Abstract
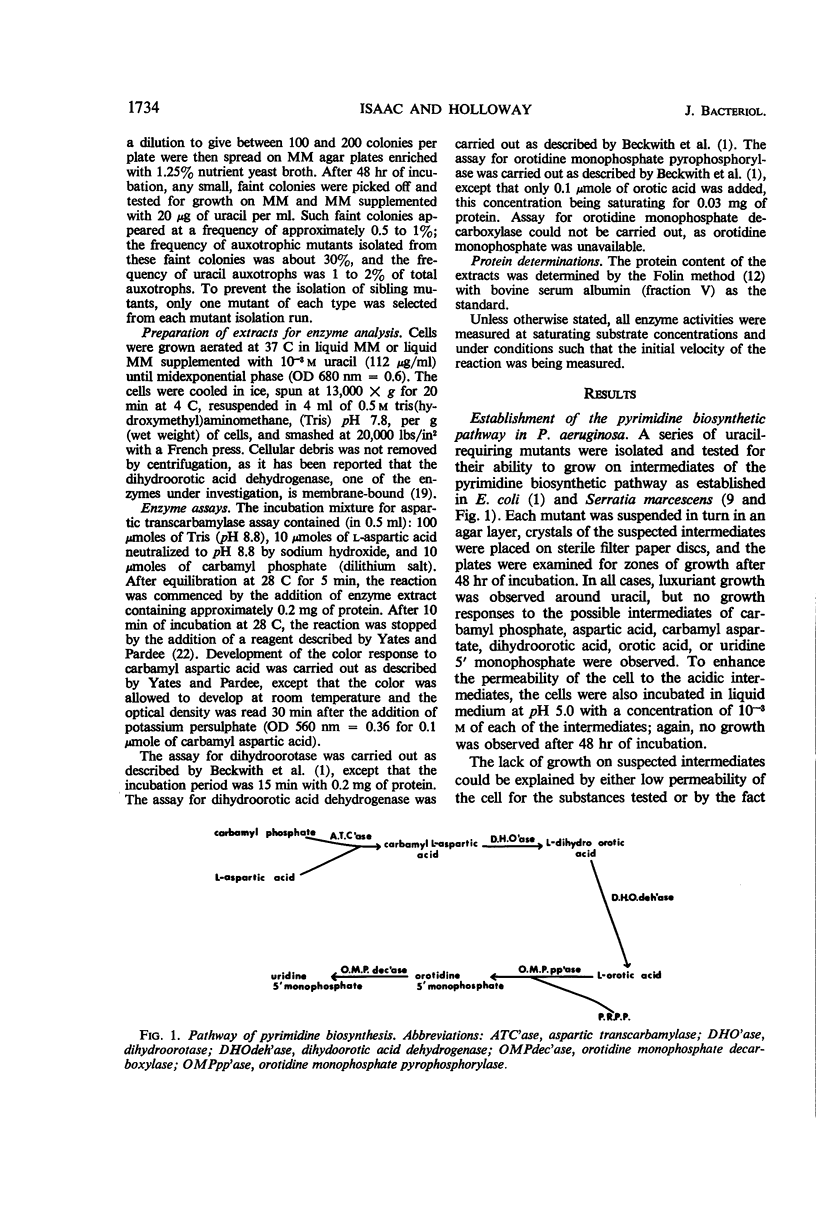
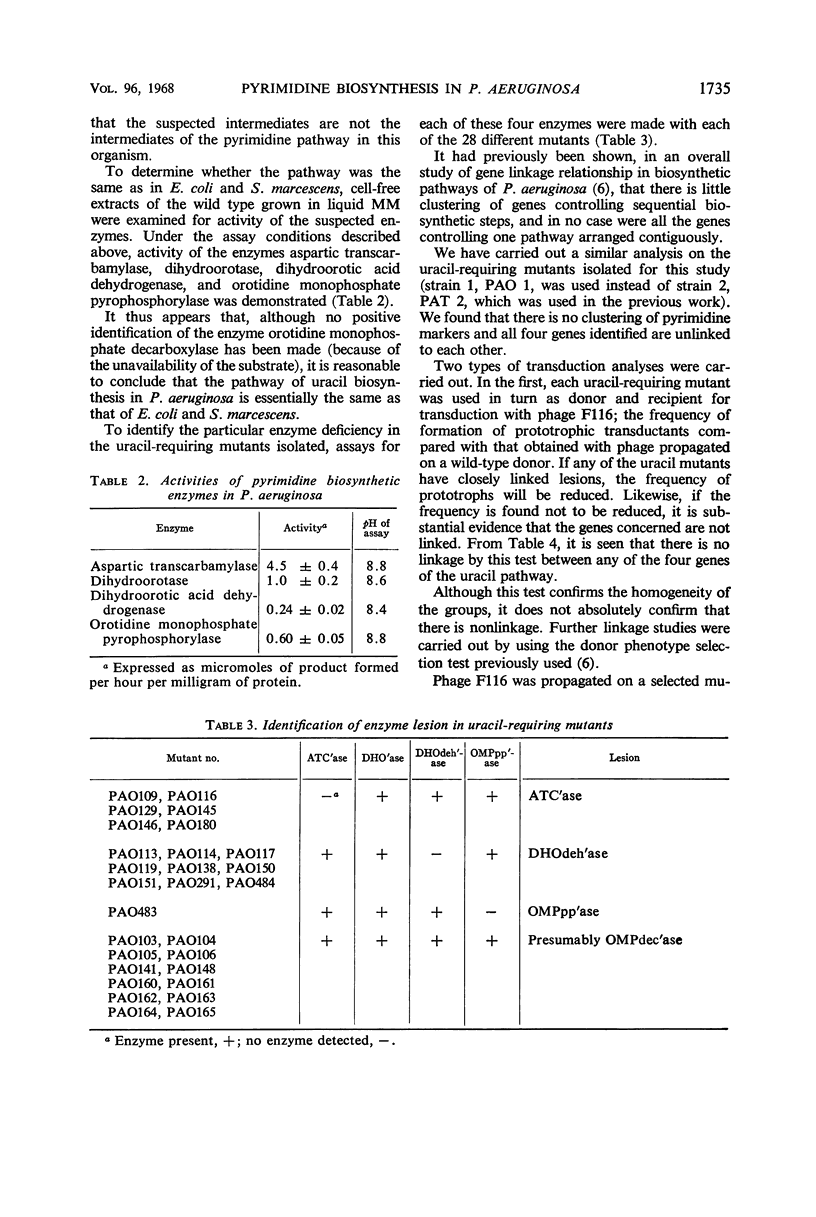
The pathway of pyrimidine biosynthesis in Pseudomonas aeruginosa has been shown to be the same as in other bacteria. Twenty-seven mutants requiring uracil for growth were isolated and the mutant lesions were identified. Mutants lacking either dihydroorotic acid dehydrogenase, orotidine monophosphate pyrophosphorylase, orotidine monophosphate decarboxylase, or aspartic transcarbamylase were isolated; none lacking dihydroorotase were found. By using transduction and conjugation, four genes affecting pyrimidine biosynthetic enzymes have been identified and shown to be unlinked to each other. The linkage of pyrB to met-28 and ilv-2 was shown by contransduction. Repression by uracil alone or by broth could not be demonstrated for any enzymes of this pathway, in contrast to the situation in Escherichia coli and Serratia marcescens. In addition, derepression of these enzymes could not be demonstrated. A low level of feedback inhibition of aspartic transcarbamylase was found to occur. It is suggested that the control of such constitutive biosynthetic enzymes in P. aeruginosa may be related to the comprehensive metabolic activities of this organism.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BECKWITH J. R., PARDEE A. B., AUSTRIAN R., JACOB F. Coordination of the synthesis of the enzymes in the pyrimidine pathway of E. coli. J Mol Biol. 1962 Dec;5:618–634. doi: 10.1016/s0022-2836(62)80090-4. [DOI] [PubMed] [Google Scholar]
