Abstract
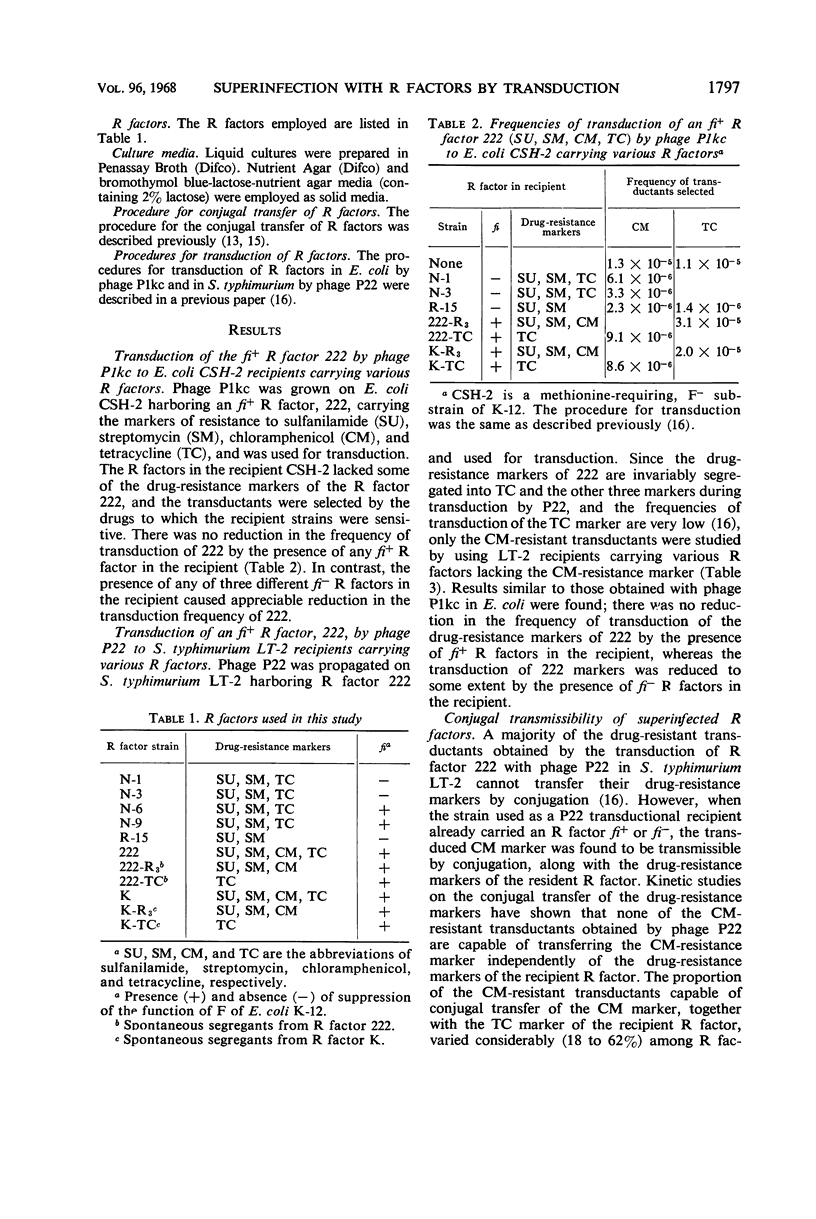
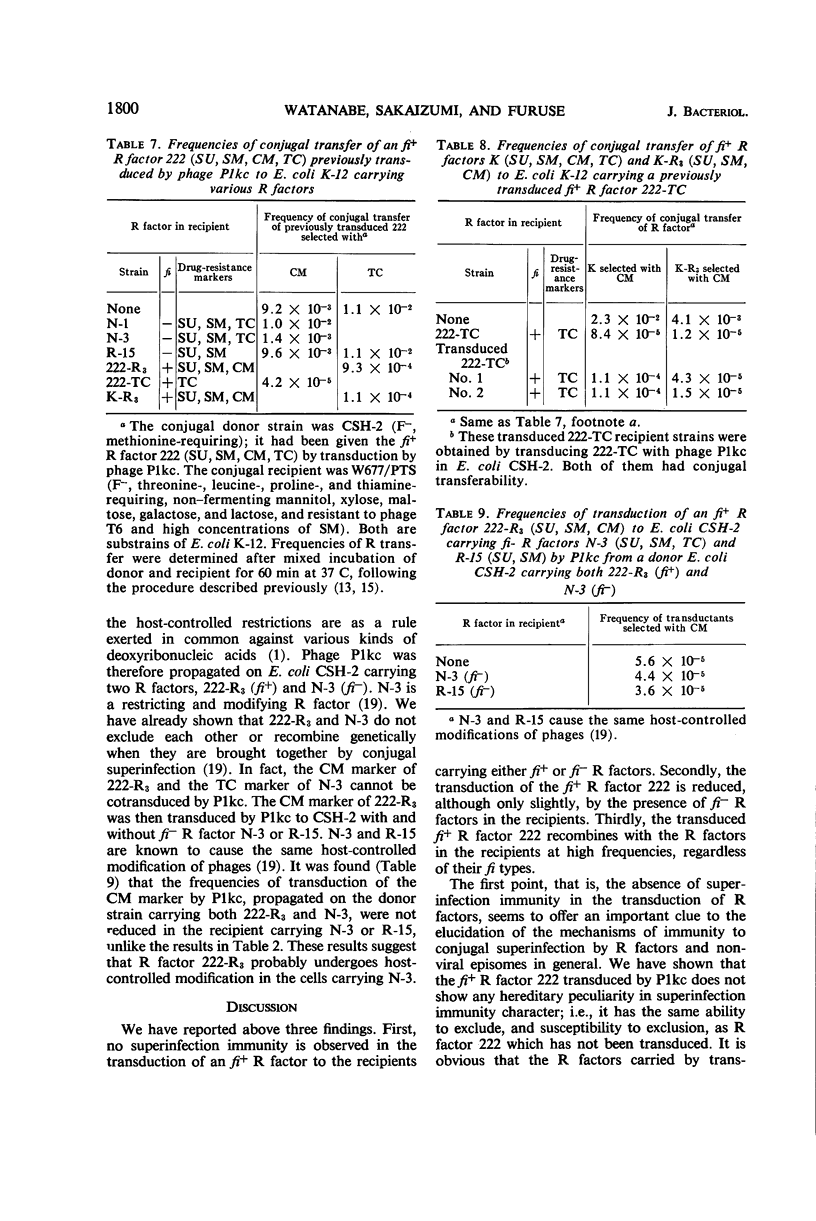
Superinfection immunity is found in the conjugal transfer of R factors between two fi+ R factors and between two fi− R factors (fi = fertility inhibition), as we reported previously. In contrast, no reduction in the frequencies of transduction of an fi+ R factor 222 was caused by the presence of fi+ R factors in the recipients in transduction systems with phage P1kc in Escherichia coli K-12 and with phage P22 in Salmonella typhimurium LT-2. The absence of superinfection immunity in transduction may be due to the difference in the route of entry of the R factor. The frequencies of transduction of an fi+ R factor were reduced, although slightly, by the presence of fi− R factors in the recipients. This reduction is probably due to host-controlled restriction of the entering fi+ R factor by the fi− R factors in the recipients, since transduction of an fi+ R factor by the transducing phage propagated on the strain carrying both fi+ and fi− R factors was not reduced by the presence of homologous fi− R factors in the recipients. The fi+ R factor 222, when transduced to the recipient strains carrying other R factors, recombined genetically at high frequencies with these resident R factors, regardless of their fi type.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARBER W., MORSE M. L. HOST SPECIFICITY OF DNA PRODUCED BY ESCHERICHIA COLI. VI. EFFECTS ON BACTERIAL CONJUGATION. Genetics. 1965 Jan;51:137–148. doi: 10.1093/genetics/51.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arber W. Host-controlled modification of bacteriophage. Annu Rev Microbiol. 1965;19:365–378. doi: 10.1146/annurev.mi.19.100165.002053. [DOI] [PubMed] [Google Scholar]
