Abstract
The pregnane X receptor (PXR, also known as SXR) is a nuclear hormone receptor activated by xenobiotics as well as diverse sterols and their metabolites. PXR functions as a xenobiotic sensor to coordinately regulate xenobiotic metabolism via transcriptional regulation of xenobiotic-detoxifying enzymes and transporters. Recent evidence indicates that PXR may also play an important role in lipid homeostasis and atherosclerosis. To define the role of PXR in atherosclerosis, we generated PXR and apoE double knockout (PXR−/−apoE−/−) mice. Here we show that deficiency of PXR did not alter plasma triglyceride and cholesterol levels in apoE−/− mice. However, PXR−/−apoE−/− mice had significantly decreased atherosclerotic cross-sectional lesion area in both the aortic root and brachiocephalic artery by 40% (P < 0.01) and 60% (P < 0.001), respectively. Interestingly, deficiency of PXR reduced the expression levels of CD36, lipid accumulation, and CD36-mediated oxidized LDL uptake in peritoneal macrophages of PXR−/−apoE−/− mice. Furthermore, immunofluorescence staining showed that PXR and CD36 were expressed in the atherosclerotic lesions of apoE−/− mice, and the expression levels of PXR and CD36 were diminished in the lesions of PXR−/−apoE−/− mice. Our findings indicate that deficiency of PXR attenuates atherosclerosis development, which may result from decreased CD36 expression and reduced lipid uptake in macrophages.
Keywords: pregnane X receptor, macrophage, foam cell, apolipoprotein E, lipid homeostasis
Despite enormous research efforts and advances in treatments over the past few decades, atherosclerotic cardiovascular disease remains the leading cause of death in the developed world (1, 2). Nuclear receptors have become attractive targets for the development of therapeutic agents for treatment of atherosclerosis (3, 4). A number of nuclear receptors, such as liver X receptor (LXR) and peroxisome proliferator-activated receptors (PPARs), are key regulators of lipid homeostasis and inflammation and play important roles in atherosclerosis development (3–6).
The pregnane X receptor (PXR; also known as steroid and xenobiotic receptor, or SXR) is a nuclear receptor activated by a diverse array of endogenous hormones, dietary steroids, pharmaceutical agents, and xenobiotic compounds (7–9). PXR functions as a xenobiotic sensor that regulates clearance via induction of genes involved in drug and xenobiotic metabolism, including oxidation (e.g., cytochrome P450), conjugation (e.g., glutathione transferase), and transport (e.g., multidrug resistance 1) (9, 10). The broad response profile of PXR has led to the development of “the steroid and xenobiotic sensor hypothesis.” In the past decade, PXR's function in drug and xenobiotic metabolism has been extensively studied by many laboratories (9).
Recent evidence indicates that PXR may also play an important role in lipid homeostasis and atherosclerosis. Many clinically relevant PXR ligands (e.g., rifampicin, ritonavir) have been shown to affect lipid levels in patients and may have pro-atherogenic effects (11–15). In addition to playing an important role in xenobiotic metabolism, PXR can mediate sterol-regulatory element binding protein (SREBP)-independent lipogenic pathways by activating the FFA uptake transporter CD36 and several accessory lipogenic enzymes, such as stearoyl CoA desaturase-1 and long-chain FFA elongase (16, 17). We also found that chronic activation of PXR increases CD36 expression and lipid accumulation in peritoneal macrophages of wild-type (WT) and apoE knockout (apoE−/−) mice (15). Moreover, acute PXR activation can regulate SREBP-dependent lipogenic pathways by inducing Insig-1 expression, resulting in decreased levels of active SREBP-1 and reduced triglyceride synthesis (18). We and other groups have shown that activation of PXR also affects the expression of other hepatic genes involved in lipid homeostasis, including apoA-IV, oxysterol 7α-hydroxylase (CYP39A1), and 7-dehydrocholesterol reductase (DHCR7) in several animal models including WT, apoE−/−, or LDL receptor knockout (LDLR−/−) mice (9, 19, 20). These studies indicate that PXR can mediate cholesterol and lipid homeostasis at multiple levels.
Modulation of PXR activity has been found to affect plasma lipid levels in several animal models. Activation of PXR by pregnenolone 16α-carbonitrile (PCN) treatment can increase plasma total cholesterol and VLDL levels in apoE*3-Leiden (E3L) mice and decrease HDL levels in E3L mice with human cholesteryl ester transfer protein (E3L.CETP)-transgenic mice, a well-established model for human-like lipoprotein metabolism (19). We found that chronic activation of PXR by feeding apoE−/− mice the PXR agonist PCN can decrease HDL levels without affecting total cholesterol levels (15). Another report showed that short-term activation of PXR increases plasma triglyceride levels but decreases LDL cholesterol levels in LDLR−/− mice (20). Similar treatment also caused increased plasma triglyceride levels in apoE−/− mice (20). Interestingly, a recent meta-analysis of seven genome-wide association studies indicates that common genetic variants in PXR can strongly affect plasma lipid levels in humans, and 19 PXR single nucleotide polymorphisms were determined to significantly affect plasma LDL cholesterol levels (21). All of the evidence suggests that modulation of PXR activity may regulate lipid homeostasis and affect atherosclerosis development.
The identification of PXR as a xenobiotic sensor has provided an important tool for studying new mechanisms through which diet, drugs, and chemical exposures might impact human health. Several nuclear receptors (LXR, PPAR) that regulate lipid metabolism and modulate inflammation have been extensively studied for their roles in atherosclerosis (3, 4). However, the role of PXR in atherosclerosis remains to be determined. In our previous study, we found that chronic activation of PXR by feeding apoE−/− mice the PXR agonist PCN significantly increases atherosclerosis (15). To further define the role of PXR in atherosclerosis, we generated PXR and apoE double knockout (PXR−/−apoE−/−) mice. Here we show that PXR−/−apoE−/− mice had significantly decreased atherosclerotic cross-sectional lesion area in both the aortic root and brachiocephalic artery, without altering plasma lipid levels. Fresh isolated peritoneal macrophages from PXR−/−apoE−/− mice showed reduced expression of CD36 mRNA and lipid accumulation compared with apoE−/− mice. Deficiency of PXR also decreased CD36-mediated oxidized LDL (oxLDL) uptake in peritoneal macrophages. Furthermore, immunofluorescence staining showed that PXR was present in the atherosclerotic lesions of apoE−/− mice, and the expression levels of CD36 were diminished in the lesions of PXR−/−apoE−/− mice. Our findings indicate that deficiency of PXR attenuates atherosclerosis development, which may result from decreased expression of CD36 and reduced lipid uptake in macrophages.
