Abstract
Background aims
Oligodendrocyte precursor cells (OPC) hold promise as a cellular therapy for demyelinating diseases. The feasibility of using OPC-based therapies in humans depends upon a reliable, readily available source. We have previously described the isolation, expansion and characterization of oligodendrocyte-like cells from fresh human umbilical cord blood (UCB). We now describe the isolation and expansion of OPC from thawed, cryopreserved UCB.
Methods
We thawed cryopreserved UCB units employing a standard clinical protocol, then isolated and plated mononuclear cells under previously established culture conditions. All OPC cultures were trypsinized at 21 days, counted, then characterized by flow cytometry after fixation, permeablization and labeling with the following antibodies: anti-oligodendrocyte marker 4 (O4), anti-oligodendrocyte marker 1 (O1) and anti-myelin basic protein (MBP). OPC were also placed in co-culture with shiverer mouse neuronal cells then stained in situ for beta tubulin III (BT3) and MBP as a functional assay of myelination.
Results
The average OPC yield per cryopreserved UCB unit was 64% of that seen with fresh UCB. On flow cytometric analysis, 74% of thawed UCB units yielded cells with an O4-expression level of at least 20% of total events, compared with 95% of fresh UCB units. We observed myelination of shiverer neurons in our functional assay, which could be used as a potency assay for release of OPC cells in phase I human clinical trials.
Conclusions
Our results demonstrate that OPC can be derived reliably from thawed, cryopreserved UCB units, and support the feasibility of using these cells in human clinical trials.
Keywords: cord blood stem cell transplantation, fetal blood, hematopoietic stem cells, inherited metabolic diseases, oligodendrocytes
Introduction
Oligodendrocyte progenitor cells (OPC) hold great potential as a targeted cellular therapy for neural cell repair and regeneration in patients with acquired and genetic neurodegenerative disorders. These diseases, which include pediatric leukodystrophies resulting from defects in genes regulating myelination (such as Pelizaeus–Merzbacher disease) and lysosomal storage diseases (such as Krabbe's disease and metachromatic leukodystrophy, MLD), are marked by profound neurologic deterioration and premature death. Although transplantation of unrelated umbilical cord blood (UCB) halts the progression of neurologic damage in certain metabolic diseases, it usually does not reverse the damage sustained before transplant, as illustrated in newborns transplanted for early infantile Krabbe's disease (1). Currently, there is no available therapy to reverse pre-existing damage caused by demyelinating diseases. However, OPC derived from various sources has been shown to remyelinate the congenitally hypomyelinated shiverer mouse brain (2–5) and to migrate appropriately to sites of injury in mouse models of Krabbe's disease and MLD (6,7).
Human UCB holds great potential as a source for cellular therapy. Mesenchymal stromal cells (MSC) and unrestricted somatic stem cells (USSC) have been reported to differentiate in vivo into osteoblasts, adipocytes and neural progenitor cells (8). Human UCB cells have been used in high doses to delay symptom progression in a mouse model of amyotrophic lateral sclerosis (ALS) (9) and have been identified within the central nervous system of a patient with Krabbe's disease 10 months post-UCB transplant (10), suggesting the persistence of these cells as a possible contributor to cellular repair in patients with neurodegenerative diseases.
These observations have sparked interest in developing a safe, available and reliable source of OPC cells for clinical trials in humans (11–13). The most widely described sources of OPC have been neural stem cells (NSC) and embryonic stem cells (ESC), which can be expanded into OPC cultures with high purity (4,13–16). However, the limited availability of these sources, questions surrounding their long-term safety (17), and ethical questions surrounding the use of ESC have led to the exploration of alternative sources of OPC. Our laboratory has a long-standing interest in bringing UCB-derived neural precursor cells to the clinic. While we have previously described the isolation, expansion and characterization of oligodendrocyte-like cells from fresh human UCB (18), we had not derived these cells from cryopreserved UCB prior to this series. However, isolation of these cells from cryopreserved donor UCB is crucial in bringing this therapy to the patient, because it allows for planned preparation of cells from already existing UCB banks. Additionally, cryopreserved donor UCB will allow HLA-matching. The objective of the present study was to determine the feasibility of isolating and expanding OPC from a series of thawed, cryopreserved UCB units and compare those cells with OPC derived from fresh UCB units.
