Abstract
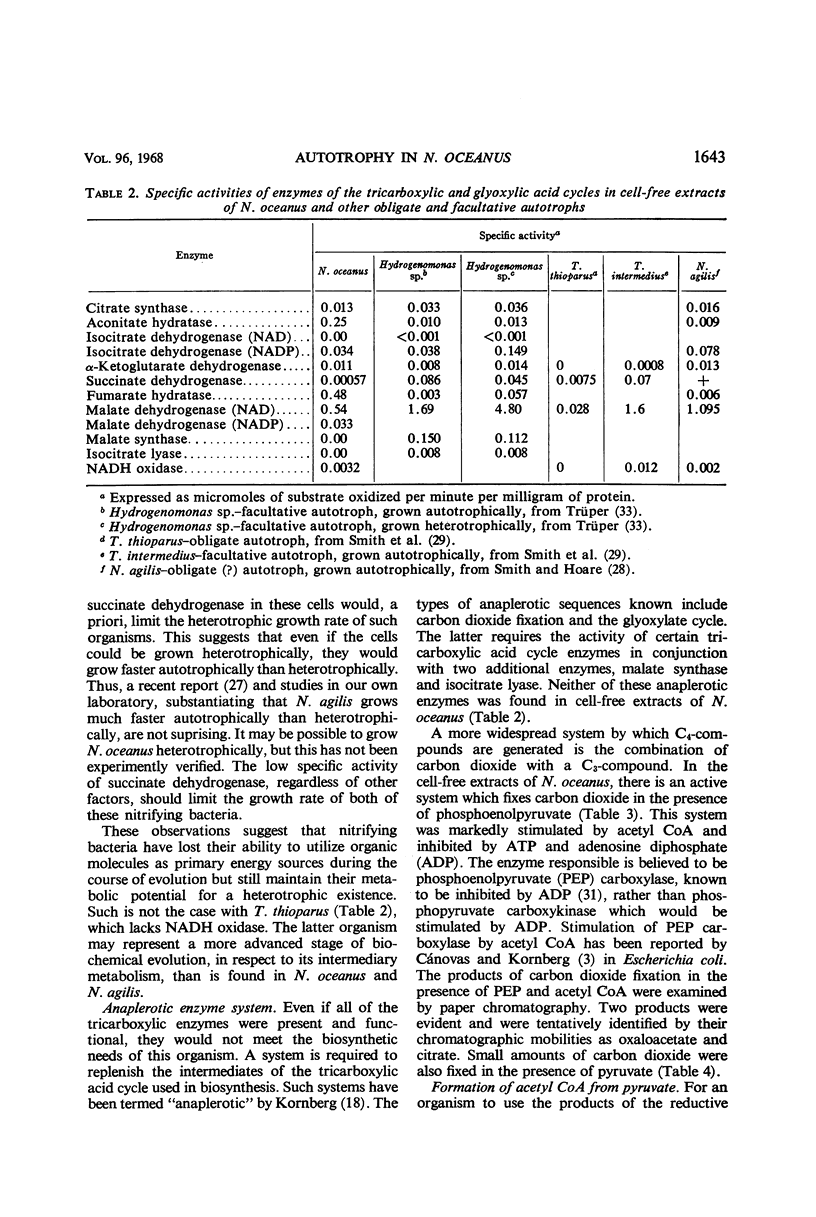
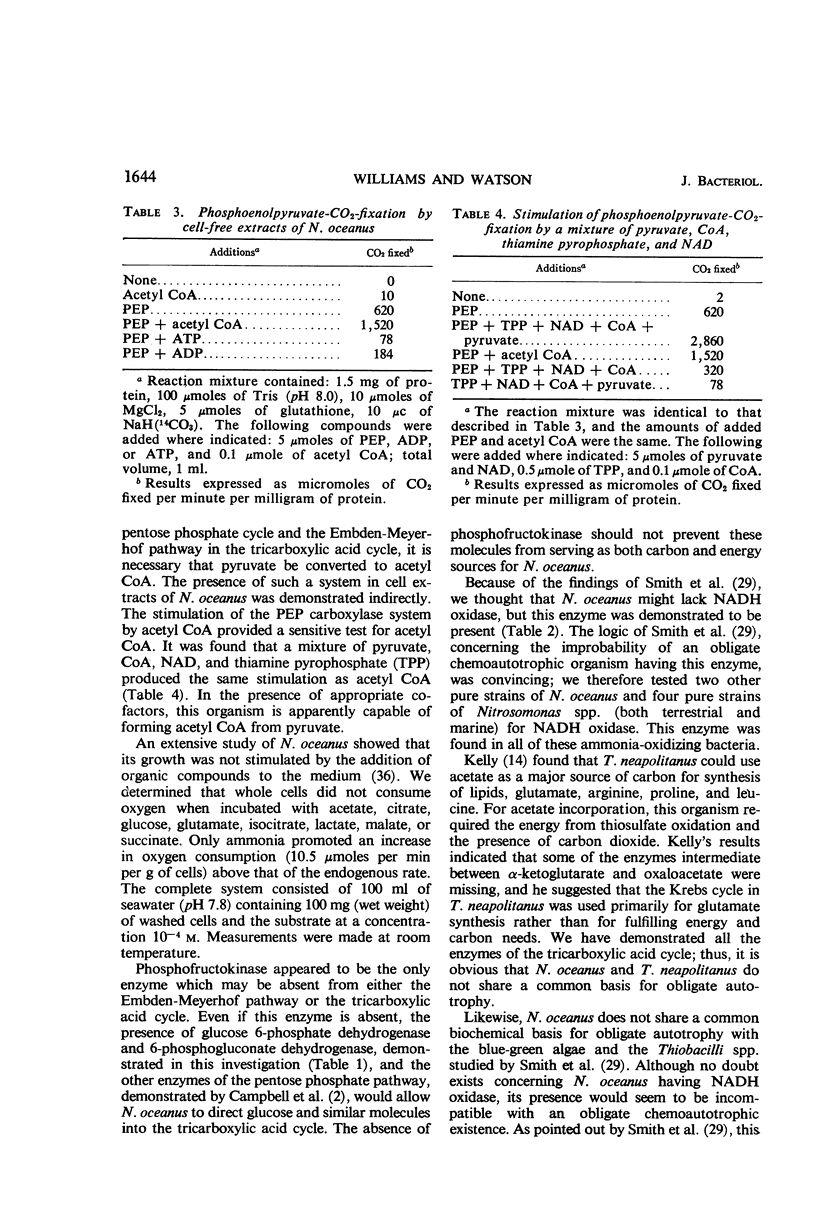
Enzymatic assays of cell-free extracts of the ammonia-oxidizing bacterium Nitrosocystis oceanus failed to establish that the biochemical basis of its obligate autotrophy stemmed solely from a metabolic defect. All of the Embden-Meyerhof enzymes except phosphofructokinase, and all of the tricarboxylic acid-cycle enzymes, as well as reduced nicotinamide adenine dinucleotide oxidase, were found in these extracts. A phosphoenolpyruvate-CO2-fixing system was also demonstrated. Resting cells incubated with 14C-d-glucose and 14C-l-glutamate and cells grown in the presence of 14C-labeled glucose, glutamate, pyruvate, and methionine incorporated these compounds into cellular material, but at a level too low to provide the cells' major carbon and energy needs.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CANOVAS J. L., KORNBERG H. L. FINE CONTROL OF PHOSPHOPYRUVATE CARBOXYLASE ACTIVITY IN ESCHERICHIA COLI. Biochim Biophys Acta. 1965 Jan;96:169–172. doi: 10.1016/0005-2787(65)90624-6. [DOI] [PubMed] [Google Scholar]
- Campbell A. E., Hellebust J. A., Watson S. W. Reductive pentose phosphate cycle in Nitrosocystis oceanus. J Bacteriol. 1966 Mar;91(3):1178–1185. doi: 10.1128/jb.91.3.1178-1185.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark C., Schmidt E. L. Growth response of Nitrosomonas europaea to amino acids. J Bacteriol. 1967 Apr;93(4):1302–1308. doi: 10.1128/jb.93.4.1302-1308.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark C., Schmidt E. L. Uptake and utilization of amino acids by resting cells of Nitrosomonas europaea. J Bacteriol. 1967 Apr;93(4):1309–1315. doi: 10.1128/jb.93.4.1309-1315.