctadecadienoic acid as a marker for lipid peroxidation in vivo. Biofactors. 24: 7–15. [DOI] [PubMed] [Google Scholar]
- 25.Rinella M. E., Green R. M. 2004. The methionine-choline deficient dietary model of steatohepatitis does not exhibit insulin resistance. J. Hepatol. 40: 47–51. [DOI] [PubMed] [Google Scholar]
- 26.Gupte A. A., Liu J. Z., Ren Y., Minze L. J., Wiles J. R., Collins A. R., Lyon C. J., Pratico D., Finegold M. J., Wong S. T., et al. 2010. Rosiglitazone attenuates age- and diet-associated nonalcoholic steatohepatitis in male low-density lipoprotein receptor knockout mice. Hepatology. 52: 2001–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weisberg S. P., McCann D., Desai M., Rosenbaum M., Leibel R. L., Ferrante A. W., Jr 2003. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 112: 1796–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu H., Barnes G. T., Yang Q., Tan G., Yang D., Chou C. J., Sole J., Nichols A., Ross J. S., Tartaglia L. A., et al. 2003. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Invest. 112: 1821–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kassel K. M., Guo G. L., Tawfik O., Luyendyk J. P. Monocyte chemoattractant protein-1 deficiency does not affect steatosis or inflammation in livers of mice fed a methionine-choline-deficient diet. Lab Invest. 90: 1794–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Day C. P., James O. F. 1998. Steatohepatitis: a tale of two “hits”? Gastroenterology. 114: 842–845. [DOI] [PubMed] [Google Scholar]
- 31.Choi S. S., Diehl A. M. 2008. Hepatic triglyceride synthesis and nonalcoholic fatty liver disease. Curr. Opin. Lipidol. 19: 295–300. [DOI] [PubMed] [Google Scholar]
- 32.Nehra V., Angulo P., Buchman A. L., Lindor K. D. 2001. Nutritional and metabolic considerations in the etiology of nonalcoholic steatohepatitis. Dig. Dis. Sci. 46: 2347–2352. [DOI] [PubMed] [Google Scholar]
- 33.Xu Z. J., Fan J. G., Ding X. D., Qiao L., Wang G. L. 2010. Characterization of high-fat, diet-induced, non-alcoholic steatohepatitis with fibrosis in rats. Dig. Dis. Sci. 55: 931–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kleemann R., Verschuren L., van Erk M. J., Nikolsky Y., Cnubben N. H., Verheij E. R., Smilde A. K., Hendriks H. F., Zadelaar S., Smith G. J., et al. 2007. Atherosclerosis and liver inflammation induced by increased dietary cholesterol intake: a combined transcriptomics and metabolomics analysis. Genome Biol. 8: R200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vergnes L., Phan J., Strauss M., Tafuri S., Reue K. 2003. Cholesterol and cholate components of an atherogenic diet induce distinct stages of hepatic inflammatory gene expression. J. Biol. Chem. 278: 42774–42784. [DOI] [PubMed] [Google Scholar]
- 36.Mari M., Caballero F., Colell A., Morales A., Caballeria J., Fernandez A., Enrich C., Fernandez-Checa J. C., Garcia-Ruiz C. 2006. Mitochondrial free cholesterol loading sensitizes to TNF- and Fas-mediated steatohepatitis. Cell Metab. 4: 185–198. [DOI] [PubMed] [Google Scholar]
- 37.Feng B., Yao P. M., Li Y., Devlin C. M., Zhang D., Harding H. P., Sweeney M., Rong J. X., Kuriakose G., Fisher E. A., et al. 2003. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat. Cell Biol. 5: 781–792. [DOI] [PubMed] [Google Scholar]
- 38.Malhi H., Gores G. J. 2008. Molecular mechanisms of lipotoxicity in nonalcoholic fatty liver disease. Semin. Liver Dis. 28: 360–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feldstein A. E., Canbay A., Angulo P., Taniai M., Burgart L. J., Lindor K. D., Gores G. J. 2003. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 125: 437–443. [DOI] [PubMed] [Google Scholar]
- 40.Malhi H., Bronk S. F., Werneburg N. W., Gores G. J. 2006. Free fatty acids induce JNK-dependent hepatocyte lipoapoptosis. J. Biol. Chem. 281: 12093–12101. [DOI] [PubMed] [Google Scholar]
- 41.Oliveira C. P., da Costa Gayotto L. C., Tatai C., Della Bina B. I., Janiszewski M., Lima E. S., Abdalla D. S., Lopasso F. P., Laurindo F. R., Laudanna A. A. 2002. Oxidative stress in the pathogenesis of nonalcoholic fatty liver disease, in rats fed with a choline-deficient diet. J. Cell. Mol. Med. 6: 399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roskams T., Yang S. Q., Koteish A., Durnez A., DeVos R., Huang X., Achten R., Verslype C., Diehl A. M. 2003. Oxidative stress and oval cell accumulation in mice and humans with alcoholic and nonalcoholic fatty liver disease. Am. J. Pathol. 163: 1301–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Videla L. A., Rodrigo R., Araya J., Poniachik J. 2004. Oxidative stress and depletion of hepatic long-chain polyunsaturated fatty acids may contribute to nonalcoholic fatty liver disease. Free Radic. Biol. Med. 37: 1499–1507. [DOI] [PubMed] [Google Scholar]
- 44.Feldstein A. E., Lopez R., Tamimi T. A., Yerian L., Chung Y. M., Berk M., Zhang R., McIntyre T. M., Hazen S. L. 2010. Mass spectrometric profiling of oxidized lipid products in human nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. J. Lipid Res. 51: 3046–3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hampel J. K., Brownrigg L. M., Vignarajah D., Croft K. D., Dharmarajan A. M., Bentel J. M., Puddey I. B., Yeap B. B. 2006. Differential modulation of cell cycle, apoptosis and PPARgamma2 gene expression by PPARgamma agonists ciglitazone and 9-hydroxyoctadecadienoic acid in monocytic cells. Prostaglandins Leukot. Essent. Fatty Acids. 74: 283–293. [DOI] [PubMed] [Google Scholar]
- 46.Addis P. B. 1986. Occurrence of lipid oxidation products in foods. Food Chem. Toxicol. 24: 1021–1030. [DOI] [PubMed] [Google Scholar]
- 47.Brown A. J., Jessup W. 2009. Oxysterols: sources, cellular storage and metabolism, and new insights into their roles in cholesterol homeostasis. Mol. Aspects Med. 30: 111–122. [DOI] [PubMed] [Google Scholar]
- 48.Shibata N., Glass C. K. 2010. Macrophages, oxysterols and atherosclerosis. Circ. J. 74: 2045–2051. [DOI] [PubMed] [Google Scholar]
- 49.Ferre N., Martinez-Clemente M., Lopez-Parra M., Gonzalez-Periz A., Horrillo R., Planaguma A., Camps J., Joven J., Tres A., Guardiola F., et al. 2009. Increased susceptibility to exacerbated liver injury in hypercholesterolemic ApoE-deficient mice: potential involvement of oxysterols. Am. J. Physiol. Gastrointest. Liver Physiol. 296: G553–G562. [DOI] [PubMed] [Google Scholar]