Abstract
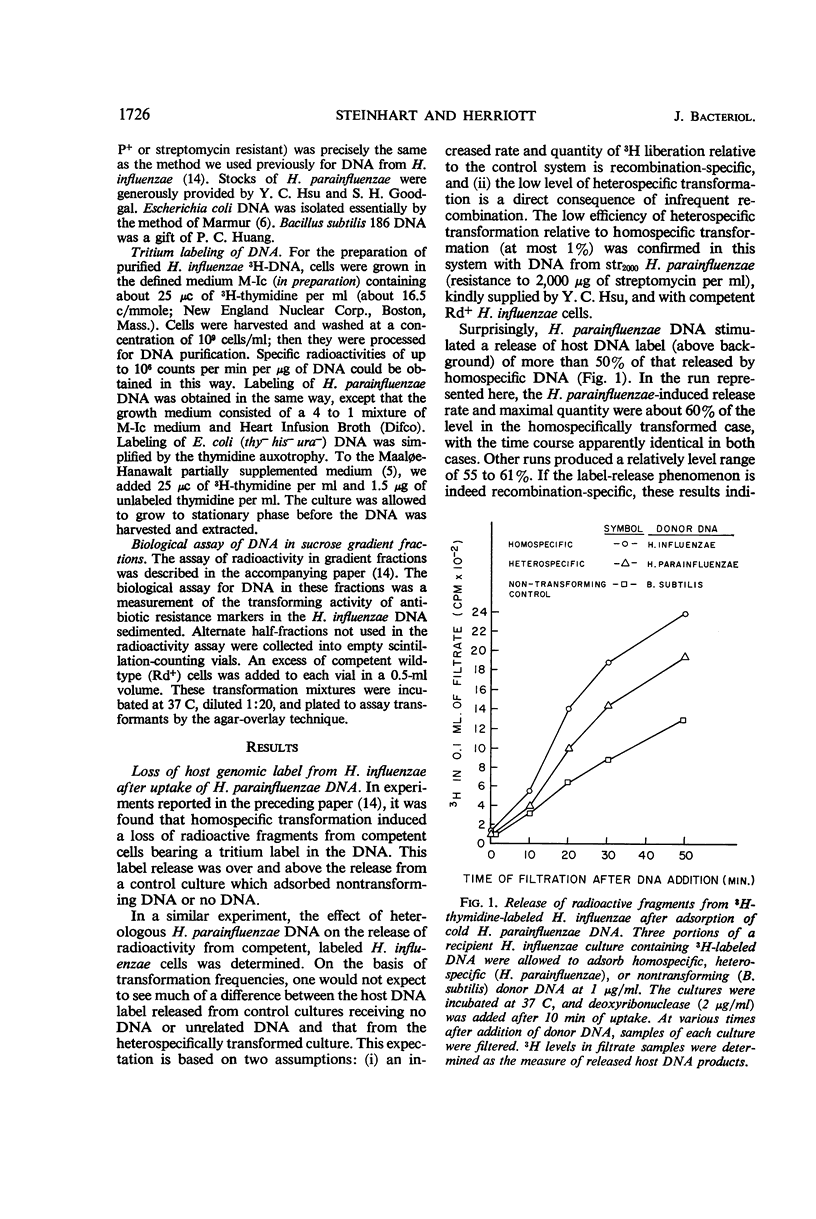
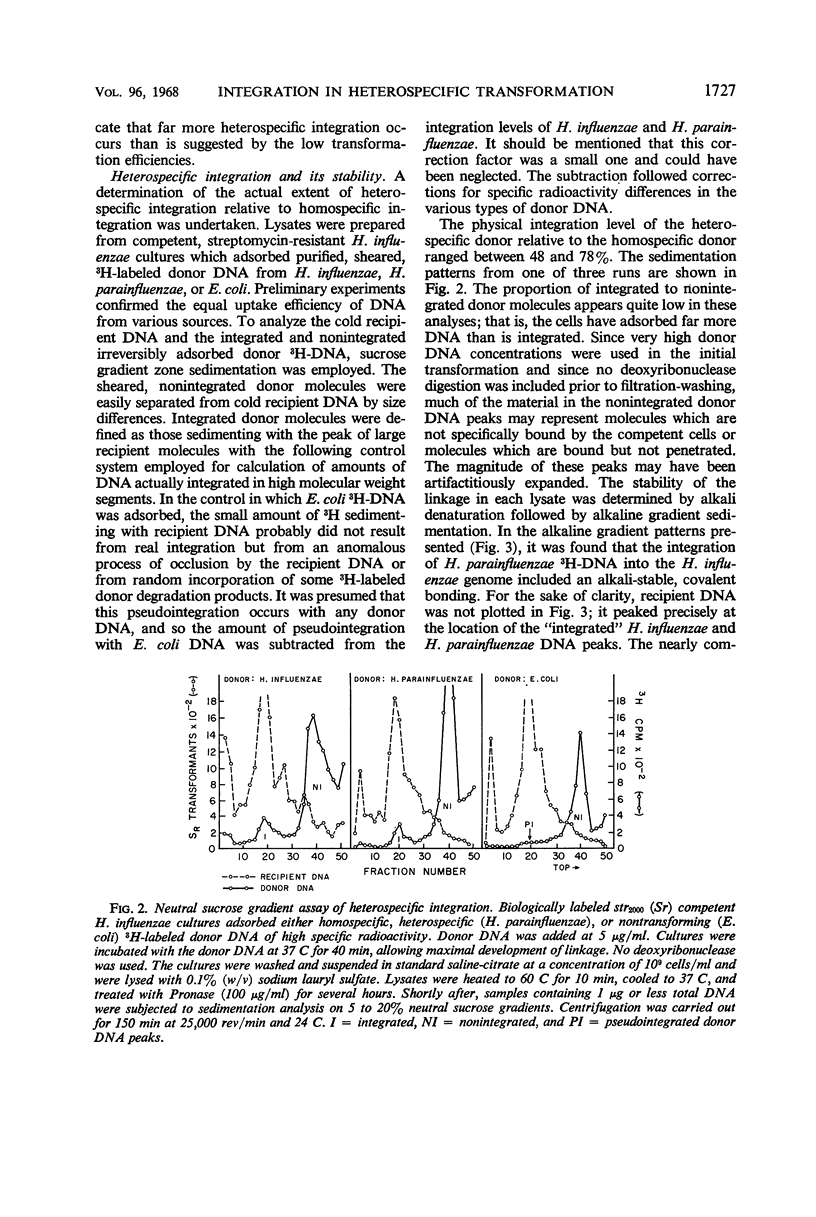
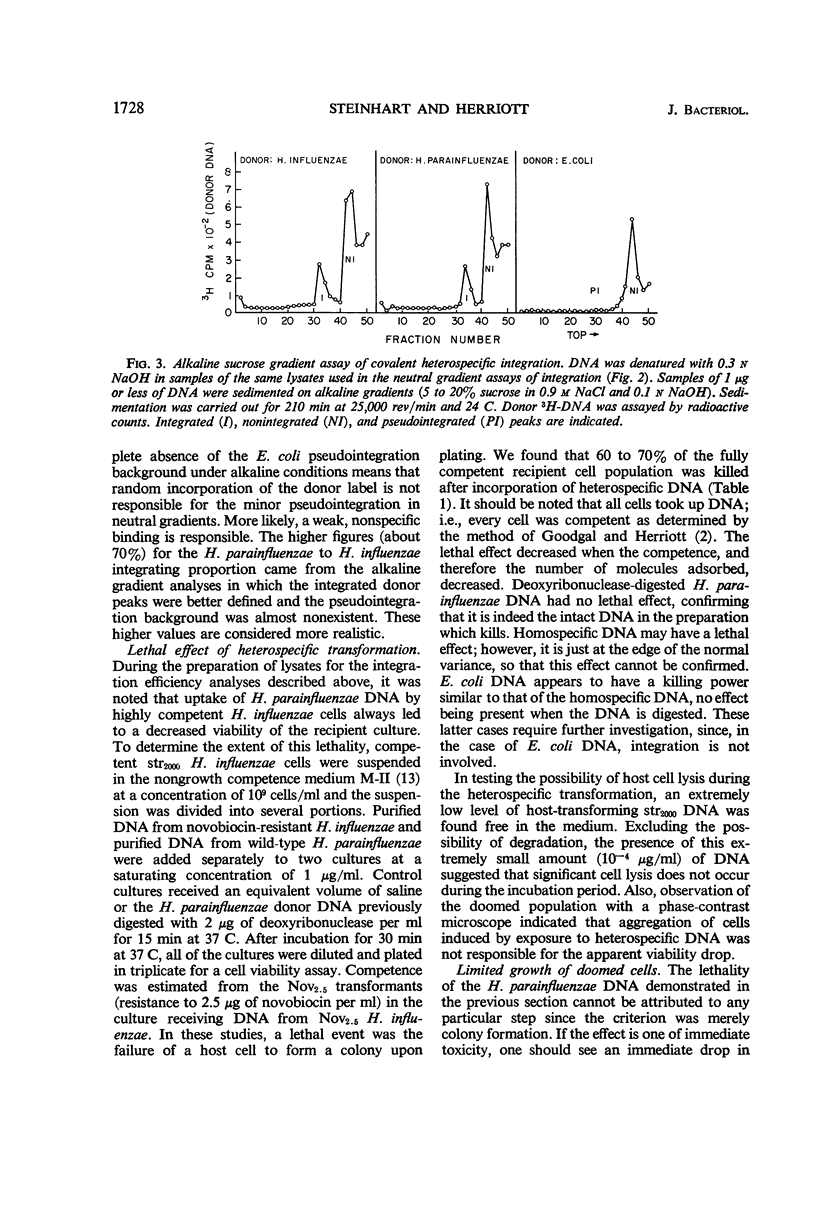
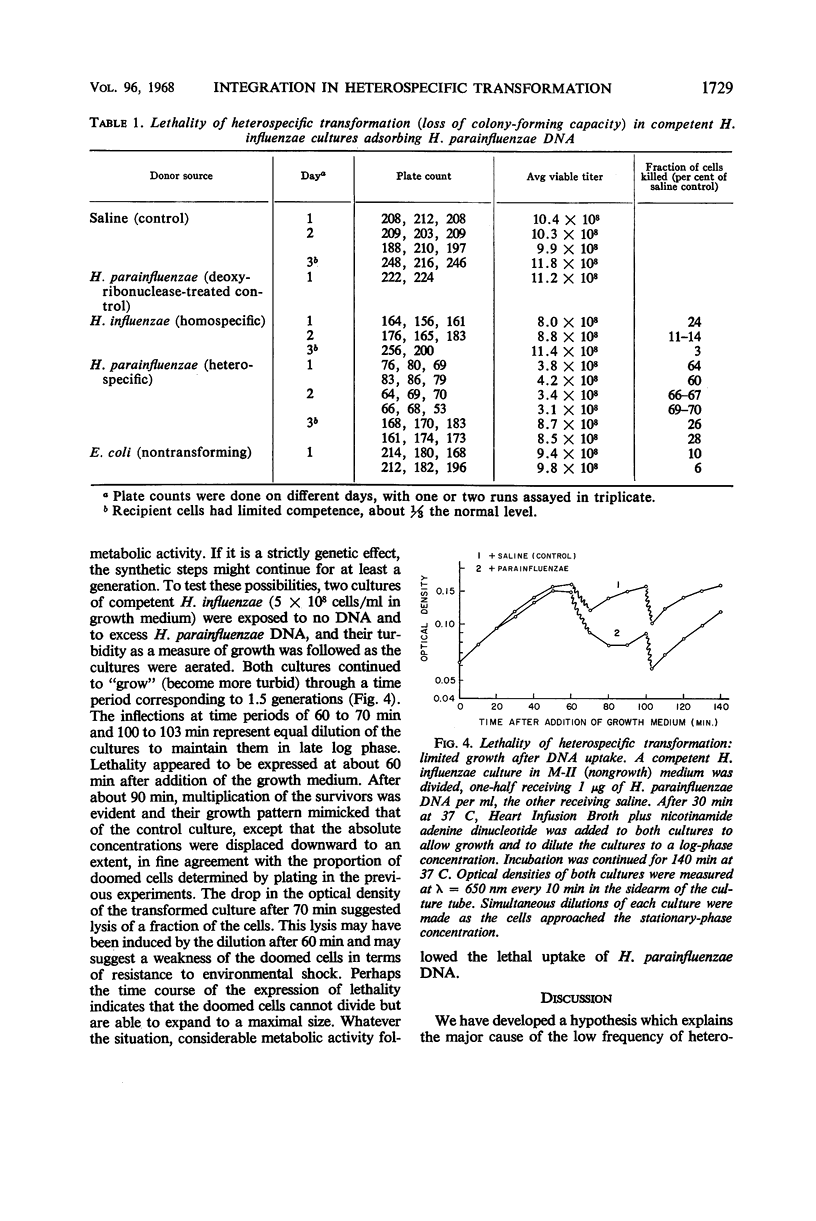
The in vivo chemical linkage of Haemophilus parainfluenzae deoxyribonucleic acid (DNA) with the H. influenzae genome has been found to occur at a much higher level than is suggested by the low efficiency of the heterospecific transformation of an antibiotic resistance marker. This linkage, about 60% of the level with homospecific DNA, was found to involve alkali-stable bonding. The amount of host DNA label released (about 60%) was about the same as that released during homospecific transformation. Also, over 60% of the H. influenzae cells adsorbing H. parainfluenzae DNA could not form colonies upon plating. This lethality of the heterospecific transformation was not immediate but followed considerable metabolic activity of the host cells. These data are presented