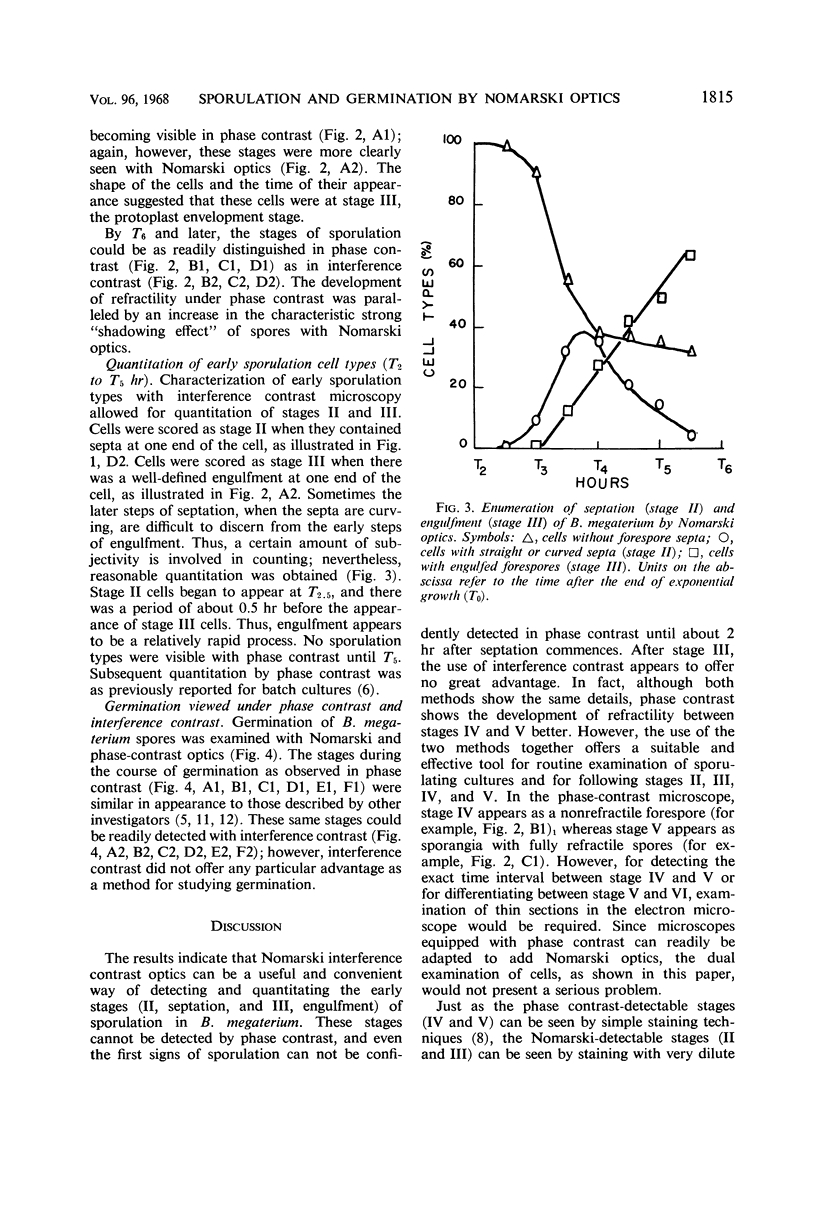
Abstract
The techniques of Nomarski interference contrast microscopy and phase-contrast microscopy were compared for their utility in monitoring sporulation and germination in Bacillus megaterium. The Nomarski technique permitted rapid and easy delineation of septation and engulfment during sporulation, whereas with phase contrast microscopy these stages were not detected at all. The later stages of sporulation were easily seen by either technique. Thus, of the seven stages of sporulation as recognized by the electron microscopy of thin sections, five can now be routinely detected quantitatively by optical microscopy: septation (stage II), engulfment (stage III), phase-dark forespore (corresponding to cortex formation, stage IV), phase-bright spore in a sporangium (corresponding to coat formation, stage V), and the free spore (stage VII). This means that now only stage I (axial filament) and stage VI (maturation of the refractile spore) require electron microscopy for routine detection. There was no advantage in using Nomarski optics for germination studies.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bajer A., Allen R. D. Structure and organization of the living mitotic spindle of Haemanthus endosperm. Science. 1966 Feb 4;151(3710):572–574. doi: 10.1126/science.151.3710.572. [DOI] [PubMed] [Google Scholar]
- Ellar D. J., Lundgren D. G., Slepecky R. A. Fine structure of Bacillus megaterium during synchronous growth. J Bacteriol. 1967 Oct;94(4):1189–1205. doi: 10.1128/jb.94.4.1189-1205.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon R. A., Murrell W. G. Simple method of detecting spore septum formation and synchrony of sporulation. J Bacteriol. 1967 Jan;93(1):495–496. doi: 10.1128/jb.93.1.495-496.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASHIMOTO T., BLACK S. H., GERHARDT P. Development of fine structure, thermostability, and dipicolinate during sporogenesis in a bacillus. Can J Microbiol. 1960 Apr;6:203–212. doi: 10.1139/m60-022. [DOI] [PubMed] [Google Scholar]
- HITCHINS A. D., GOULD G. W., HURST A. The swelling of bacterial spores during germination and outgrowth. J Gen Microbiol. 1963 Mar;30:445–453. doi: 10.1099/00221287-30-3-445. [DOI] [PubMed] [Google Scholar]
- Imanaka H., Gillis J. R., Slepecky R. A. Synchronous growth and sporulation of Bacillus megaterium. J Bacteriol. 1967 May;93(5):1624–1630. doi: 10.1128/jb.93.5.1624-1630.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire M. P. Nomarski interference contrast resolution of subchromatid structure. Proc Natl Acad Sci U S A. 1968 Jun;60(2):533–536. doi: 10.1073/pnas.60.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PULVERTAFT R. J. V., HAYNES J. A. Adenosine and spore germination; phase-contrast studies. J Gen Microbiol. 1951 Oct;5(4):657–663. doi: 10.1099/00221287-5-4-657. [DOI] [PubMed] [Google Scholar]
- SLEPECKY R., FOSTER J. W. Alterations in metal content of spores of Bacillus megaterium and the effect on some spore properties. J Bacteriol. 1959 Jul;78(1):117–123. doi: 10.1128/jb.78.1.117-123.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SZULMAJSTER J., SCHAEFFER P. [Augmentation of the DPNH-oxidase activity during sporulation of Bacillus subtilis]. C R Hebd Seances Acad Sci. 1961 Jan 4;252:220–222. [PubMed] [Google Scholar]
- Slepecky R. A., Law J. H. SYNTHESIS AND DEGRADATION OF POLY-beta-HYDROXYBUTYRIC ACID IN CONNECTION WITH SPORULATION OF BACILLUS MEGATERIUM. J Bacteriol. 1961 Jul;82(1):37–42. doi: 10.1128/jb.82.1.37-42.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VINTER V. Spores of microorganisms. Chloramphenicol-sensitive and penicillin-resistant incorporation of 14C-diaminopimelic acid into sporulating cells of Bacillus cereus. Experientia. 1963 Jun 15;19:307–308. doi: 10.1007/BF02150422. [DOI] [PubMed] [Google Scholar]