MATERIALS AND METHODS
Animals
ApoE−/− mice on the C57BL/6 background (stock no. 002052) were purchased from The Jackson Laboratory. PXR−/− mice were originally generated in the laboratory of Dr. Ronald Evans at Salk Institute (22) and were backcrossed with C57BL/6 for several generations before we obtained them. These animals were backcrossed five additional generations onto the C57BL/6 background (>99% C57BL/6) using the marker-assisted Microsatellite Genotyping technique (15). PXR−/−apoE−/− mice were generated by crossing PXR−/− with apoE−/− mice and maintained on standard chow diet. All animals were housed by the Division of Laboratory Animal Resources, University of Kentucky, in a specific pathogen-free environment with a 12 h light-dark cycle, and approved by the Institutional Animal Care and Use Committee. Four week-old experimental male apoE−/− or PXR−/−apoE−/− littermates were weaned and fed a modified semisynthetic low-fat AIN76 diet containing 0.02% cholesterol (Research Diet; New Brunswick, NJ) (23, 24) for 12 weeks until euthanasia at 16 weeks of age.
Tissue preparation
On the day of euthanasia, mice were fasted for 6 h following the dark (feeding) cycle. Immediately prior to euthanasia,the fasting plasma glucose was measured, and mice were then anesthetized by intraperitoneal injection with sodium pentobarbital (Henry Schein; Melville, NY). Mice were exsanguinated by left ventricular puncture, and blood was collected into EDTA-containing syringes. Plasma was prepared by spinning at 16,000 g for 10 min. The circulation was flushed with PBS, and the heart was removed and stored frozen in Tissue-Tek OCT compound as described previously (15). Liver and other tissues were collected and stored in RNAlater solution (Life Technologies; Carlsbad, CA).
Plasma analysis
Plasma total cholesterol and triglyceride concentrations were determined enzymatically by colorimetric methods as described previously (Roche; Indianapolis, IN) (15). Plasma from multiple mice (n = 6) was pooled, and plasma lipoprotein cholesterol distributions were determined by fast-performance liquid chromatography (FPLC). Lipoprotein fractions were also isolated by centrifuging at 70,000 rpm for 3 h in a Beckman Optima TL-100 tabletop ultracentrifuge at its own density (1.006 g/ml). Then the infranatant was adjusted to a density of 1.063 g/ml with solid KBr in order to harvest the HDL fraction by spinning at 70,000 rpm for 18 h. The cholesterol content of HDL infranatant was measured enzymatically.
Quantification of atherosclerosis
To quantify the lesion areas at the aortic root, OCT compound-embedded hearts were sectioned at a 12 µm thickness, keeping all three valves of the aortic root in the same plane, and stained with Oil Red O as described previously (15, 23). To quantify atherosclerosis at the brachiocephalic artery, the OCT-embedded brachiocephalic artery was sectioned distal to proximal at a thickness of 10 µm. Atherosclerotic lesions lumenal to the internal elastic lamina were quantified in three equidistant Oil Red O-stained sections 200, 400, and 600 µm proximal from the branching point of the brachiocephalic artery into the carotid and subclavian arteries (15).
RNA isolation and quantitative real-time PCR Analysis
Total RNA was isolated from mouse tissues using TRIzol Reagent (Life Technologies) as per the manufacturer-supplied protocol. Quantitative real-time PCR (QPCR) was performed using gene-specific primers and the SYBR green PCR kit (Life Technologies) in an ABI 7900 system (Life Technologies) as described previously (15). All samples were quantified using the comparative Ct method for relative quantification of gene expression, and normalized to GAPDH (25). The primer sets used in this study are shown in supplementary Table I.
Peritoneal macrophage isolation and staining
Mice were injected intraperitoneally with 1 ml of 3% thioglycollate, and peritoneal macrophages were collected 4 days later as described previously (15). After centrifugation at 500 g for 5 min, the cells were resuspended in DMEM. Three to five million peritoneal macrophages from each group were allowed to attach to either coverslips or one 10 cm culture dish for 4 h. Then the culture dishes and coverslips were washed three times with PBS to remove nonadherent cells. RNA was isolated from the macrophages on culture dishes, and Oil Red O/hematoxylin staining was performed on the macrophages adhering to one coverslip. Cells containing lipid droplets (>10) were counted as foam cells and at least 10 fields per condition were counted (26, 27). The macrophages on the other coverslip were incubated with DMEM containing 100 µg/ml of oxLDL (Biomedical Technologies, Inc.; Stoughton, MA) for 24 h, followed by washing with PBS and staining with Oil Red O/hematoxylin. Macrophages were also stained with FITC-conjugated monocyte/macrophage antibody MOMA-2 (AbD Serotec; Raleigh, NC) or CD36 antibody (AbD Serotec), followed by Alexa 488-conjugated secondary antibody (Life Technologies).
Immunohistochemistry
Immunohistochemistry was performed on 12 μm sections of aortic roots freshly embedded in OCT. Sections were first fixed in 100% ice-cold acetone for 15 min and then washed with PBS for 20 min. Sections were permeabilized with PBS + 0.1% Triton X-100 (PBST) for 10 min. Nonspecific binding was reduced by incubating slides in 10% rabbit sera diluted in PBST for 20 min at room temperature. Sections were then incubated with rat anti-mouse monocyte/macrophage marker MOMA-2 antibody conjugated with FITC (1:100; AbD Serotec), rabbit anti-mouse PXR antibody (1:100; Santa Cruz Biotechnology, Santa Cruz, CA), or rat anti-mouse CD36 antibody (1:100; AbD Serotec) at 4°C for 12–15 h. Sections were rinsed with PBS and incubated with Alexa 594-labeled goat anti-rabbit (for PXR) or Alexa 488-labeled donkey anti-rat (for CD36) secondary antibodies (1:500; Life Technologies). The nuclei were stained by mounting the slides with 4’,6-diamidino-2-phenylindole (DAPI) medium (Vector Laboratories; Burlingame, CA). Images were acquired with a Nikon fluorescence microscope (Nikon; Melville, NY).
Statistical analysis
All data are expressed as mean ± SD unless indicated otherwise. Statistically significant differences between two groups were analyzed by t-test for data normally distributed and by the Mann-Whitney test for data not normally distributed, using Prism software, version 5.0. A P value of less than 0.05 was considered to be significant.