Methods
Preparation of UCB
We obtained cryopreserved UCB units from the Carolinas Cord Blood Bank at Duke University (Durham, NC, USA) (CCBB) after obtaining written, informed consent for donation to research by mothers delivering healthy term infants. The cryopreserved UCB was removed from storage under liquid nitrogen and thawed to a thick slurry in a 37°C water bath, diluted in dextran +5% human serum albumin and washed either (a) using a standard protocol employed in clinical transplantation (dextran–albumin thaw; CCBB master file) (18) or with (b) serial washes with 1% human serum albumin (HSA; Octapharma, Centreville, VA, USA) in phosphate-buffered saline (PBS; Mediatech, Manasas, VA, USA), referred to as the HSA–PBS thaw. The cryopreserved units subjected to the dextran–albumin thaw were also separated further by density-gradient centrifugation (1.077 Ficoll; Axis Shield, Oslo, Norway) to isolate mononuclear cells, which were then cultured as described below. Cryopreserved units thawed by the HSA–PBS required no further separation; mononuclear cells from these units were cultured as described below.
Fresh UCB units underwent volume reduction and red blood cells (RBC) depletion using an automated Sepax system (Biosafe, Eysins, Switzerland) after the addition of hetastarch Hespan (6% Hetastarch, B. Braun, Irvine, CA, USA). The volume-reduced, RBC-depleted product underwent mononuclear cell isolation via density-gradient centrifugation with Ficoll as described previously (17) and was cultured as described below.
Cell culture
After optimizing thaw and culture conditions, we cultured OPC from a series of 31 cryopreserved and 20 fresh UCB units. Our culture conditions were the same as previously reported except for the concentration of mononuclear cells per milliliter. Isolated mononuclear cells from each UCB unit were plated in 75-mm3 flasks (Corning Inc., Corning, NY, USA) at a concentration of 3 × 106 cells/mL (for fresh UCB) or 5 × 106 (for cryopreserved UCB) in 15 mL growth medium containing alpha-MEM (Modified Eagle Medium) (Gibco, Grand Island, NY, USA), 10% fetal calf serum (FCS; HyClone, Logan, UT, USA), insulin–transferrin–selenium supplementation, penicillin/streptomycin (Gibco), 2 mm l-glutamine (Gibco), 5 ng/mL platelet-derived growth factor (PDGF; PeproTech, Rocky Hill, NJ, USA), 1 ng/mL neurotrophin-3 (NT-3; PeproTech), 10 ng/mL vascular endothelial growth factor (VEGF; PeproTech) and 30 ng/mL triiodothyronine (T3 ; Sigma, St Louis, MO, USA). The FCS was from a single lot and manufactured under Good Manufacturing Practice (GMP) standards conditions. At day 7, half the medium was exchanged for fresh growth medium. Starting at day 14, the following 7-day media change cycle was initiated. On the first day of the cycle, half the volume of medium in each flask was exchanged for an equal volume of medium containing neurocult NS-A basal medium (Stem Cell Technologies), neurocult NS-A proliferation supplement (Stem Cell Technologies, Vancouver, BC, Canada), PDGF, VEGF and NT-3 in concentrations above. On the third day of the cycle, half the volume of medium in each flask was removed and an equal volume of the growth medium described initially added. This 7-day cycle was repeated over the life of the cultured cells. Cells were observed twice weekly for morphologic changes. Cultures containing greater than 25% of cells/flask with morphologic changes of oligodendrocytes (initial bipolar morphology with branching processes developing by day 21) were considered to be ‘morphologically consistent’ with OPC cells. Yields were compared by Student's t-test with significance defined as P < 0.05.
Flow cytometry analysis
For phenotypic analysis, 3-week old OPC cultures from each fresh or cryopreserved UCB units were dissociated by incubation with 0.05% trypsin– Ethylenediaminetetraacetic acid (EDTA) (Gibco), washed and resuspended in 2 mL PBS (Gibco), passed through a 40-μm cell filter (BD Bioscience, San Diego, CA, USA) to remove clumps and debris, then counted to obtain a total cell count for each unit. Cells were fixed and permeabilized using a commercially available kit (Invitrogen, Carlsbad, CA, USA). Samples were incubated for 12–24 h with the following primary antibodies, myelin basic protein (MBP; polyclonal; Stem Cell Technologies), oligodendrocyte marker 1 (O1; clone 59; Millipore, Billerica, MA, USA) and oligodendrocyte marker 4 (O4; clone 81, Stem Cell Technologies), then washed with PBS + 5% FCS (Gibco). Cells were incubated for 20 min with appropriate secondary antibodies, washed, then analyzed on a FACSCaliber system with CellQuest Pro software (BD Bioscience).
For further analysis of phenotypic changes over time, cultured cells from two cryopreserved UCB units and one fresh UCB unit control were analyzed weekly over a period of 4 weeks. Day 0 was defined as the day on which the culture was initiated, day 2 was 2 days after the initiation of the culture, etc.