- Chakrabarty A. M., Gunsalus C. F., Gunsalus I. C. Transduction and the clustering of genes in fluorescent Pseudomonads. Proc Natl Acad Sci U S A. 1968 May;60(1):168–175. doi: 10.1073/pnas.60.1.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford I. P., Gunsalus I. C. Inducibility of tryptophan synthetase in Pseudomonas putida. Proc Natl Acad Sci U S A. 1966 Aug;56(2):717–724. doi: 10.1073/pnas.56.2.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOY C. H. THE BIOCHEMICAL DIFFERENCE BETWEEN CERTAIN PHENOTYPICALLY SIMILAR, BUT GENOTYPICALLY DIFFERENT, TRYPTOPHAN AUXOTROPHS OF PSEUDOMONAS AERUGINOSA. Biochim Biophys Acta. 1964 Jul 15;90:180–183. doi: 10.1016/0304-4165(64)90136-9. [DOI] [PubMed] [Google Scholar]
- Demerec M., Adelberg E. A., Clark A. J., Hartman P. E. A proposal for a uniform nomenclature in bacterial genetics. Genetics. 1966 Jul;54(1):61–76. doi: 10.1093/genetics/54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARGIE B., HOLLOWAY B. W. ABSENCE OF CLUSTERING OF FUNCTIONALLY RELATED GENES IN PSEUDOMONAS AERUGINOSA. Genet Res. 1965 Jul;6:284–299. doi: 10.1017/s0016672300004158. [DOI] [PubMed] [Google Scholar]
- GERHART J. C., PARDEE A. B. The enzymology of control by feedback inhibition. J Biol Chem. 1962 Mar;237:891–896. [PubMed] [Google Scholar]
- HANDSCHUMACHER R. E., PASTERNAK C. A. Inhibition of orotidylic acid decarboxylase, a primary site of carcinostasis by 6-azauracil. Biochim Biophys Acta. 1958 Nov;30(2):451–452. doi: 10.1016/0006-3002(58)90088-x. [DOI] [PubMed] [Google Scholar]
- HOLLOWAY B. W. Genetic recombination in Pseudomonas aeruginosa. J Gen Microbiol. 1955 Dec;13(3):572–581. doi: 10.1099/00221287-13-3-572. [DOI] [PubMed] [Google Scholar]
- HOLLOWAY B. W., ROLFE B. HOST GENOME CONTROL IN HOST-INDUCED MODIFICATION OF PSEUDOMONAS AERUGINOSA PHAGES. Virology. 1964 Aug;23:595–602. doi: 10.1016/0042-6822(64)90244-2. [DOI] [PubMed] [Google Scholar]