RESULTS
Deficiency of PXR does not affect plasma lipid profile in apoE−/− mice
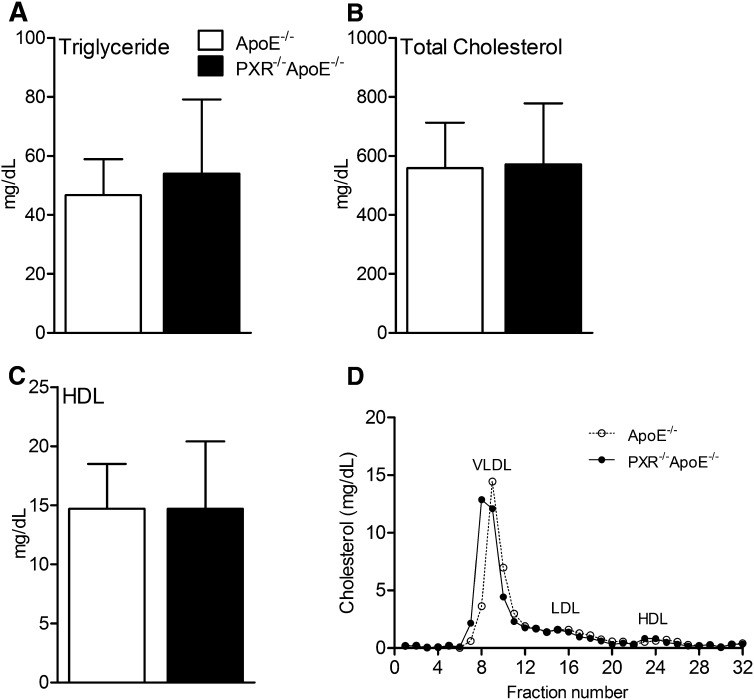
To determine the role of PXR in the development of atherosclerosis, we generated PXR−/−apoE−/− double knockout mice by crossing PXR−/− mice with apoE−/− mice. PXR−/−apoE−/− mice are variable and appear indistinguishable from apoE−/− mice. To investigate whether deficiency of PXR affects atherosclerosis, male apoE−/− and PXR−/−apoE−/− mice were fed a low-fat semi-synthetic AIN76a diet containing 0.02% cholesterol for 12 weeks. The low-fat and low-cholesterol AIN76a diet has been successfully used to induce atherosclerosis development in apoE−/− or LDLR−/− mice without increasing obesity and insulin resistance (15, 23, 24). At 16 weeks of age, apoE−/− and PXR−/−apoE−/− mice had similar body weights and fasting blood glucose levels (see supplementary Fig. I). The effect of deficiency of PXR on plasma lipid and lipoprotein levels was next examined. As shown in Fig. 1, deficiency of PXR did not affect plasma triglyceride and total and HDL cholesterol levels in PXR−/−apoE−/− mice. In addition, FPLC analysis showed that apoE−/− and PXR−/−apoE−/− mice had similar plasma cholesterol distribution patterns (Fig. 1D).
Fig.1.
Plasma lipid profiles of apoE−/− and PXR−/−apoE−/− mice. Four week-old male apoE−/− and PXR−/−apoE−/− mice were fed a semi-synthetic 0.02% cholesterol AIN76a diet for 12 weeks. The plasma triglyceride (A), total cholesterol (B), and HDL cholesterol (C) levels were measured (n = 8–10 mice/group). D: FPLC analysis of plasma cholesterol distribution of apoE−/− and PXR−/−apoE−/− mice. Error bars indicate SD.
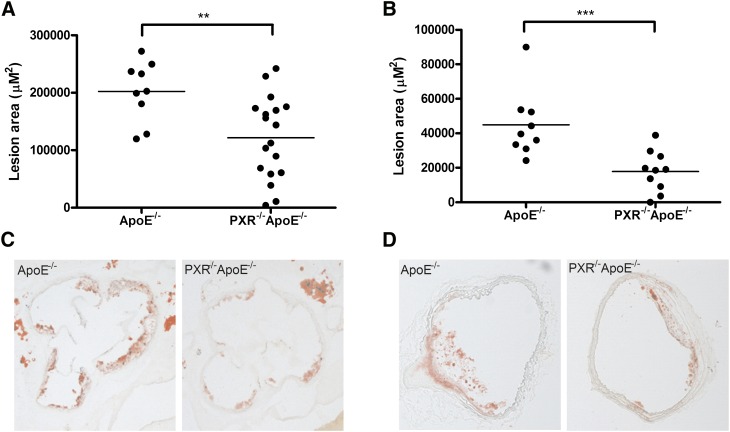
Deficiency of PXR significantly decreases atherosclerosis in apoE−/− mice
The apoE−/− and PXR−/−apoE−/− mice were euthanized at 16 weeks of age, and atherosclerotic lesion areas were determined in the aortic root and brachiocephalic artery as shown in Fig. 2. ApoE−/− mice have a mean atherosclerotic lesion area of 202,405 ± 49,489 μM2 in the aortic root and 44,945 ± 18,344 μM2 in the brachiocephalic artery. Compared with apoE−/− mice, PXR−/−apoE−/− mice had significantly decreased cross-sectional lesion areas of 40% at the aortic root (121,713 ± 69,511 μM2; P < 0.01) and 60% at the brachiocephalic artery (17,899 ± 11,918 μM2; P < 0.001) (Fig. 2A, B, respectively). Thus, deficiency of PXR decreases atherosclerosis in apoE−/− mice.
Fig.2.
Decreased atherosclerotic lesions in PXR−/−apoE−/− mice. Four week-old male apoE−/− and PXR−/−apoE−/− mice were fed a semi-synthetic 0.02% cholesterol AIN76a diet for 12 weeks. A, B: Quantitative analysis of the lesion size in the aortic root (A) and brachiocephalic artery (B) of apoE−/− and PXR−/−apoE−/− mice (n = 9–17; **P < 0.01 and ***P < 0.001). C, D: Representative Oil Red O-stained sections of atherosclerotic lesion area in the aortic root (C) and brachiocephalic artery (D) from apoE−/− and PXR−/−apoE−/− mice.
