Abstract
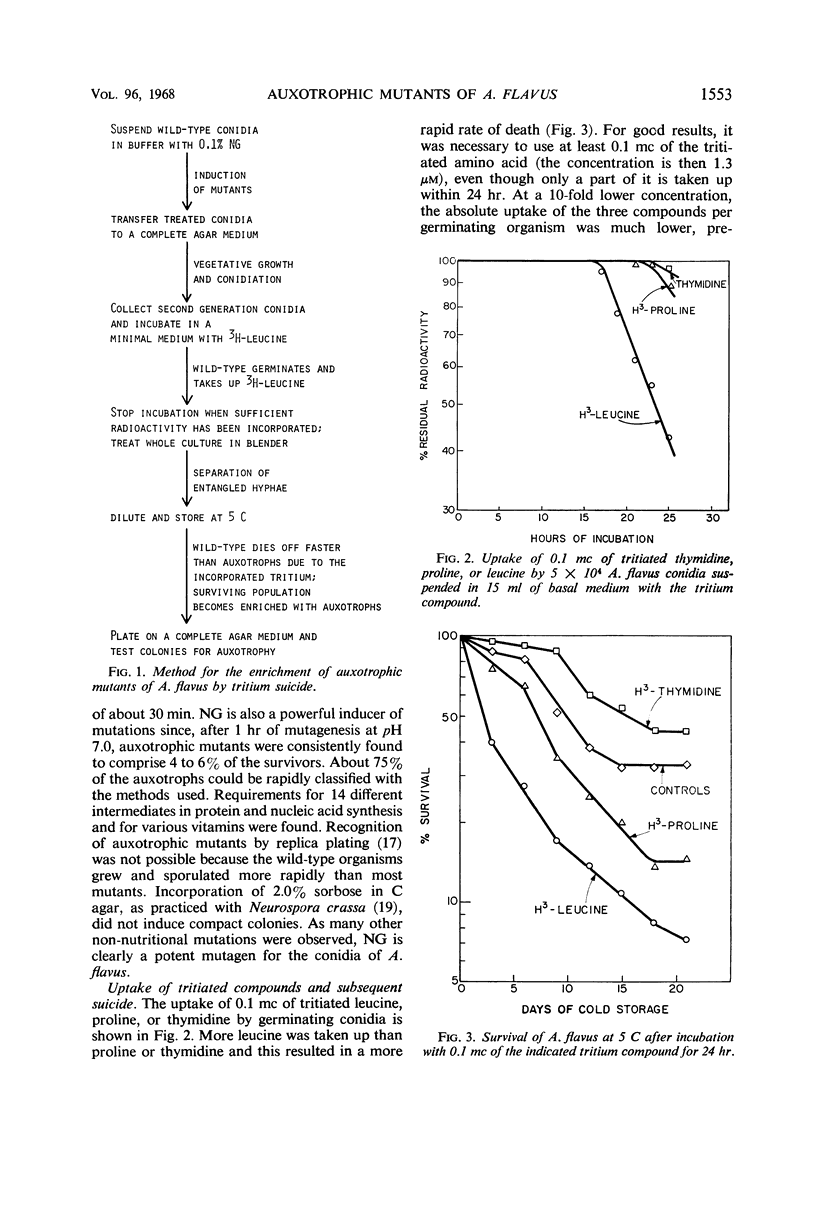
A method based on tritium suicide was developed to enrich auxotrophic mutants of Aspergillus flavus. N-methyl-N′-nitro-N-nitrosoguanidine (NG) was chosen as a mutagen, since a wide variety of mutations were induced by the action of 0.1% NG on A. flavus conidia suspended in phosphate buffer (pH 7.0). The decimal reduction time under these conditions was about 30 min, and the surviving population contained 4 to 6% auxotrophs after 1 hr of mutagenesis. This proportion was then increased by tritium suicide of wild-type cells. At a concentration of 1.3 μm, 3H-leucine was incorporated better than 3H-proline or 3H-thymidine into the germinating conidia. With about 20 hr of incubation and a short treatment in a high-speed mixer to disentangle mycelia and conidia, a 5- to 20-fold decrease in the number of survivors resulted from the incorporated 3H-leucine (5 c/mmole) after 1 week of storage at 5 C. At a 10-fold lower concentration, the uptake of radioactivity and the subsequent suicide rate were much lower. With 3H-leucine, the proportion of auxotrophs in the surviving population rose from 5 to about 20% during 2 weeks of storage at 5 C. Mutants requiring various intermediates for protein or nucleic acid synthesis or requiring vitamins were isolated. Finally, it was noted that A. flavus shows a much higher resistance to tritium suicide than does Escherichia coli.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Darlington A. J., Scazzocchio C. Use of analogues and the substrate-sensitivity of mutants in analysis of purine uptake and breakdown in Aspergillus nidulans. J Bacteriol. 1967 Mar;93(3):937–940. doi: 10.1128/jb.93.3.937-940.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn G., Rivera W. Kinetics of fungal growth and phosphatase formation in Aspergillus nidulans. J Bacteriol. 1966 Dec;92(6):1618–1622. doi: 10.1128/jb.92.6.1618-1622.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENBERG J., MORRIS J. The protection of Escherichia coli against inhibition by 1-methyl-3-nitro-1-nitrosoguanidine. Antibiot Chemother (Northfield) 1961 Jan;11:52–57. [PubMed] [Google Scholar]
- LESTER H. E., GROSS S. R. Efficient method for selection of auxotrophic mutants of Neurospora. Science. 1959 Feb 27;129(3348):572–572. doi: 10.1126/science.129.3348.572. [DOI] [PubMed] [Google Scholar]