- Hayward W. S., Belser W. L. Regulation of pyrimidine biosynthesis in Serratia marcescens. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1483–1489. doi: 10.1073/pnas.53.6.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mee B. J., Lee B. T. An analysis of histidine requiring mutants in Pseudomonas aeruginosa. Genetics. 1967 Apr;55(4):709–722. doi: 10.1093/genetics/55.4.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEUMANN J., JONES M. E. END-PRODUCT INHIBITION OF ASPARTATE TRANSCARBAMYLASE IN VARIOUS SPECIES. Arch Biochem Biophys. 1964 Mar;104:438–447. doi: 10.1016/0003-9861(64)90487-4. [DOI] [PubMed] [Google Scholar]
- PEARCE L. E., LOUTIT J. S. BIOCHEMICAL AND GENETIC GROUPING OF ISOLEUCINE-VALINE MUTANTS OF PSEUDOMONAS AERUGINOSA. J Bacteriol. 1965 Jan;89:58–63. doi: 10.1128/jb.89.1.58-63.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinsky L., Krooth R. S. Studies on the control of pyrimidine biosynthesis in human diploid cell strains, I. Effect of 6-azauridine on cellular phenotype. Proc Natl Acad Sci U S A. 1967 Apr;57(4):925–932. doi: 10.1073/pnas.57.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Revised linkage map of Escherichia coli. Bacteriol Rev. 1967 Dec;31(4):332–353. doi: 10.1128/br.31.4.332-353.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor W. H., Taylor M. L., Eames D. F. Two functionally different dihydroorotic dehydrogenases in bacteria. J Bacteriol. 1966 Jun;91(6):2251–2256. doi: 10.1128/jb.91.6.2251-2256.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Waltho J. A., Holloway B. W. Suppression of fluorophenylalanine resistance by mutation to streptomycin resistance in Pseudomonas aeruginosa. J Bacteriol. 1966 Jul;92(1):35–42. doi: 10.1128/jb.92.1.35-42.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YATES R. A., PARDEE A. B. Control by uracil of formation of enzymes required for orotate synthesis. J Biol Chem. 1957 Aug;227(2):677–692. [PubMed] [Google Scholar]