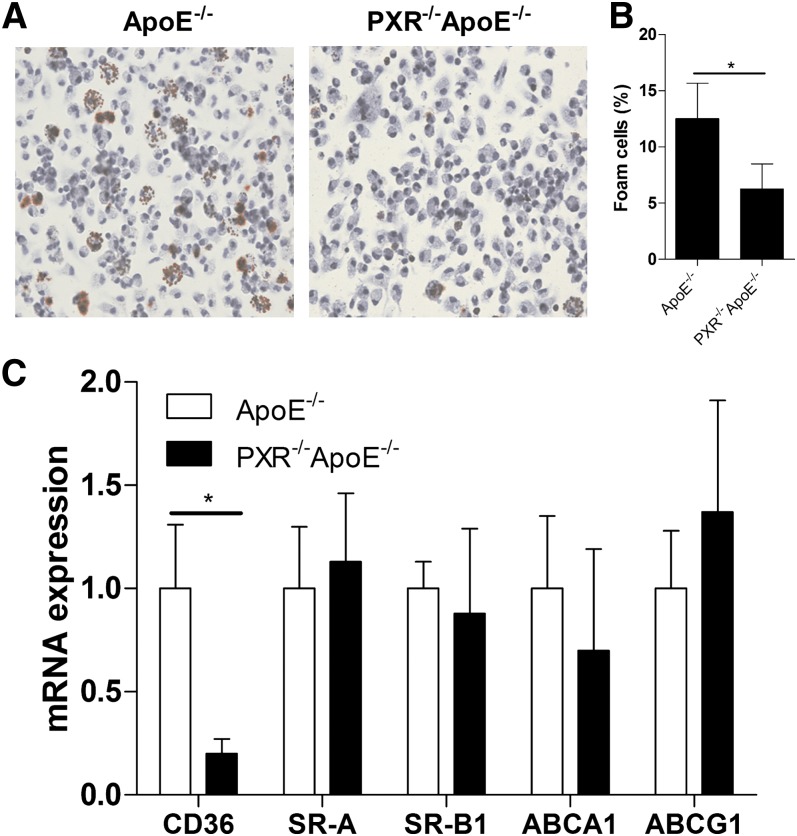
Peritoneal macrophages of PXR−/−apoE−/− mice have reduced lipid accumulation and CD36 mRNA levels
To elucidate possible molecular mechanisms through which PXR deficiency decreases atherosclerosis, a panel of hepatic atherosclerosis modifier genes were measured by QPCR. As shown in supplementary Fig. II, the expression levels of the selected genes, including SREBP1a, SREBP1c, SREBP2, HMGCR, LDLR, and SR-BI, did not change in the liver of PXR−/−apoE−/− mice.
In addition to liver, PXR is also expressed in immune cells such as T cells, B cells, monocytes, and peritoneal macrophages (15, 28–31). Macrophages play a critical role in atherogenesis, and accumulation of lipid-loaded macrophages is a hallmark or atherosclerosis (1, 32). We have previously reported that activation of PXR increases CD36 expression and lipid accumulation in peritoneal macrophages of apoE−/− mice, which may contribute to the pro-atherogenic effects of PXR (15). To investigate whether deficiency of PXR affects macrophage function, peritoneal macrophages from 16 week-old apoE−/− and PXR−/−apoE−/− mice fed an AIN76a diet for 12 weeks were isolated and allowed to adhere to coverslips for 4 h. We performed Oil Red O staining to assess neutral lipid levels and foam cell formation in peritoneal macrophages. As shown in Fig. 3A, B, the lipid accumulation and foam cell formation in peritoneal macrophages were substantially reduced in peritoneal macrophages of PXR−/−apoE−/− mice. Gene expression analysis indicates that the CD36 mRNA levels were significantly decreased in fresh isolated macrophages from PXR−/−apoE−/− mice (Fig. 3C). Consistent with QPCR results, immunofluorescence staining showed that CD36 expression was decreased in macrophages from PXR−/−apoE−/− mice (see supplementary Fig. III). CD36 is a member of the scavenger receptor class B family and might play an important role in atherosclerosis-related processes (33, 34). The decreased CD36 expression is consistent with decreased lipid accumulation in those cells. In contrast, the expression levels of another scavenger receptor, SR-A, and ABC transporters ABCA1 and ABCG1 were not significantly affected in macrophages derived from apoE−/− and PXR−/−apoE−/− mice (Fig. 3C).
Fig.3.
Reduced lipid accumulation and CD36 mRNA levels in peritoneal macrophages of PXR−/−apoE−/− mice. A: Fresh isolated peritoneal macrophages from apoE−/− and PXR−/−apoE−/− mice fed AIN76a diet for 12 weeks were stained with Oil Red O and hematoxylin. Magnification, ×400. B: Foam cell quantification from peritoneal macrophages in studies described in A (n = 5, *P < 0.05). C: Total RNAs were isolated from peritoneal macrophages of apoE−/− and PXR−/−apoE−/− mice. The expression levels of indicated genes were analyzed by QPCR (n = 5 per group; *P < 0.05). Error bars indicate SD.
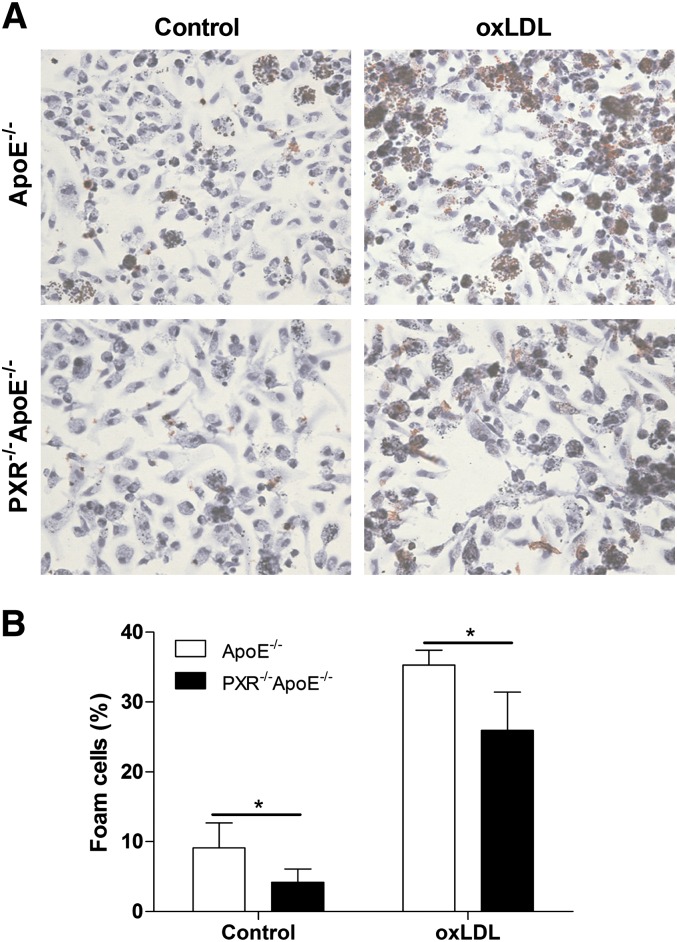
Deficiency of PXR prevents oxLDL uptake and foam cell formation in peritoneal macrophages
In vitro CD36 was shown to mediate uptake of oxLDL by macrophages, a critical step in foam cell formation (26). oxLDL is a modified form of LDL that has been considered the most important atherogenic LDL (35). To determine whether disruption of the PXR gene affects foam cell formation, peritoneal macrophages were isolated from PXR−/−apoE−/− and apoE−/− mice and incubated with oxLDL overnight. Oil Red O staining showed that the uptake of oxLDL was significantly decreased in peritoneal macrophages isolated from PXR−/−apoE−/− mice, which indicates that deficiency of PXR prevents foam cell formation in vitro (Fig. 4A, B).