- BRINTON C. C., Jr, GEMSKI P., Jr, CARNAHAN J. A NEW TYPE OF BACTERIAL PILUS GENETICALLY CONTROLLED BY THE FERTILITY FACTOR OF E. COLI K 12 AND ITS ROLE IN CHROMOSOME TRANSFER. Proc Natl Acad Sci U S A. 1964 Sep;52:776–783. doi: 10.1073/pnas.52.3.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton C. C., Jr The structure, function, synthesis and genetic control of bacterial pili and a molecular model for DNA and RNA transport in gram negative bacteria. Trans N Y Acad Sci. 1965 Jun;27(8):1003–1054. doi: 10.1111/j.2164-0947.1965.tb02342.x. [DOI] [PubMed] [Google Scholar]
- Falkow S., Citarella R. V., Wohlhieter J. A. The molecular nature of R-factors. J Mol Biol. 1966 May;17(1):102–116. doi: 10.1016/s0022-2836(66)80097-9. [DOI] [PubMed] [Google Scholar]
- Lawn A. M., Meynell G. G., Meynell E., Datta N. Sex pili and the classification of sex factors in the enterobacteriaceae. Nature. 1967 Oct 28;216(5113):343–346. doi: 10.1038/216343a0. [DOI] [PubMed] [Google Scholar]
- Meynell E., Datta N. Functional homology of the sex-factor and resistance transfer factors. Nature. 1965 Aug 21;207(999):884–885. doi: 10.1038/207884a0. [DOI] [PubMed] [Google Scholar]
- Meynell E., Datta N. Mutant drug resistant factors of high transmissibility. Nature. 1967 May 27;214(5091):885–887. doi: 10.1038/214885a0. [DOI] [PubMed] [Google Scholar]
- Meynell E., Datta N. The relation of resistance transfer factors to the F-factor (sex-factor) of Escherichia coli K12. Genet Res. 1966 Feb;7(1):134–140. doi: 10.1017/s0016672300009538. [DOI] [PubMed] [Google Scholar]
- Okada M., Watanabe T., Miyake T. On the nature of the recipient ability of Salmonella typhimurium for foreign deoxyribonucleic acids. J Gen Microbiol. 1968 Feb;50(2):241–252. doi: 10.1099/00221287-50-2-241. [DOI] [PubMed] [Google Scholar]
- Rownd R., Nakaya R., Nakamura A. Molecular nature of the drug-resistance factors of the Enterobacteriaceae. J Mol Biol. 1966 Jun;17(2):376–393. doi: 10.1016/s0022-2836(66)80149-3. [DOI] [PubMed] [Google Scholar]