to show that the “limited-pairing” hypothesis may be only a partial explanation for the low efficiency of heterospecific transformation. Another hypothesis is presented which takes into account the lethal effect of this kind of transformation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALEXANDER H. E., HAHN E., LEIDY G. On the specificity of the desoxyribonucleic acid which induces streptomycin resistance in Hemophilus. J Exp Med. 1956 Sep 1;104(3):305–320. doi: 10.1084/jem.104.3.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CABRERA JUAREZ E., HERRIOTT R. M. ULTRAVIOLET IRRADIATION OF NATIVE AND DENATURED TRANSFORMING DEOXYRIBONUCLEIC ACID FROM HAEMOPHILUS INFLUENZAE. J Bacteriol. 1963 Mar;85:671–675. doi: 10.1128/jb.85.3.671-675.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODGAL S. H., HERRIOTT R. M. Studies on transformations of Hemophilus influenzae. I. Competence. J Gen Physiol. 1961 Jul;44:1201–1227. doi: 10.1085/jgp.44.6.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEIDY G., HAHN E., ALEXANDER H. E. Interspecific transformation in Hemophilus: a possible index of relationship between H. influenzae and H. aegyptius. Proc Soc Exp Biol Med. 1959 Oct;102:86–88. doi: 10.3181/00379727-102-25151. [DOI] [PubMed] [Google Scholar]
- MAALOE O., HANAWALT P. C. Thymine deficiency and the normal DNA replication cycle. I. J Mol Biol. 1961 Apr;3:144–155. doi: 10.1016/s0022-2836(61)80041-7. [DOI] [PubMed] [Google Scholar]
- MARMUR J., FALKOW S., MANDEL M. NEW APPROACHES TO BACTERIAL TAXONOMY. Annu Rev Microbiol. 1963;17:329–372. doi: 10.1146/annurev.mi.17.100163.001553. [DOI] [PubMed] [Google Scholar]
- PENE J. J., ROMIG W. R. ON THE MECHANISM OF GENETIC RECOMBINATION IN TRANSFORMING BACILLUS SUBTILIS. J Mol Biol. 1964 Jul;9:236–245. doi: 10.1016/s0022-2836(64)80103-0. [DOI] [PubMed] [Google Scholar]
- Piechowska M., Shugar D. Influence of DNA on growth and viability of transformable group H streptococci. Acta Biochim Pol. 1967;14(2):277–291. [PubMed] [Google Scholar]
- Ravin A. W., Chen K. C. Heterospecific transformation of pneumococcus and streptococcus. 3. Reduction of linkage. Genetics. 1967 Dec;57(4):851–864. doi: 10.1093/genetics/57.4.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHAEFFER P. Interspecific reactions in bacterial transformation. Symp Soc Exp Biol. 1958;12:60–74. [PubMed] [Google Scholar]
- Spencer H. T., Herriott R. M. Development of competence of Haemophilus influenzae. J Bacteriol. 1965 Oct;90(4):911–920. doi: 10.1128/jb.90.4.911-920.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhart W. L., Herriott R. M. Fate of recipient deoxyribonucleic acid during transformation in Haemophilus influenzae. J Bacteriol. 1968 Nov;96(5):1718–1724. doi: 10.1128/jb.96.5.1718-1724.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]