Fig.4.
Decreased lipid uptake and foam cell formation in peritoneal macrophages of PXR−/−apoE−/− mice. A: Fresh isolated peritoneal macrophages from apoE−/− and PXR−/−apoE−/− mice were incubated with oxLDL (100 µg/ml) for 24 h, and stained with Oil Red O and hematoxylin. Magnification, ×400. B: Foam cell quantification from peritoneal macrophages in studies described in A (n = 3–5; *P < 0.05).
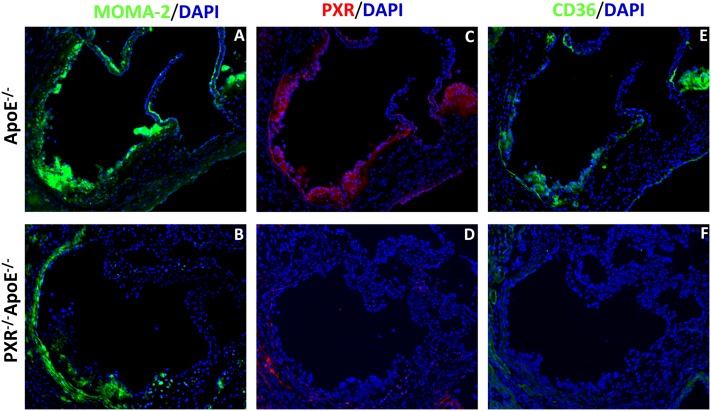
PXR−/−apoE−/− mice have diminished CD36 expression in the atherosclerotic lesions
The significantly decreased CD36 mRNA levels and reduced lipid accumulation and uptake in the peritoneal macrophages of PXR−/−apoE−/− mice prompted us to investigate the protein content of CD36 in the atherosclerotic lesions. PXR has been found to be expressed in vascular tissue (36), but it is not clear whether PXR is present in atherosclerotic lesions. Immunofluorescence staining showed that PXR is present in the lesions of apoE−/− mice but not in those of PXR−/−apoE−/− mice. Moreover, the protein levels of CD36 were substantially decreased in the atherosclerotic lesions of PXR−/−apoE−/− mice compared with apoE−/− mice (Fig. 5). Thus, the decreased atherosclerotic lesions in PXR−/−apoE−/− mice may be associated with decreased CD36 expression and inhibited foam cell formation in macrophages.
Fig.5.
Immunohistochemistry of PXR and CD36 in atherosclerotic lesions of apoE−/− and PXR−/−apoE−/− mice. Sections of atherosclerotic lesion area in the aortic root of apoE−/− and PXR−/−apoE−/− mice were stained with anti-monocytes/macrophages (MOMA-2) (A, B), anti-PXR (C, D), or anti-CD36 (E, F) primary antibodies, followed by fluorescein-labeled secondary antibodies. The nuclei were stained with DAPI (blue). Magnification, ×100.
DISCUSSION
It was previously shown that clinically relevant PXR ligands affect plasma lipid levels in patients and that activation of PXR may have pro-atherogenic effects in animals (11, 13, 15, 16, 37, 38). However, the role of PXR itself in atherosclerosis has not been thoroughly investigated. In the current study, we investigate the pathological effects of PXR deficiency on the development of atherosclerosis in apoE−/− mice. PXR−/−apoE−/− double knockout mice were generated, and the atherosclerosis lesion areas were compared between apoE−/− and PXR−/−apoE−/− mice in a well-controlled feeding study. PXR−/−apoE−/− mice have similar plasma lipid levels and cholesterol distribution patterns compared with apoE−/− mice but significantly decreased atherosclerotic lesion areas in both the aortic root and brachiocephalic artery. We found that CD36 expression and lipid accumulation were reduced in fresh isolated peritoneal macrophages from PXR−/−apoE−/− mice. Deficiency of PXR also decreased oxLDL uptake in peritoneal macrophages, which may be due to diminished CD36 expression. We also found that PXR and CD36 were expressed in the atherosclerotic lesions of apoE−/− mice, but the expression levels of CD36 were diminished in the lesions of PXR−/−apoE−/− mice. To our knowledge, this is the first study to investigate the effects of PXR deficiency on atherosclerosis in animal models.
We previously reported that chronic activation of PXR increases atherosclerosis in ApoE−/− mice (15). Activation of PXR reduces HDL cholesterol levels without altering plasma triglyceride and total cholesterol levels (15). The decreased atherosclerosis in PXR−/−apoE−/− mice reported here confirms the pro-atherogenic effects of PXR. However, we found that the plasma lipid levels and cholesterol distribution pattern were not altered in PXR−/−apoE−/− mice. Furthermore, PXR deficiency did not alter expression levels of hepatic genes such as SREBP1, SREBP2, HMGCR, and LDLR involved in lipid homeostasis. Therefore, the decreased atherosclerosis in PXR−/−apoE−/− mice could not be explained by unchanged plasma lipid levels and unaffected hepatic lipid homeostasis.