- LUBIN M. Selection of auxotrophic bacterial mutants by tritium-labeled thymidine. Science. 1959 Mar 27;129(3352):838–839. doi: 10.1126/science.129.3352.838. [DOI] [PubMed] [Google Scholar]
- MANDELL J. D., GREENBERG J. A new chemical mutagen for bacteria, 1-methyl-3-nitro-1-nitrosoguanidine. Biochem Biophys Res Commun. 1960 Dec;3:575–577. doi: 10.1016/0006-291x(60)90064-4. [DOI] [PubMed] [Google Scholar]
- MATELES R. I., ADYE J. C. PRODUCTION OF AFLATOXINS IN SUBMERGED CULTURE. Appl Microbiol. 1965 Mar;13:208–211. doi: 10.1128/am.13.2.208-211.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERSON S., BOCKRATH R. C., Jr DIFFERENTIAL MUTATION PRODUCTION BY THE DECAY OF INCORPORATED TRITIUM COMPOUNDS IN E. COLI. Biophys J. 1964 Sep;4:355–365. doi: 10.1016/s0006-3495(64)86788-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PONTECORVO G., ROPER J. A., HEMMONS L. M., MACDONALD K. D., BUFTON A. W. J. The genetics of Aspergillus nidulans. Adv Genet. 1953;5:141–238. doi: 10.1016/s0065-2660(08)60408-3. [DOI] [PubMed] [Google Scholar]
- ROBERTS C. F. A replica plating technique for the isolation of nutritionally exacting mutants of a filamentous fungus (Aspergillus nidulans). J Gen Microbiol. 1959 Jun;20(3):540–548. doi: 10.1099/00221287-20-3-540. [DOI] [PubMed] [Google Scholar]
- Sinha U. Aromatic amino acid biosynthesis and para-fluorophenylalanine resistance in Aspergillus nidulans. Genet Res. 1967 Dec;10(3):261–272. doi: 10.1017/s0016672300011022. [DOI] [PubMed] [Google Scholar]
- Tatum E. L., Barratt R. W., Cutter V. M., Jr Chemical Induction of Colonial Paramorphs in Neurospora and Syncephalastrum. Science. 1949 May 20;109(2838):509–511. doi: 10.1126/science.109.2838.509. [DOI] [PubMed] [Google Scholar]
- WESTERGAARD M. Chemical mutagenesis in relation to the concept of the gene. Experientia. 1957 Jun 15;13(6):224–234. doi: 10.1007/BF02157427. [DOI] [PubMed] [Google Scholar]
- Woodward V. W., De Zeeuw J. R., Srb A. M. THE SEPARATION AND ISOLATION OF PARTICULAR BIOCHEMICAL MUTANTS OF NEUROSPORA BY DIFFERENTIAL GERMINATION OF CONIDIA, FOLLOWED BY FILTRATION AND SELECTIVE PLATING. Proc Natl Acad Sci U S A. 1954 Mar;40(3):192–200. doi: 10.1073/pnas.40.3.192. [DOI] [PMC free article] [PubMed] [Google Scholar]