Functional assay of myelination
With approval of our Duke University Institutional Committee on the Care and Use of Animals (Durham, NC, USA), we harvested neural tissue from the brains of homozygous shiverer mice (MBP <shi>/J; Jackson Lab, Bar Harbor, ME, USA) in a sterile fashion. The brain tissue was dissociated into a cell suspension using a sterile 40-μm filter (BD Bioscience). The suspension from each brain was divided among three cell culture dishes (VWR) in 10 mL zinc option medium (Gibco) + 20% FCS (HyClone). Upon reaching 50–80% confluence, cells were transferred to 75-cm3 cellbind culture flasks (Corning Inc.). Co-cultures of shiverer neuronal cells and cryopreserved UCB-derived OPC were prepared by dissociation of each cell type with 0.05% trypsin–EDTA (Gibco) then adding 1 × 105 cells of each type to each well of a LabTek II Chamber Slide (Nalge Nunc, Naperville, IL, USA) in 1.5 mL zinc option medium with 20% FCS. Co-cultures were maintained with weekly changes of conditioned medium.
After 1–6 weeks in co-culture, media were removed and cells were washed three times in PBS (Gibco) and then fixed with 3.7% formaldehyde (VWR) at room temperature. Blocking was performed for 1 h at room temperature with normal goat serum (BD Bioscience). Cells were then incubated with mouse anti-human MBP (polyclonal, 1:200; Stem Cell Technologies) and anti-mouse beta tubulin III (BT3; Tuj1, 1:1000; Stem Cell Technologies) for 12–24 h at 4°C and washed three times with PBS. Samples were incubated with appropriate secondary antibodies for 30 min in the dark. After three washes with PBS, vectashield anti-fade medium with DAPI (Vector Laboratories, Servion, Switzerland) was added to each slide before a cover slip was applied. Slides were stored in a humidified light-blocked chamber at 4°C until imaging. Imaging was carried out using a Leica SP5 confocal microscope (Leica Microsystems, Wetzlar, Germany) at 100 × under oil immersion. Positive cells were scored if MBP staining was observed on axonal surfaces of shiverer neurons.
Results
Isolation and expansion of cryopreserved UCB-derived OPC
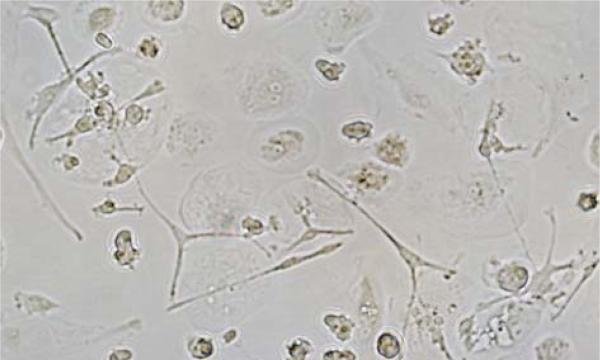
We prepared cell cultures from 31 cryopreserved and 20 fresh UCB units. Of the cell cultures prepared from cryopreserved UCB units, 29 of 31 (94%) were morphologically consistent with OPC cells by day 21 of culture, compared with 20 of 20 (100%) cell cultures prepared from fresh UCB units. The average yield of cryopreserved UCB units was 64% of that seen for fresh UCB units, although the mean number of mononuclear cells plated per unit was higher for the cryopreserved units (Table I). OPC derived from cryopreserved UCB displayed initial bipolar morphology, then developed branching processes radiating from the cell body over subsequent weeks, consistent with our observations of OPC derived from fresh UCB (Figure 1). By the third week in culture, OPC were developing multiple branching processes. When left in culture beyond 3 weeks, this process of arborization continued. After 6–8 weeks in culture, OPC began to senesce and failed to proliferate further. Passage of OPC was difficult after this time-point as well.
Table I.
Morphology and yield of fresh and cryopreserved UCB-derived OPC at 21 days.
| Cryopreserved UCB | Fresh UCB | P-value | |
|---|---|---|---|
| Total UCB units cultured | 31 | 20 | |
| Mean storage time of UCB units | 12.5 ± 4 months | 0–48 h | |
| Mean number of mononuclear cells plated per UCB unit | 3.58 × 108 ± 2.3 × 108 | 1.60 × 108 ± 1.3 × 108 | <0.01 |
| Mean final oligodendrocyte cell count per UCB unit | 2.18 × 106 ± 3.2 × 106 | 3.42 × 106 ± 4.1 × 106 | = 0.18 |
| Range of final oligodendrocyte cell count per UCB unit | 4.0 × 104–1.0 × 107 | 2.4 × 105–1.1 × 107 | |
| UCB units producing displaying oligodendrocyte morphology (%) | 29 (94) | 20 (100) |
Figure 1.
Morphology of cryopreserved UCB-derived OPC. In culture, cryopreserved UCB-derived OPC initially displayed bipolar morphology, then developed branching processes radiating from the cell body over the subsequent 2–3 weeks in culture.