PXR can directly regulate the transcription of CD36, a FA transporter, and activation of PXR promotes CD36-mediated hepatic lipid accumulation (16). CD36 is also a member of the scavenger receptor class B family and might play an important role in atherosclerosis-related processes such as macrophage lipid uptake and foam cell formation (33). We previously found that activation of PXR increases CD36 expression and lipid accumulation in peritoneal macrophages of apoE−/− mice (15). In the current study, the expression levels of CD36 and lipid accumulation were significantly decreased in the peritoneal macrophages of PXR−/−apoE−/− mice. Deficiency of PXR prevented the modified lipid uptake and foam cell formation of peritoneal macrophages in apoE−/− mice. It was previously reported that PXR ligands can induce ABCA1 and ABCG1 expression and stimulate cholesterol efflux in intestinal cells, but not in other cells, such as liver cells (39). We found that deficiency of PXR did not affect the basal expression levels of ABCA1 and ABCG1 in macrophages. The decreased CD36 expression in macrophages may contribute to the decreased atherosclerosis in PXR−/−apoE−/− mice.
The in vivo role of CD36 in atherosclerosis remains unclear and controversial. Febbraio and colleagues reported that targeted disruption of CD36 in vivo protects against atherosclerotic lesion development in apoE−/− mice (35, 40). However, Moore et al. showed that CD36−/−apoE−/− mice have increased atherosclerosis in the aortic sinus areas (41). More recently, the same group reported that loss of CD36 and scavenger receptor A activity reduces atherosclerotic lesion complexity without affecting atherosclerotic lesion size in apoE−/− mice (42). These data suggest that the role of CD36 in atherosclerosis remains unresolved. However, it is undisputable that CD36 plays an important role in macrophage lipid accumulation in vitro and in vivo (26, 41). Furthermore, a recent report indicates that CD36 signaling in response to oxLDL modulates macrophage migration and contributes to macrophage trapping in atherosclerotic lesions (43). We also observed decreased CD36 expression in the atherosclerotic lesions of PXR−/−apoE−/− mice. Therefore, a plausible explanation for the decreased atherosclerosis observed in PXR−/−apoE−/− mice is the reduction of CD36 expression and its mediated macrophage lipid uptake or migration.
It is intriguing that deficiency of PXR only affects the expression levels of CD36 in the macrophages but not in other tissues such as liver. In addition to PXR, the CD36 gene can be regulated by many other transcription factors or mediators, including PPARγ, LXR, transforming growth factor-β, interferon-γ, and glucocorticoids (17, 44–46). For example, PPARγ is required for basal expression of CD36 in macrophages, and expression of CD36 is diminished in PPARγ-null mice (46, 47). Therefore, the altered environment and other transcriptional factors may also be involved in the downregulation of CD36 in the macrophages of PXR−/−apoE−/− mice. Furthermore, we have previously reported that PXR can regulate target gene expression in a tissue-specific manner (48). PXR may be important for basal expression of CD36 in the macrophage but not in the liver. Interestingly, deletion of FXR, a PXR closely related nuclear receptor, also decreases CD36 expression and reduces lipid accumulation in the macrophages of LDLR−/− (49) or apoE−/− (50) mice. Unlike PXR-deficient mice, deficiency of FXR increases plasma cholesterol and triglyceride levels in apoE−/− mice (50), and increases plasma triglyceride levels but decreases LDL and HDL levels in LDLR−/− mice fed a high-fat diet (49). However, both studies showed that deficiency of FXR reduces atherosclerosis in these mice. It is likely that decreased atherosclerosis in PXR- or FXR-deficient apoE−/− mice is mediated by similar mechanisms involving macrophage lipid uptake, and additional studies will be necessary to determine the role of those nuclear receptors in foam cell formation and atherosclerosis development.
The precise mechanisms through which PXR modulates atherosclerosis in different animal models and in humans remain to be determined. It would also be interesting to investigate the effects of deficiency of PXR on lipid levels and atherosclerosis in different animal models such as LDLR−/− mice. Nevertheless, our study provides critical insight for understanding the role of PXR in atherosclerosis and, for the first time, showed that deficiency of PXR decreases atherosclerosis in animal models.
In summary, we generated PXR−/−apoE−/− double knockout mice to study the role of PXR in atherosclerosis development. PXR−/−apoE−/− mice had significantly reduced atherosclerosis, which may result from decreased expression of CD36, and reduced lipid uptake in macrophages. Our findings suggest an important role of PXR in atherosclerosis and provide evidence for a potential link between xenobiotic metabolism and atherosclerosis. The discovery and pharmacological development of new selective PXR antagonists could represent an interesting and innovative therapeutic approach to combating atherosclerosis.
Supplementary Material
Acknowledgments
The authors thank Dr. Jan Breslow for invaluable advice, discussion, and technical support; Drs. Ronald Evans and Bruce Blumberg for PXR−/− mice; Dr. Alan Daugherty and Ms. Deborah Howatt for help with FPLC analysis; and Dr. Florence Gizard for critical reading of the manuscript.
Footnotes
Abbreviations:
- CYP
- cytochrome P450
- FPLC
- fast-performance liquid chromatography
- LXR
- liver X receptor
- oxLDL
- oxidized LDL
- PBST
- PBS + 0.1% Triton X-100
- PCN
- pregnenolone 16α-carbonitrile
- PPAR
- peroxisome proliferator-activated receptor
- PXR
- pregnane X receptor
- QPCR
- quantitative real-time PCR
- SREBP
- sterol-regulatory element binding protein
- SXR
- steroid and xenobiotic receptor
- WT
- wild type
This work was supported in part by National Institutes of Health Grant P30HL101300 and American Heart Association Grant 09SDG2150176. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or other granting agencies.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of three figures and one table.