- WATANABE T., FUKASAWA T. Episome-mediated transfer of drug resistance in Enterobacteriaceae. I. Transfer of resistance factors by conjugation. J Bacteriol. 1961 May;81:669–678. doi: 10.1128/jb.81.5.669-678.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATANABE T., FUKASAWA T. Episome-mediated transfer of drug resistance in Enterobacteriaceae. III. Transduotion of resistance factors. J Bacteriol. 1961 Aug;82:202–209. doi: 10.1128/jb.82.2.202-209.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATANABE T. Infective heredity of multiple drug resistance in bacteria. Bacteriol Rev. 1963 Mar;27:87–115. doi: 10.1128/br.27.1.87-115.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATANABE T., LYANG K. W. Episome-mediated transfer of drug resistance in Enterobacteriaceae. V. Spontaneous segregation and recombination of resistance factors in Salmonella typhimurium. J Bacteriol. 1962 Sep;84:422–430. doi: 10.1128/jb.84.3.422-430.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATANABE T., NISHIDA H., OGATA C., ARAI T., SATO S. EPISOME-MEDIATED TRANSFER OF DRUG RESISTANCE IN ENTEROBACTERIACEAE. VII. TWO TYPES OF NATURALLY OCCURRING R FACTORS. J Bacteriol. 1964 Sep;88:716–726. doi: 10.1128/jb.88.3.716-726.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATANABE T. SELECTED METHODS OF GENETIC STUDY OF EPISOME-MEDIATED DRUG RESISTANCE IN BACTERIA. Methods Med Res. 1964;10:202–220. [PubMed] [Google Scholar]
- Watanabe T. Evolutionary relationships of R factors with other episomes and plasmids. Fed Proc. 1967 Jan-Feb;26(1):23–28. [PubMed] [Google Scholar]
- Watanabe T., Furuse C., Sakaizumi S. Transduction of various R factors by phage P1 in Escherichia coli and by phage P22 in Salmonella typhimurium. J Bacteriol. 1968 Nov;96(5):1791–1795. doi: 10.1128/jb.96.5.1791-1795.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T., Takano T., Arai T., Nishida H., Sato S. Episome-mediated Transfer of Drug Resistance in Enterobacteriaceae X. Restriction and Modification of Phages by fi R Factors. J Bacteriol. 1966 Aug;92(2):477–486. doi: 10.1128/jb.92.2.477-486.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]