Cryopreserved UCB-derived OPC yield improves when RBC are minimized
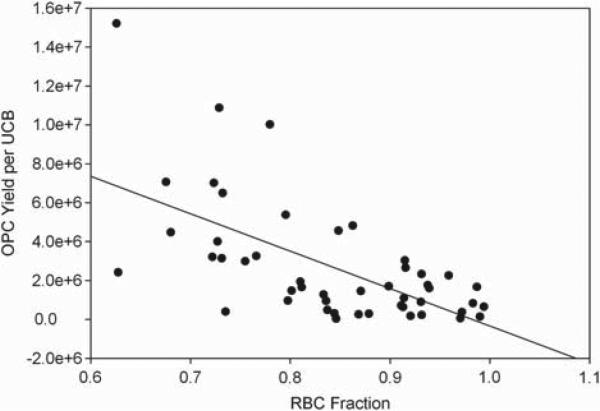
In our initial attempts to culture OPC from cryopreserved UCB units using our previously described culture conditions (18), we found that the overall yield of cells with OPC morphology and oligodendrocyte marker expression was lower than our previous results from fresh UCB units. When we reviewed our preliminary data, we noticed higher yields for the units thawed by the HSA–PBS (4 × 106 ± 2.8 × 106, n = 11 units) than by the dextran–albumin (1 × 106 ± 8 × 105, n = 20 units) thaw. After further analysis, we observed that UCB units thawed by HAS–PBS had a much smaller fraction of RBC compared with cultures established from UCB thawed with dextran–albumin. We also noticed that both cultures from fresh and cryopreserved units exhibited more robust and reliable OPC growth when fewer RBC were present relative to mononuclear cells, and our data showed an inverse correlation between the percentage of RBC in the cell suspension relative to mononuclear cells (Figure 2). When we compared the final yields for the cryopreserved UCB-derived OPC cultures plated with 80% or fewer RBC with those that had more than 80% RBC, we found that the mean yields were higher in the group with 80% or fewer RBC. Yields from cryopreserved UCB increased almost six-fold as the percentage of RBC decreased (6.0 × 10 × 106 versus 1.1 × 10 × 106). A similar effect was seen with fresh UCB-derived OPC cultures, although more of these cultures had fewer than 80% RBC. This finding was consistent with previous findings that heme is inhibitory to oligodendrocyte growth, especially during periods of oxidative stress (20).
Figure 2.
Percentage of RBC in mononuclear cell suspension versus OPC yield. We found an inverse correlation between RBC percentage and OPC yield from both fresh and thawed cryopreserved UCB units. Yields from thawed, cryopreserved UCB increased almost 6-fold (6.0 × 10e6 versus 1.1 × 10e6) when units with a low RBC percentage were compared with those with high RBC percentages. R2 = 0.386.
Expression of oligodendrocyte markers by cryopreserved UCB-derived OPC is consistent with fresh UCB-derived OPC
To characterize the phenotype of cultured OPC with greater precision and accuracy than direct observation under a microscope, we developed an assay using intracellular flow cytometry, based on previous work by Zeigler & Hall (21). We assayed expression of O4 (expressed by OPC), O1 (expressed by immature oligodendrocytes) and MBP (expressed in more mature, myelin-producing oligodendrocytes). Upon flow cytometric analysis, expression of O1, O4 and MBP was similar between thawed and fresh cords after 3 weeks in culture (Table II). The average O4 expression was 84% of the gated population for cryopreserved UCB-derived OPC compared with 86% for fresh UCB-derived OPC. The average O1 expression was 91% of the gated population for cryopreserved UCB-derived OPC compared with 89% for fresh UCB-derived OPC. Twenty-three of 31 (74%) cryopreserved UCB-derived OPC cultures demonstrated an O4 expression of 20% or greater of total events compared with 18 of 20 (90%) fresh UCB-derived OPC cultures.
Table II.
Analysis of oligodendrocyte marker expression by flow cytometry.