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delwiche C. C., Finstein M. S. Carbon and Energy Sources for the Nitrifying Autotroph Nitrobacter. J Bacteriol. 1965 Jul;90(1):102–107. doi: 10.1128/jb.90.1.102-107.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ida S., Alexander M. Permeability of Nitrobacter agilis to Organic Compounds. J Bacteriol. 1965 Jul;90(1):151–156. doi: 10.1128/jb.90.1.151-156.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORNBERG A., PRICER W. E., Jr Enzymatic phosphorylation of adenosine and 2,6-diaminopurine riboside. J Biol Chem. 1951 Dec;193(2):481–495. [PubMed] [Google Scholar]
- KORNBERG H. L. The metabolism of C2 compounds in micro-organisms. I. The incorporation of [2-14C] acetate by Pseudomonas fluorescens, and by a Corynebacterium, grown on ammonium acetate. Biochem J. 1958 Mar;68(3):535–542. doi: 10.1042/bj0680535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly D. P. The incorporation of acetate by the chemoautotroph Thiobacillus neapolitanus strain C. Arch Mikrobiol. 1967;58(2):99–116. doi: 10.1007/BF00406671. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MURRAY R. G., WATSON S. W. STRUCTURE OF NITROSOCYSTIS OCEANUS AND COMPARISON WITH NITROSOMONAS AND NITROBACTER. J Bacteriol. 1965 Jun;89:1594–1609. doi: 10.1128/jb.89.6.1594-1609.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RACKER E. Spectrophotometric measurements of the enzymatic formation of fumaric and cis-aconitic acids. Biochim Biophys Acta. 1950 Jan;4(1-3):211–214. doi: 10.1016/0006-3002(50)90026-6. [DOI] [PubMed] [Google Scholar]
- Remsen C. C., Valois F. W., Watson S. W. Fine structure of the cytomembranes of Nitrosocystis oceanus. J Bacteriol. 1967 Aug;94(2):422–433. doi: 10.1128/jb.94.2.422-433.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANADI D. R., LITTLEFIELD J. W. Studies on alpha-ketoglutaric oxidase. I. Formation of "active" succinate. J Biol Chem. 1951 Dec;193(2):683–689. [PubMed] [Google Scholar]