REFERENCES
- 1.Lusis A. J. 2000. Atherosclerosis. Nature. 407: 233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanz J., Fayad Z. A. 2008. Imaging of atherosclerotic cardiovascular disease. Nature. 451: 953–957. [DOI] [PubMed] [Google Scholar]
- 3.Barish G. D., Evans R. M. 2004. PPARs and LXRs: atherosclerosis goes nuclear. Trends Endocrinol. Metab. 15: 158–165. [DOI] [PubMed] [Google Scholar]
- 4.Glass C. K. 2006. Going nuclear in metabolic and cardiovascular disease. J. Clin. Invest. 116: 556–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hageman J., Herrema H., Groen A. K., Kuipers F. 2010. A role of the bile salt receptor FXR in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 30: 1519–1528. [DOI] [PubMed] [Google Scholar]
- 6.Huang W., Glass C. K. 2010. Nuclear receptors and inflammation control: molecular mechanisms and pathophysiological relevance. Arterioscler. Thromb. Vasc. Biol. 30: 1542–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blumberg B., Sabbagh W., Jr, Juguilon H., Bolado J., Jr, van Meter C. M., Ong E. S., Evans R. M. 1998. SXR, a novel steroid and xenobiotic-sensing nuclear receptor. Genes Dev. 12: 3195–3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kliewer S. A., Moore J. T., Wade L., Staudinger J. L., Watson M. A., Jones S. A., McKee D. D., Oliver B. B., Willson T. M., Zetterstrom R. H., et al. 1998. An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell. 92: 73–82. [DOI] [PubMed] [Google Scholar]
- 9.Zhou C., Verma S., Blumberg B. 2009. The steroid and xenobiotic receptor (SXR), beyond xenobiotic metabolism. Nucl. Recept. Signal. 7: e001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kliewer S. A., Goodwin B., Willson T. M. 2002. The nuclear pregnane X receptor: a key regulator of xenobiotic metabolism. Endocr. Rev. 23: 687–702. [DOI] [PubMed] [Google Scholar]
- 11.Khogali A. M., Chazan B. I., Metcalf V. J., Ramsay J. H. 1974. Hyperlipidaemia as a complication of rifampicin treatment. Tubercle. 55: 231–233. [DOI] [PubMed] [Google Scholar]
- 12.Carr A., Samaras K., Burton S., Law M., Freund J., Chisholm D. J., Cooper D. A. 1998. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS. 12: F51–F58. [DOI] [PubMed] [Google Scholar]
- 13.Shafran S. D., Mashinter L. D., Roberts S. E. 2005. The effect of low-dose ritonavir monotherapy on fasting serum lipid concentrations. HIV Med. 6: 421–425. [DOI] [PubMed] [Google Scholar]
- 14.Barbaro G. 2006. Metabolic and cardiovascular complications of highly active antiretroviral therapy for HIV infection. Curr. HIV Res. 4: 79–85. [DOI] [PubMed] [Google Scholar]
- 15.Zhou C., King N., Chen K. Y., Breslow J. L. 2009. Activation of PXR induces hypercholesterolemia in wild-type and accelerates atherosclerosis in apoE deficient mice. J. Lipid Res. 50: 2004–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou J., Zhai Y., Mu Y., Gong H., Uppal H., Toma D., Ren S., Evans R. M., Xie W. 2006. A novel pregnane X receptor-mediated and sterol regulatory element-binding protein-independent lipogenic pathway. J. Biol. Chem. 281: 15013–15020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou J., Febbraio M., Wada T., Zhai Y., Kuruba R., He J., Lee J. H., Khadem S., Ren S., Li S., et al. 2008. Hepatic fatty acid transporter Cd36 is a common target of LXR, PXR, and PPARgamma in promoting steatosis. Gastroenterology. 134: 556–567. [DOI] [PubMed] [Google Scholar]
- 18.Roth A., Looser R., Kaufmann M., Blaettler S., Rencurel F., Huang W., Moore D. D., Meyer U. A. 2008. Regulatory cross-talk between drug metabolism and lipid homeostasis: constitutive androstane receptor and pregnane X receptor increase Insig-1 expression. Mol Pharmacol. 73: 1282–1289. [DOI] [PubMed] [Google Scholar]
- 19.de Haan W., de Vries-van der Weij J., Mol I. M., Hoekstra M., Romijn J. A., Jukema J. W., Havekes L. M., Princen H. M., Rensen P. C. 2009. PXR agonism decreases plasma HDL levels in ApoE3-Leiden.CETP mice. Biochim. Biophys. Acta. 1791: 191–197. [DOI] [PubMed] [Google Scholar]
- 20.Hoekstra M., Lammers B., Out R., Li Z., Van Eck M., Van Berkel T. J. 2009. Activation of the nuclear receptor PXR decreases plasma LDL-cholesterol levels and induces hepatic steatosis in LDL receptor knockout mice. Mol. Pharm. 6: 182–189. [DOI] [PubMed] [Google Scholar]
- 21.Lu Y., Feskens E. J., Boer J. M., Muller M. 2010. The potential influence of genetic variants in genes along bile acid and bile metabolic pathway on blood cholesterol levels in the population. Atherosclerosis. 210: 14–27. [DOI] [PubMed] [Google Scholar]
- 22.Xie W., Barwick J. L., Downes M., Blumberg B., Simon C. M., Nelson M. C., Neuschwander-Tetri B. A., Brunt E. M., Guzelian P. S., Evans R. M. 2000. Humanized xenobiotic response in mice expressing nuclear receptor SXR. Nature. 406: 435–439. [DOI] [PubMed] [Google Scholar]
- 23.Teupser D., Persky A. D., Breslow J. L. 2003. Induction of atherosclerosis by low-fat, semisynthetic diets in LDL receptor-deficient C57BL/6J and FVB/NJ mice: comparison of lesions of the aortic root, brachiocephalic artery, and whole aorta (en face measurement). Arterioscler. Thromb. Vasc. Biol. 23: 1907–1913. [DOI] [PubMed] [Google Scholar]
- 24.Wolfrum S., Teupser D., Tan M., Chen K. Y., Breslow J. L. 2007. The protective effect of A20 on atherosclerosis in apolipoprotein E-deficient mice is associated with reduced expression of NF-kappaB target genes. Proc. Natl. Acad. Sci. USA. 104: 18601–18606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Livak K. J., Schmittgen T. D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 26.Rahaman S. O., Lennon D. J., Febbraio M., Podrez E. A., Hazen S. L., Silverstein R. L. 2006. A CD36-dependent signaling cascade is necessary for macrophage foam cell formation. Cell Metab. 4: 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Z., Klipfell E., Bennett B. J., Koeth R., Levison B. S., Dugar B., Feldstein A. E., Britt E. B., Fu X., Chung Y. M., et al. 2011. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 472: 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dubrac S., Elentner A., Ebner S., Horejs-Hoeck J., Schmuth M. 2010. Modulation of T lymphocyte function by the pregnane X receptor. J. Immunol. 184: 2949–2957. [DOI] [PubMed] [Google Scholar]