| Cryopreserved UCB | Fresh UCB | |
|---|---|---|
| Total number of UCB units cultured | 31 | 20 |
| Events in oligodendrocyte gate as % total events | 53.08 | 76.91 |
| O4 expression (% of gated events) | 84.41 | 85.92 |
| O1 expression (% of gated events) | 90.82 | 88.88 |
| MBP expression (% of gated events) | 32.71 | 30.51 |
| Co-expression of MBP + O1 (% of gated events) | 30.43 | 30.50 |
| Units with O4 expression > 20% of total events (%) | 23 (74) | 18 (95) |
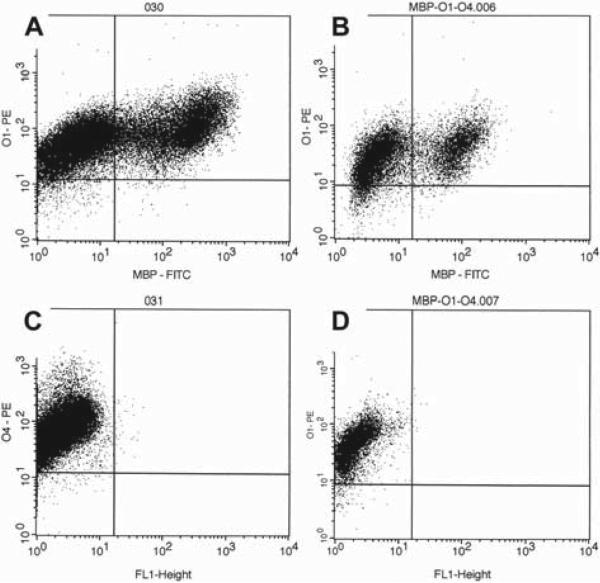
We also demonstrated myelin expression by a subpopulation of cryopreserved UCB-derived OPC, a finding consistent with that found in fresh UCB-derived OPC. Mean expression of MBP alone was 33% of the gated population for cryopreserved UCB-derived OPC compared with 31% for fresh UCB-derived OPC. When we assayed specifically for cells co-expressing O1 and MBP, we found the two markers co-expressed in 30% of the gated population for cryopreserved UCB-derived OPC compared with 31% for fresh UCB-derived OPC (Figure 3).
Figure 3.
Representative OPC phenotype by flow cytometry. (A, B) Co-expression of the oligodendrocyte markers O1 and MBP in (A) oligodendrocytes derived from fresh UCB and (B) oligodendrocytes derived from thawed, cryopreserved UCB. The O1 + MBP + population can be seen in the right upper quadrant. (C, D) O4 expression in (C) oligodendrocytes derived from fresh UCB and (D) oligodendrocytes derived from thawed, cryopreserved UCB. Both demonstrate positive staining.
Along with the morphologic changes described above for cryopreserved UCB-derived OPC in culture, we observed phenotypic changes over time. The mean percentage of marker expression at sequential time-points by OPC from two separate cryopreserved UCB units and an OPC from a single fresh UCB control is displayed in Table III. At the baseline time-point (culture day 2), only 10% of total events fell in our oligodendrocyte gate (compared with 50–80% of events at subsequent time-points) for the OPC from cryopreserved UCB. Expression of all oligodendrocyte markers in this gate was low: O4 expression was 10% of gated events and co-expression of MBP + O1 was 5% of gated events. By day 7, O4 expression had risen to 53% of gated events and co-expression of MBP + O1 increased to 37% (Table III).
Table III.
Changes in oligodendrocyte marker expression in cell cultures over a 4-week period.
| Marker expression (% of gated events) |
|||
|---|---|---|---|
| O4 | MBP | O1 + MBP | |
| Cryopreserved UCB-derived OPC (n = 2 UCB units) | |||
| Day 7 | 75.01 | 10.14 | 9.45 |
| Day 21 | 98.54 | 34.44 | 31.80 |
| Day 28 | 98.92 | 51.08 | 51.04 |
| Fresh UCB-derived OPC (n = 1 UCB unit) | |||
| Day 7 | 71.99 | 12.11 | 12.11 |
| Day 21 | 99.58 | 34.75 | 34.72 |
| Day 28 | 98.29 | 24.10 | 23.40 |
Cryopreserved UCB-derived OPC myelinate shiverer neuronal cells
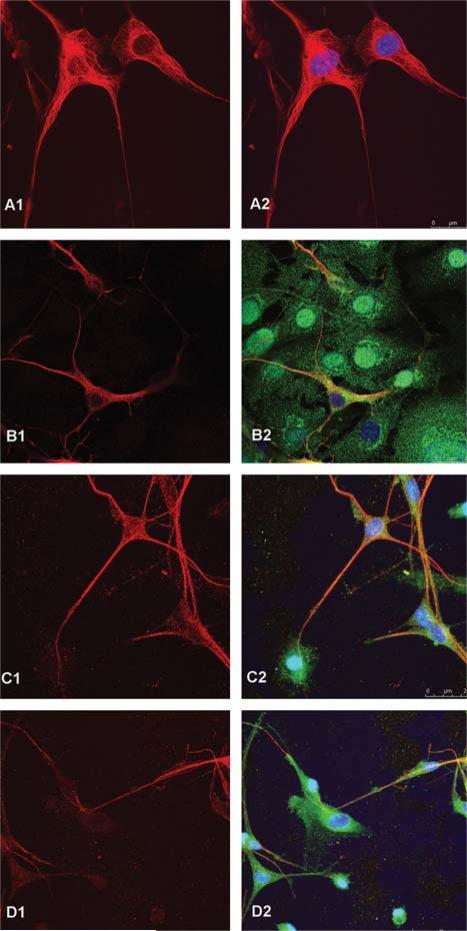
We developed an in vitro functional assay to assess the potential of cryopreserved UCB-derived OPC to facilitate myelination of unmyelinated shiverer neurons as a possible release assay for OPC in early phase I clinical trials in humans. Shiverer neurons incapable of endogenous myelination (cultured ex vivo after harvest from mouse brains) were co-cultured in vitro with cryopreserved UCB-derived OPC. Shiverer cells were identified by staining with BT3 but not MBP. On day 0 of co-culture, we observed neurons staining only with BT3, with no cells co-staining with MBP and BT3. After 1 week in co-culture, we observed at least partial co-staining of the axons with MBP and BT3 by fluorescence microscopy. By 2–3 weeks in co-culture, most shiverer neurons expressing BT3 co-expressed MBP (Figure 4). Initially, large numbers of OPC cells were associated with shiverer neurons. When images of 2–3-week co-cultures were stacked in the z-plane, the close association between OPC and shiverer neuron axons could be appreciated. By 4–6 weeks in culture, fewer OPC were associated with shiverer neurons expressing both BT3 and MBP. While MBP staining was descriptive and not formally quantitated, approximately 60% of neuronal axons appeared to be myelinated.
