Abstract
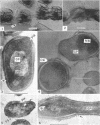
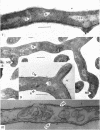
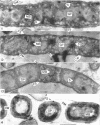
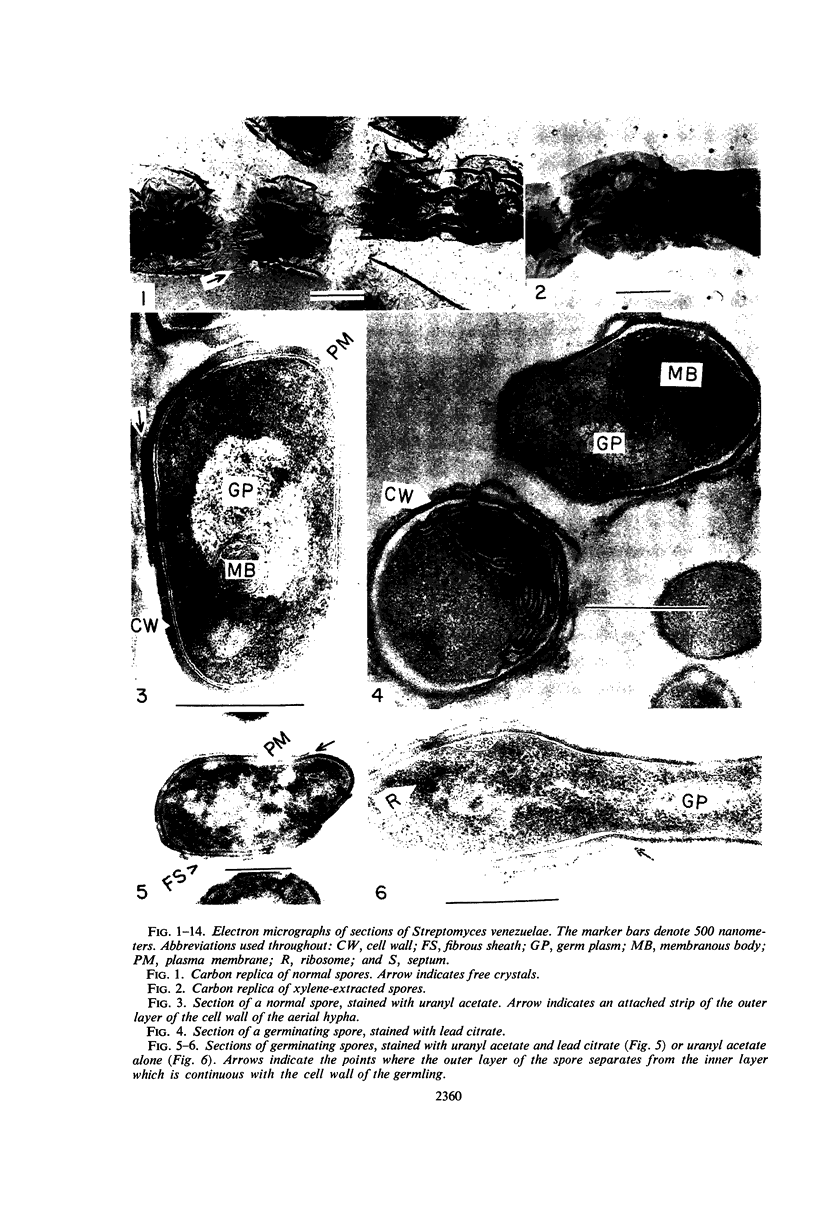
Streptomyces venezuelae is a filamentous bacterium with branching vegetative hyphae embedded in the substrate and aerial hyphae bearing spores. The exterior of the spore is inlaid with myriads of tiny rods which can be removed with xylene. The spore wall is approximately 30 nanometers thick. Occasionally, it can be seen that the plasma membrane and the membranous bodies within a spore are connected. The spore's germ plasm is not separated from the cytoplasm by a nuclear envelope. The cell walls of the vegetative hyphae, which are about 15 nanometers thick, are structurally and chemically similar to those of gram-positive bacteria. The numerous internal membranous bodies, some of which arise from the plasma membrane of the vegetative hypha, may be vesicular, whirled, or convoluted. Membranous bodies are usually prominent at the hyphal apices and are associated with septum formation. The germ plasm is an elongate, contorted, centrally placed area of lower electron density than the hyphal cytoplasm. The spores differ from the vegetative hyphae, not only in fine structure, but also in the arginine and leucine contents of their total cellular proteins.