- SUZUKI I., WERKMAN C. H. Chemoautotrophic carbon dioxide fixation by extracts of Thiobacillus thiooxidans. I. Formation of oxalacetic acid. Arch Biochem Biophys. 1958 Jul;76(1):103–111. doi: 10.1016/0003-9861(58)90124-3. [DOI] [PubMed] [Google Scholar]
- Smith A. J., Hoare D. S. Acetate assimilation by Nitrobacter agilis in relation to its "obligate autotrophy". J Bacteriol. 1968 Mar;95(3):844–855. doi: 10.1128/jb.95.3.844-855.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. J., London J., Stanier R. Y. Biochemical basis of obligate autotrophy in blue-green algae and thiobacilli. J Bacteriol. 1967 Oct;94(4):972–983. doi: 10.1128/jb.94.4.972-983.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudinger P. A., Kelly D. P. Reduced nicotinamide adenine dinucleotide oxidation by Thiobacillus neapolitanus and Thiobacillus strain C. J Bacteriol. 1968 May;95(5):1962–1963. doi: 10.1128/jb.95.5.1962-1963.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trüper H. G. Tricarboxylic acid cycle and related enzymes in Hydrogenomonas strain H16G+ grown on various carbon sources. Biochim Biophys Acta. 1965 Dec 16;111(2):565–568. doi: 10.1016/0304-4165(65)90074-7. [DOI] [PubMed] [Google Scholar]
- UNDERWOOD A. H., NEWSHOLME E. A. PROPERTIES OF PHOSPHOFRUCTOKINASE FROM RAT LIVER AND THEIR RELATION TO THE CONTROL OF GLYCOLYSIS AND GLUCONEOGENESIS. Biochem J. 1965 Jun;95:868–875. doi: 10.1042/bj0950868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIELAND O. Eine enzymatische Methode zur Bestimmung von Glycerin. Biochem Z. 1957;329(4):313–319. [PubMed] [Google Scholar]