Figure 4.
In vitro functional assay of myelination by cryopreserved UCB-derived OPC. Shiverer mouse neurons co-cultured in vitro with UCB-derived OPC were co-stained for BT3 (Texas Red) and MBP (FITC). A shiverer neuronal control had positive staining for BT3 (A1) but not MBP (A2). Shiverer neuronal cells co-cultured with cryopreserved UCB-derived OPC expressed both BT3 (B1) and MBP (B2) after 1 week in co-culture. A z-stacked projection of shiverer neuronal cells co-cultured with cryopreserved UCB-derived OPC after 3 weeks in co-culture demonstrates BT3 expression (C1) as well as close association between BT3-expressing neuronal cells and MBP-expressing cells (C2). A z-stacked projection of shiverer neuronal cells co-cultured with cryopreserved UCB-derived OPC after 3 weeks in co-culture demonstrates BT3 expression (D1) as well as close association between BT3-expressing neuronal cells and MBP-expressing cells, and with MBP expression along axonal processes (C2). Images obtained using a Leica SP5 confocal microscope.
Discussion
We have developed methods to derive reproducibly a population of cells from cryopreserved UCB obtained from the standard inventory of a public cord blood bank that have the morphology of OPC and express O4, O1 and MBP after 3 weeks in tissue culture. These cryopreserved UCB-derived OPC are morphologically and phenotypically similar to OPC cells previously isolated from fresh UCB (18). Growth was enhanced in cultures containing lower numbers of erythrocytes. Cryopreserved UCB-derived OPC also produced myelin in a functional assay developed for product release.
Cultured OPC derived from cryopreserved UCB had a similar phenotype as fresh UCB-derived OPC by flow cytometric analysis. In both groups, expression of O1 and O4 was >80%, suggesting that the majority of cultured cells progressed from OPC and pre-oligodendrocytes into immature oligodendrocytes (22–23). Expression of MBP in a large proportion of cultured cells suggests that a subset may be differentiating into mature, myelin-producing oligodendrocytes by 2–3 weeks in culture. The trend towards increasing expression of MBP in OPC cultures derived from both fresh and cryopreserved UCB is consistent with this hypothesis. Our observation that OPC demonstrated senescence and decreased proliferative capacity after 6–8 weeks in culture also suggests maturation of the cells. The optimal time for use as a cellular therapy may be between 2–4 weeks, when myelination is evident as a functional assay for release but before the proliferative potential of the cells is exhausted.
While recent reports have shown that human MBP proteins are also present in differentiated hematopoietic cells and hematopoietic progenitors (24), the co-expression of oligodendrocyte-specific O1 and MBP suggests that these cells are not hematopoietic and have truly differentiated along the oligodendrocyte lineage. The degree to which OPC are phenotypically distinct from MSC or USSC is an area for further inquiry. The presence of MBP on shiverer neuronal cells after co-culture with cryopreserved UCB-derived OPC does suggest that these cells facilitate myelination in vitro , whether through direct myelination or through signaling/simulation of native shiverer glial cells.