Full text
PDF


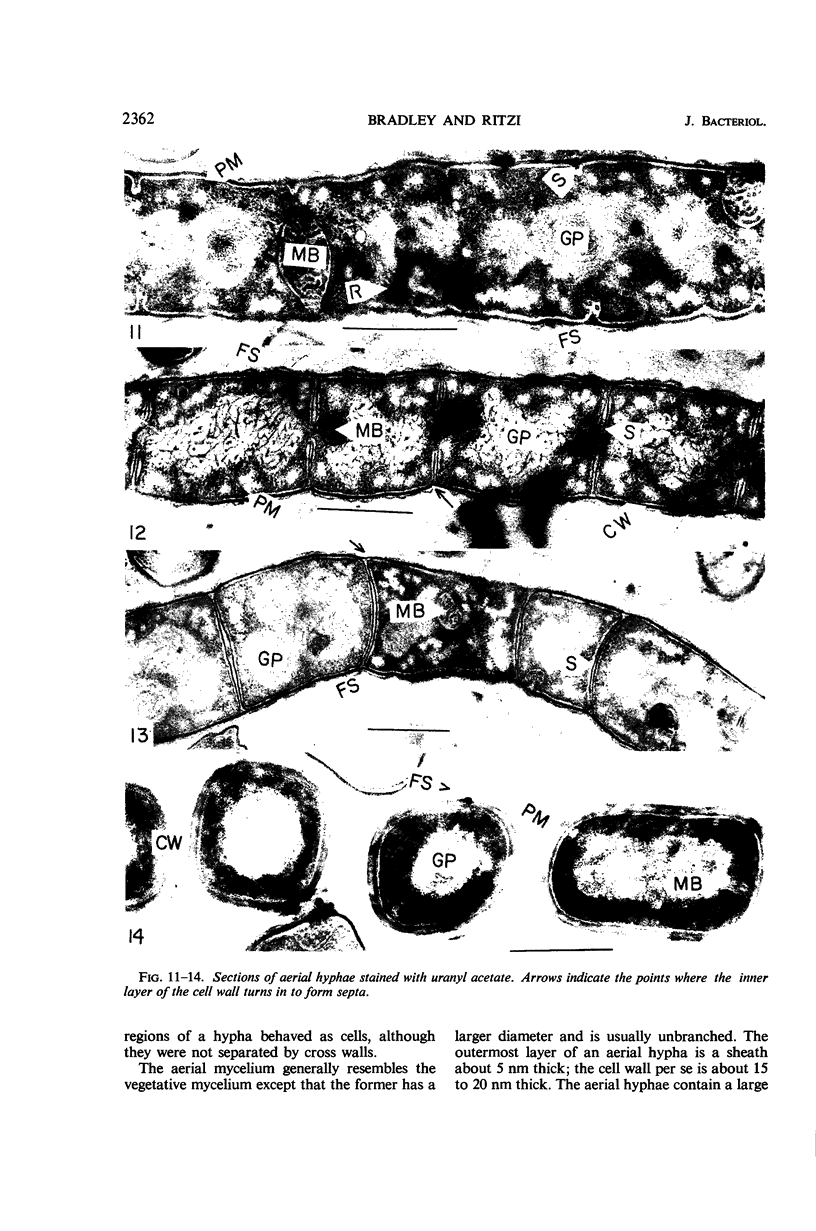
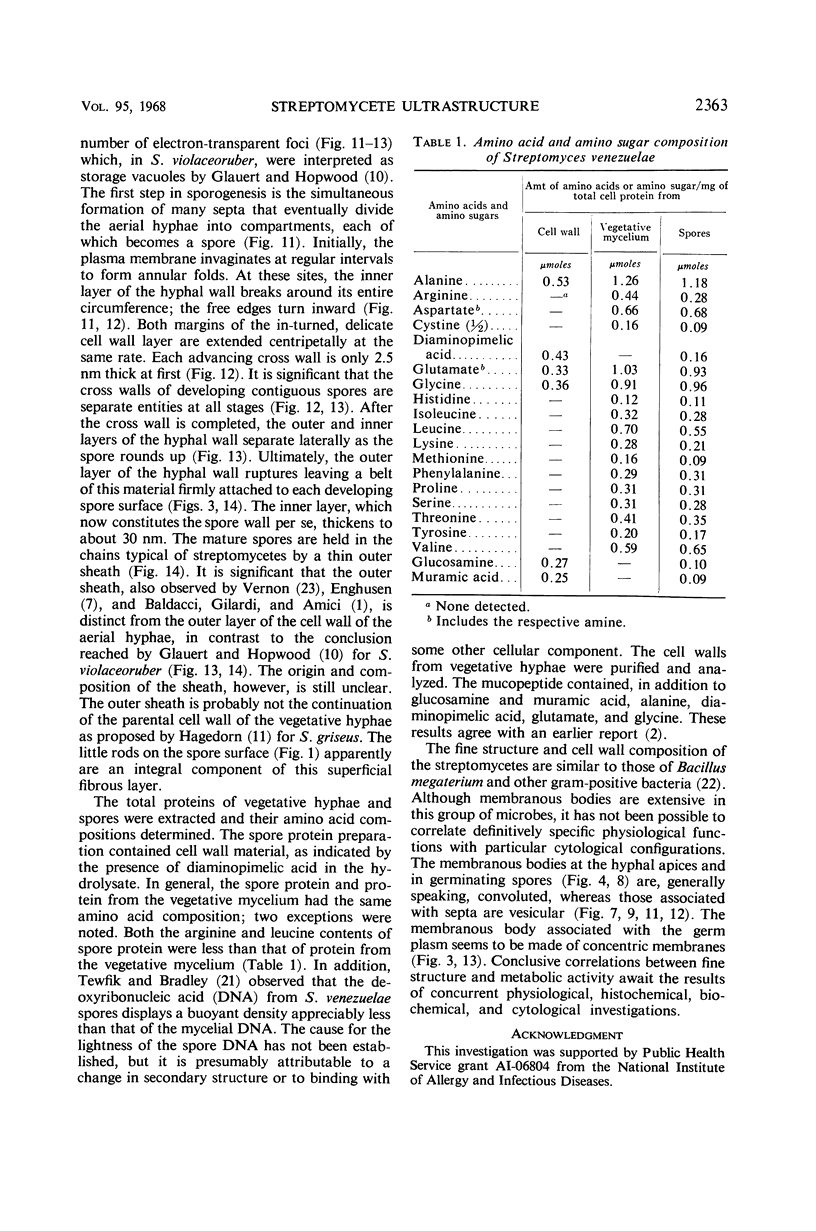

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BECKER B., LECHEVALIER M. P., LECHEVALIER H. A. CHEMICAL COMPOSITION OF CELL-WALL PREPARATIONS FROM STRAINS OF VARIOUS FORM-GENERA OF AEROBIC ACTINOMYCETES. Appl Microbiol. 1965 Mar;13:236–243. doi: 10.1128/am.13.2.236-243.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley S. G. Genetics in applied microbiology. Adv Appl Microbiol. 1966;8:29–59. doi: 10.1016/s0065-2164(08)70491-x. [DOI] [PubMed] [Google Scholar]
- Bradley S. G., Ritzi D. Electron microscopical studies of actinophage multiplication. J Gen Virol. 1967 Jul;1(3):285–290. [PubMed] [Google Scholar]
- DIETZ A., MATHEWS J. Taxonomy by carbon replication. I. An examination of Streptomyces hygroscopicus. Appl Microbiol. 1962 May;10:258–263. doi: 10.1128/am.10.3.258-263.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENGHUSEN H. Elekronenoptische Darstellungen von Streptomyceten-Sporen und-Hällen. Arch Mikrobiol. 1955;21(3):329–334. [PubMed] [Google Scholar]
- GLAUERT A. M., HOPWOOD D. A. A membranous component of the cytoplasm in Streptomyces coelicolor. J Biophys Biochem Cytol. 1959 Dec;6:515–516. doi: 10.1083/jcb.6.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLAUERT A. M., HOPWOOD D. A. The fine structure of Streptomyces coelicolor. I. The cytoplasmic membrane system. J Biophys Biochem Cytol. 1960 Jun;7:479–488. doi: 10.1083/jcb.7.3.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLAUERT A. M., HOPWOOD D. A. The fine structure of Streptomyces violaceoruber (S. coelicolor). III. The walls of the mycelium and spores. J Biophys Biochem Cytol. 1961 Aug;10:505–516. doi: 10.1083/jcb.10.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOPWOOD D. A., GLAUERT A. M. Electron microscope observations on the surface structures of Streptomyces violaceoruber. J Gen Microbiol. 1961 Oct;26:325–330. doi: 10.1099/00221287-26-2-325. [DOI] [PubMed] [Google Scholar]