- 29.Albermann N., Schmitz-Winnenthal F. H., Z'Graggen K., Volk C., Hoffmann M. M., Haefeli W. E., Weiss J. 2005. Expression of the drug transporters MDR1/ABCB1, MRP1/ABCC1, MRP2/ABCC2, BCRP/ABCG2, and PXR in peripheral blood mononuclear cells and their relationship with the expression in intestine and liver. Biochem. Pharmacol. 70: 949–958. [DOI] [PubMed] [Google Scholar]
- 30.Owen A., Chandler B., Back D. J., Khoo S. H. 2004. Expression of pregnane-X-receptor transcript in peripheral blood mononuclear cells and correlation with MDR1 mRNA. Antivir. Ther. 9: 819–821. [PubMed] [Google Scholar]
- 31.Siest G., Jeannesson E., Marteau J. B., Samara A., Marie B., Pfister M., Visvikis-Siest S. 2008. Transcription factor and drug-metabolizing enzyme gene expression in lymphocytes from healthy human subjects. Drug Metab. Dispos. 36: 182–189. [DOI] [PubMed] [Google Scholar]
- 32.Glass C. K., Witztum J. L. 2001. Atherosclerosis. The road ahead. Cell. 104: 503–516. [DOI] [PubMed] [Google Scholar]
- 33.Abumrad N. A., el-Maghrabi M. R., Amri E. Z., Lopez E., Grimaldi P. A. 1993. Cloning of a rat adipocyte membrane protein implicated in binding or transport of long-chain fatty acids that is induced during preadipocyte differentiation. Homology with human CD36. J. Biol. Chem. 268: 17665–17668. [PubMed] [Google Scholar]
- 34.Tontonoz P., Nagy L., Alvarez J. G., Thomazy V. A., Evans R. M. 1998. PPARgamma promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell. 93: 241–252. [DOI] [PubMed] [Google Scholar]
- 35.Febbraio M., Podrez E. A., Smith J. D., Hajjar D. P., Hazen S. L., Hoff H. F., Sharma K., Silverstein R. L. 2000. Targeted disruption of the class B scavenger receptor CD36 protects against atherosclerotic lesion development in mice. J. Clin. Invest. 105: 1049–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hagedorn K. A., Cooke C. L., Falck J. R., Mitchell B. F., Davidge S. T. 2007. Regulation of vascular tone during pregnancy: a novel role for the pregnane X receptor. Hypertension. 49: 328–333. [DOI] [PubMed] [Google Scholar]
- 37.Carr A., Samaras K., Chisholm D. J., Cooper D. A. 1998. Pathogenesis of HIV-1-protease inhibitor-associated peripheral lipodystrophy, hyperlipidaemia, and insulin resistance. Lancet. 351: 1881–1883. [DOI] [PubMed] [Google Scholar]
- 38.Eiris J. M., Lojo S., Del Rio M. C., Novo I., Bravo M., Pavon P., Castro-Gago M. 1995. Effects of long-term treatment with antiepileptic drugs on serum lipid levels in children with epilepsy. Neurology. 45: 1155–1157. [DOI] [PubMed] [Google Scholar]
- 39.Li T., Chen W., Chiang J. Y. 2007. PXR induces CYP27A1 and regulates cholesterol metabolism in the intestine. J. Lipid Res. 48: 373–384. [DOI] [PubMed] [Google Scholar]
- 40.Guy E., Kuchibhotla S., Silverstein R., Febbraio M. 2007. Continued inhibition of atherosclerotic lesion development in long term Western diet fed CD36o /apoEo mice. Atherosclerosis. 192: 123–130. [DOI] [PubMed] [Google Scholar]
- 41.Moore K. J., Kunjathoor V. V., Koehn S. L., Manning J. J., Tseng A. A., Silver J. M., McKee M., Freeman M. W. 2005. Loss of receptor-mediated lipid uptake via scavenger receptor A or CD36 pathways does not ameliorate atherosclerosis in hyperlipidemic mice. J. Clin. Invest. 115: 2192–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manning-Tobin J. J., Moore K. J., Seimon T. A., Bell S. A., Sharuk M., Alvarez-Leite J. I., de Winther M. P., Tabas I., Freeman M. W. 2009. Loss of SR-A and CD36 activity reduces atherosclerotic lesion complexity without abrogating foam cell formation in hyperlipidemic mice. Arterioscler. Thromb. Vasc. Biol. 29: 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park Y. M., Febbraio M., Silverstein R. L. 2009. CD36 modulates migration of mouse and human macrophages in response to oxidized LDL and may contribute to macrophage trapping in the arterial intima. J. Clin. Invest. 119: 136–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Han J., Hajjar D. P., Tauras J. M., Feng J., Gotto A. M., Jr, Nicholson A. C. 2000. Transforming growth factor-beta1 (TGF-beta1) and TGF-beta2 decrease expression of CD36, the type B scavenger receptor, through mitogen-activated protein kinase phosphorylation of peroxisome proliferator-activated receptor-gamma. J. Biol. Chem. 275: 1241–1246. [DOI] [PubMed] [Google Scholar]
- 45.Yesner L. M., Huh H. Y., Pearce S. F., Silverstein R. L. 1996. Regulation of monocyte CD36 and thrombospondin-1 expression by soluble mediators. Arterioscler. Thromb. Vasc. Biol. 16: 1019–1025. [DOI] [PubMed] [Google Scholar]
- 46.Moore K. J., Rosen E. D., Fitzgerald M. L., Randow F., Andersson L. P., Altshuler D., Milstone D. S., Mortensen R. M., Spiegelman B. M., Freeman M. W. 2001. The role of PPAR-gamma in macrophage differentiation and cholesterol uptake. Nat. Med. 7: 41–47. [DOI] [PubMed] [Google Scholar]
- 47.Akiyama T. E., Sakai S., Lambert G., Nicol C. J., Matsusue K., Pimprale S., Lee Y. H., Ricote M., Glass C. K., Brewer H. B., Jr, et al. 2002. Conditional disruption of the peroxisome proliferator-activated receptor gamma gene in mice results in lowered expression of ABCA1, ABCG1, and apoE in macrophages and reduced cholesterol efflux. Mol. Cell. Biol. 22: 2607–2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou C., Tabb M. M., Sadatrafiei A., Grun F., Blumberg B. 2004. Tocotrienols activate the steroid and xenobiotic receptor, SXR, and selectively regulate expression of its target genes. Drug Metab. Dispos. 32: 1075–1082. [DOI] [PubMed] [Google Scholar]
- 49.Zhang Y., Wang X., Vales C., Lee F. Y., Lee H., Lusis A. J., Edwards P. A. 2006. FXR deficiency causes reduced atherosclerosis in Ldlr−/− mice. Arterioscler. Thromb. Vasc. Biol. 26: 2316–2321. [DOI] [PubMed] [Google Scholar]
- 50.Guo G. L., Santamarina-Fojo S., Akiyama T. E., Amar M. J., Paigen B. J., Brewer B., Jr, Gonzalez F. J. 2006. Effects of FXR in foam-cell formation and atherosclerosis development. Biochim. Biophys. Acta. 1761: 1401–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.