Although derivation of OPC cells from ESC and NSC has been well described, safety concerns have been raised regarding the safety of such immature cells in the clinical setting (17). Further in vivo experiments must be performed to establish the safety of OPC in humans, but the theoretical risks of these lineage-committed cells are lower. The heterogeneity of the cultures may require further study to determine whether OPC cells must be purified before delivery to patients. Nevertheless, the potential to use human UCB as a source of OPC cells is appealing as cord blood banks exist throughout the country and cord blood can be readily HLA matched.
We are only aware of one report describing the use of cryopreserved UCB cells for culturing OPC (25). However, in that report mononuclear cell samples isolated from fresh UCB and frozen in small cryovials were used, not whole UCB units processed and frozen according to standard clinical protocols. Also, the expression of a ‘myelin/oligodendrocyte’ marker was reported as 5–10% for the cultures, which is significantly lower than in the present study.
The therapeutic potential of OPC has been well-established in animal models (3–7). Several investigators have outlined the requirements for the clinical feasibility of OPC-based cell therapies (12,26). These include a readily available source of cells that can be reliably expanded into quantities needed for human transplantation and easily characterized to ensure the appropriate dose of functional cells is given. Cryopreserved UCB is a promising source of human OPC because large, public cord blood banks already exist with standard procedures for screening and storing UCB. OPC for potential cellular therapy could be derived from HLA-matched UCB. Although further clinical studies are needed for confirmation of dosing, our finding that a unit of cryopreserved UCB yielded more than 2 million OPC cells in short-term cultures suggests that it has the proliferative capacity for early human clinical trials, especially for local or intrathecal administration. Optimal cell dosing, delivery routes and timing of administration is likely to vary depending on the clinical indication.
The isolation and expansion of OPC cells derived from fresh and cryopreserved UCB has great potential for clinical trials that could explore the therapeutic uses of these cells. With the development of a functional potency assay and demonstration of in vitro myelination, we have established a release assay for these cells. These steps will facilitate early clinical trials to establish the safety and feasibility of using these cells to treat human demyelinating diseases.
Footnotes
Declaration of interest: No disclosures.
References
- 1.Escolar ML, Poe MD, Provenzale JM, Richards KC, Allison J, Wood S, et al. Transplantation of umbilical-cord blood in babies with infantile Krabbe's disease. N Engl J Med. 2005;352:2069–81. doi: 10.1056/NEJMoa042604. [DOI] [PubMed] [Google Scholar]
- 2.Gomez CM, Muggleton-Harris AL, Whittingham DG, Hood LE, Readhead C. Rapid preimplantation detection of mutant (shiverer) and normal alleles of the mouse myelin basic protein gene allowing selective implantation and birth of live young. Proc Natl Acad Sci USA. 1990;87:4481–4. doi: 10.1073/pnas.87.12.4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu S, Qu Y, Stewart TJ, Howard MJ, Chakrabortty S, Holekamp TF, McDonald JW. Embryonic stem cells differentiate into oligodendrocytes and myelinate in culture and after spinal cord transplantation. Proc Natl Acad Sci USA. 2000;97:6126–31. doi: 10.1073/pnas.97.11.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nistor GI, Totoiu MO, Haque N, Carpenter MK, Keirstead HS. Human embryonic stem cells differentiate into oligodendrocytes in high purity and myelinate after spinal cord transplantation. Glia. 2005;49:385–96. doi: 10.1002/glia.20127. [DOI] [PubMed] [Google Scholar]
- 5.Windrem MS, Schanz SJ, Guo M, Tian GF, Washco V, Stanwood N, et al. Neonatal chimerization with human glial progenitor cells can both remyelinate and rescue the otherwise lethally hypomyelinated shiverer mouse. Cell Stem Cell. 2008;2:553–65. doi: 10.1016/j.stem.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Givogri MI, Galbiati F, Fasano S, Amadio S, Perani L, Superchi D, et al. Oligodendroglial progenitor cell therapy limits central neurological deficits in mice with metachromatic leukodystrophy. J Neurosci. 2006;26:3109–19. doi: 10.1523/JNEUROSCI.4366-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pellegatta S, Tunici P, Poliani PL, Dolcetta D, Cajola L, Colombelli C, et al. The therapeutic potential of neural stem/progenitor cells in murine globoid cell leukodystrophy is conditioned by macrophage/microglia activation. Neurobiol Dis. 2006;21:314–23. doi: 10.1016/j.nbd.