- HOPWOOD D. A., GLAUERT A. M. The fine structure of Streptomyces coelicolor. II. The nuclear material. J Biophys Biochem Cytol. 1960 Sep;8:267–278. doi: 10.1083/jcb.8.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L. A., Bradley S. G. The life-cycle of an actionophage for Streptomyces venezuelae. J Gen Microbiol. 1965 Aug;40(2):191–198. doi: 10.1099/00221287-40-2-191. [DOI] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolstad R. A., Bradley S. G. Purification of Streptomyces venezuelae phage. J Bacteriol. 1964 May;87(5):1157–1161. doi: 10.1128/jb.87.5.1157-1161.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOORE R. T., CHAPMAN G. B. Observations of the fine structure and modes of growth of a streptomycete. J Bacteriol. 1959 Dec;78:878–885. doi: 10.1128/jb.78.6.878-885.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUART D. C., Jr Fine structure of the nucleoid and internal membrane systems of Streptomyces. J Bacteriol. 1959 Aug;78:272–281. doi: 10.1128/jb.78.2.272-281.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewfik E. M., Bradley S. G. Characterization of deoxyribonucleic acids from streptomycetes and nocardiae. J Bacteriol. 1967 Dec;94(6):1994–2000. doi: 10.1128/jb.94.6.1994-2000.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VERNON T. R. Spore formation in the genus Streptomyces. Nature. 1955 Nov 12;176(4489):935–936. doi: 10.1038/176935a0. [DOI] [PubMed] [Google Scholar]
- van Iterson W. Symposium on the fine structure and replication of bacteria and their parts. II. Bacterial cytoplasm. Bacteriol Rev. 1965 Sep;29(3):299–325. doi: 10.1128/br.29.3.299-325.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]