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 8.Garbuzova-Davis S, Sanberg CD, Kuzmin-Nichols N, Willing AE, Gemma C, Bickford PC, et al. Human umbilical cord blood treatment in a mouse model of ALS: optimization of cell dose. PLoS ONE. 2008;3:e2494. doi: 10.1371/journal.pone.0002494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kurtzberg J, Kosaras B, Stephens C, Snyder EY. Umbilical cord blood cells engraft and differentiate into neural tissue after human transplantation. Biol Blood Marrow Transplant. 2003;9:128. [Google Scholar]
- 10.Goldman SA, Schanz S, Windrem MS. Stem cell-based strategies for treating pediatric disorders of myelin. Hum Mol Genet. 2008;17:R76–83. doi: 10.1093/hmg/ddn052. [DOI] [PubMed] [Google Scholar]
- 11.Keirstead HS. Stem cell transplantation into the central nervous system and the control of differentiation. J Neurosci Res. 2001;63:233–6. doi: 10.1002/1097-4547(20010201)63:3<233::AID-JNR1016>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 12.Keirstead HS. Stem cells for the treatment of myelin loss. Trends Neurosci. 2005;28:677–83. doi: 10.1016/j.tins.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Brüstle O, Jones KN, Learish RD, Karram K, Choudhary K, Wiestler OD, et al. Embryonic stem cell-derived glial precursors: a source of myelinating transplants. Science. 1999;285:754–6. doi: 10.1126/science.285.5428.754. [DOI] [PubMed] [Google Scholar]
- 14.Galli R, Gritti A, Bonfanti L, Vescovi AL. Neural stem cells: an overview. Circ Res. 2003;92:598–608. doi: 10.1161/01.RES.0000065580.02404.F4. [DOI] [PubMed] [Google Scholar]
- 15.Kang SH, Bergles DE. Glial progenitor cells in the adult brain reveal their alternate fate. Nat Neurosci. 2008;11:1365–7. doi: 10.1038/nn1208-1365. [DOI] [PubMed] [Google Scholar]
- 16.Amariglio N, Hirshberg A, Scheithauer BW, Cohen Y, Loewenthal R, Trakhtenbrot L. Donor derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient. PLoS Med. 2009;17:222–230. doi: 10.1371/journal.pmed.1000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tracy E, Aldrink J, Panosian J, Beam D, Thacker J, Reese M, Kurtzberg J. Isolation of oligodendrocyte-like cells from human umbilical cord blood. Cytotherapy. 2008;10:518–25. doi: 10.1080/14653240802154586. [DOI] [PubMed] [Google Scholar]
- 18.Rubinstein P, Dobrila L, Rosenfield RE, Adamson JW, Migliaccio G, Migliaccio AR, et al. Processing and cryopreservation of placental/umbilical cord blood for unrelated bone marrow reconstitution. Proc Natl Acad Sci USA. 1995;92:10119–22. doi: 10.1073/pnas.92.22.10119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Back SA, Volpe JJ. Approaches to the study of disease involving oligodendroglial death. In: Koliastsos V, Ratan R, editors. Cell Death and Diseases of the Nervous System. Humana Press; Totowa: 1999. pp. 401–28. [Google Scholar]
- 20.Zeigler F, Hall SG. Isolation of oligodendroglial cells from cultured neural stem/progenitors. Methods Mol Biol. 2007;407:323–31. doi: 10.1007/978-1-59745-536-7_22. [DOI] [PubMed] [Google Scholar]
- 21.Mokrý J, Karbanová J, Filip S, Cízková D, Pazour J, English D. Phenotypic and morphological characterization of in vitro oligodendrogliogenesis. Stem Cells Dev. 2008;17:333–41. doi: 10.1089/scd.2007.0091. [DOI] [PubMed] [Google Scholar]
- 22.Nicolay DJ, Doucette JR, Nazarali AJ. Transcriptional control of oligodendrogenesis. Glia. 2007;55:1287–99. doi: 10.1002/glia.20540. [DOI] [PubMed] [Google Scholar]
- 23.Marty MC, Alliot F, Rutin J, Fritz R, Trisler D, Pessac B. The myelin basic protein gene is expressed in differentiated blood cell lineages and in hemopoietic progenitors. Proc Natl Acad Sci USA. 2002;99:8856–61. doi: 10.1073/pnas.122079599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee MW, Moon YJ, Yang MS, Kim SK, Jang IK, et al. Neural differentiation of novel multipotent progenitor cells from cryopreserved human umbilical cord blood. Biochem Biophys Res Commun. 2007;358:637–43. doi: 10.1016/j.bbrc.2007.04.181. [DOI] [PubMed] [Google Scholar]
- 25.Sharp J, Keirstead HS. Therapeutic applications of oligoden drocyte precursors derived from human embryonic stem cells. Curr Opin Biotechnol. 2007;18:434–40. doi: 10.1016/j.copbio.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 26.Arien-Zakay H, Lazarovici P, Nagler A. Tissue regeneration potential in umbilical cord blood. Best Prac Res Clin Hematol. 2010;23:291–303. doi: 10.1016/j.beha.2010.04.001. [DOI] [PubMed] [Google Scholar]