Abstract
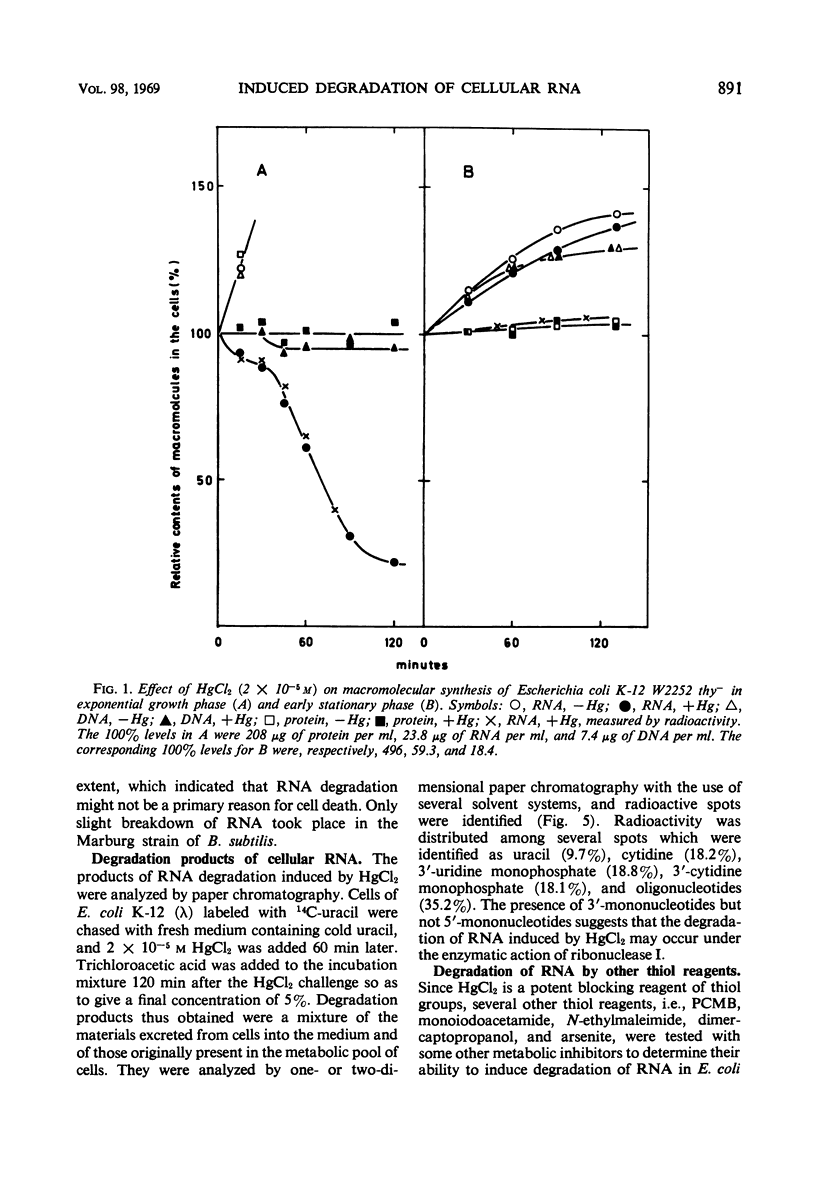
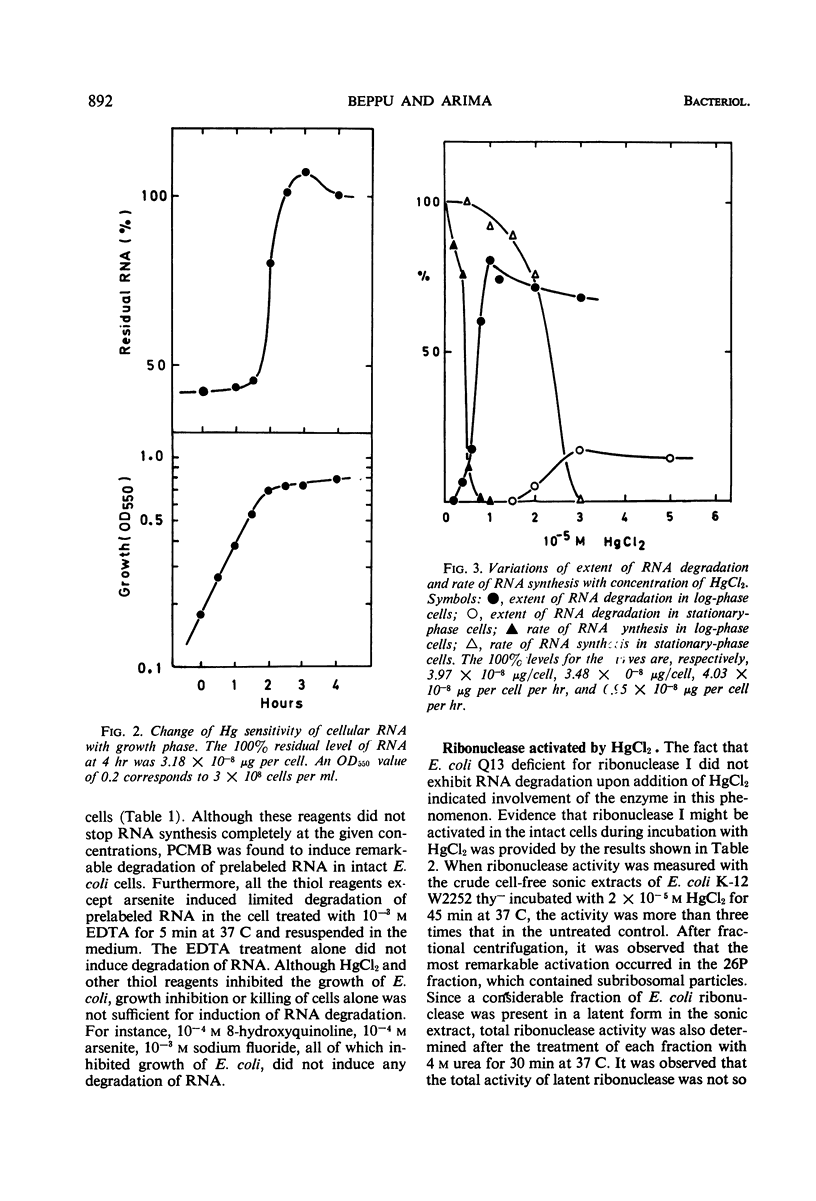
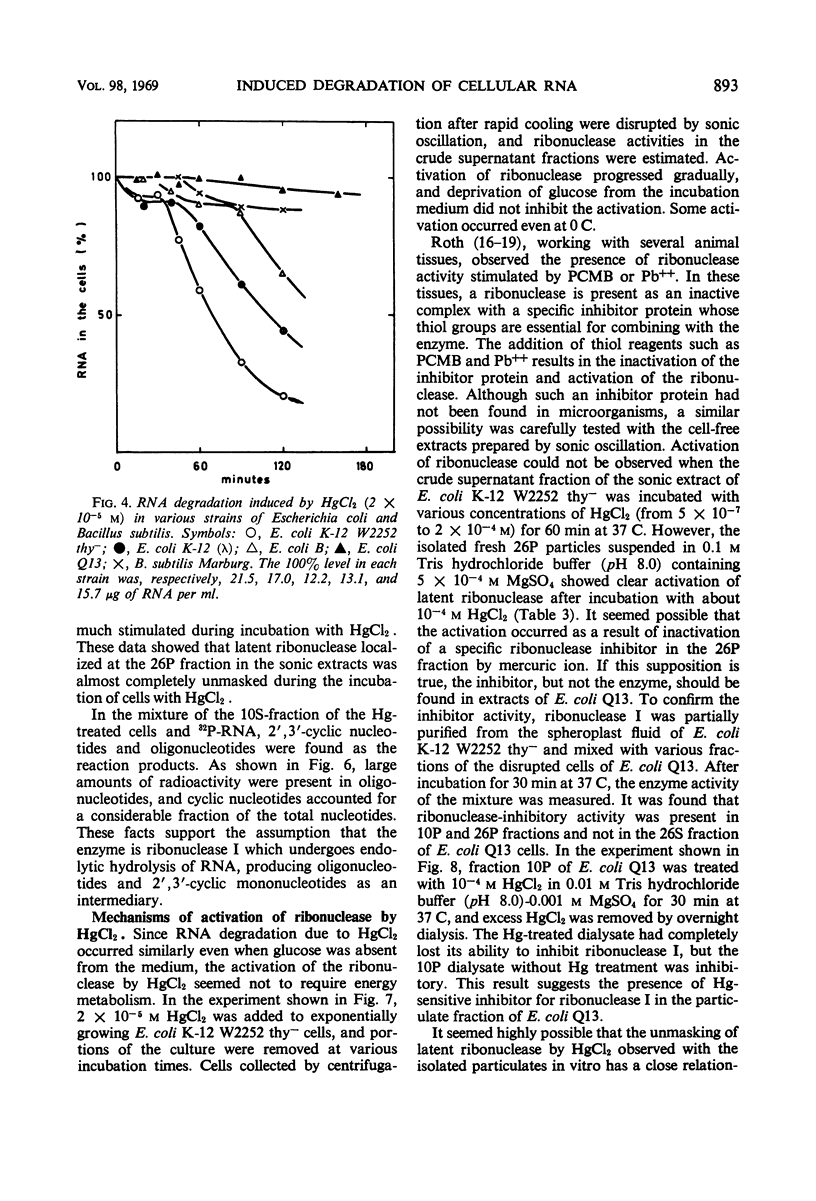
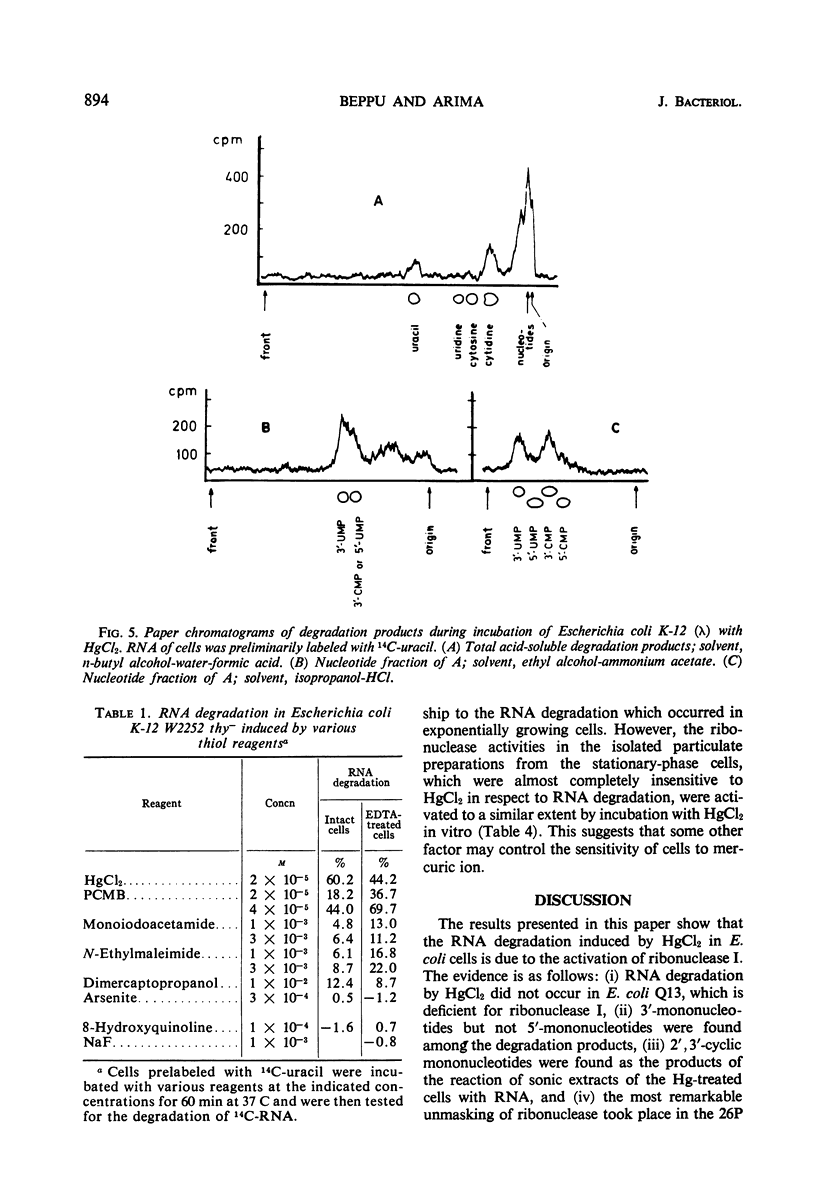
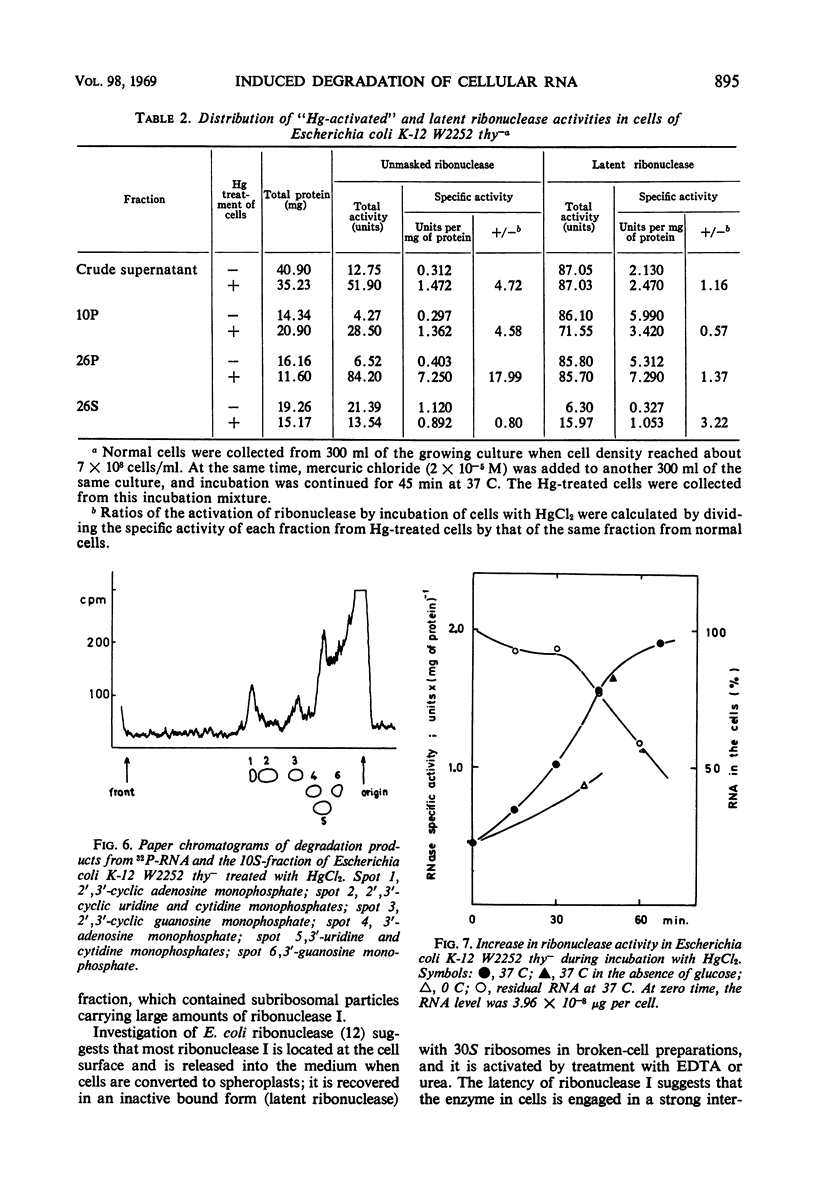
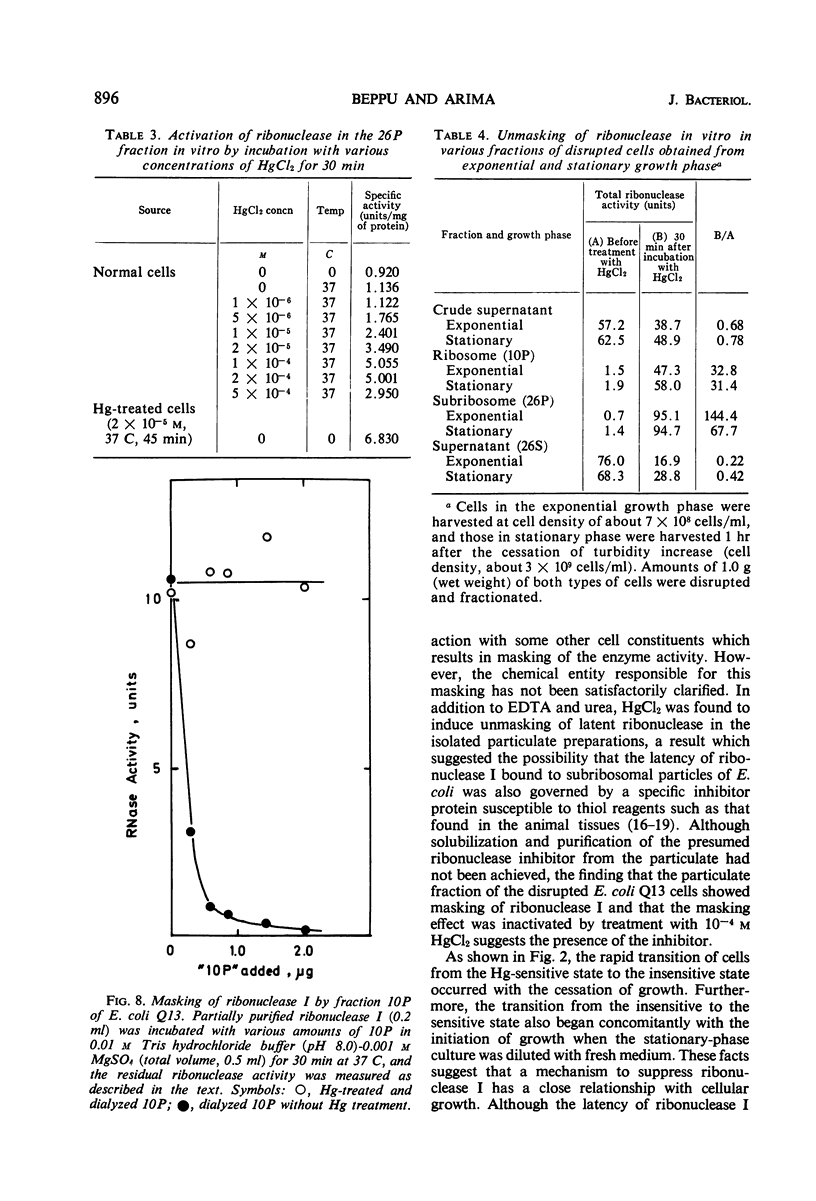
Low concentrations of HgCl2 were found to induce extensive degradation of ribonucleic acid (RNA) in exponentially growing Escherichia coli cells but not in stationary-phase cells. Whereas 80% of cellular RNA was degraded during 90 min of incubation with 10−5m HgCl2 at 37 C, HgCl2 caused only slight degradation in stationary cells, even when present at concentrations higher than 5 × 10−5m. Inhibition of RNA synthesis occurred at almost the same concentration of HgCl2 as degradation, and the ability of stationary-phase cells to synthesize RNA was also resistant to HgCl2. The transition of cells from complete sensitivity to HgCl2 to a fully insensitive state took place simultaneously with the cessation of growth. p-Chloromercuribenzoate was also found to induce remarkable degradation of RNA. In E. coli Q13, a mutant deficient for ribonuclease I, no degradation of RNA was evident, even in the exponential growth phase. 3′-Mononucleotides but not 5′-mononucleotides were found among the degradation products of cellular RNA. 2′,3′-Cyclic mononucleotides were produced when RNA was degraded by the cell-free extracts of the Hg treated cells. Almost complete unmasking of the latent ribonuclease occurred in the particle fraction containing subribosomal particles of the Hg-treated cells. These data suggest that the incubation of exponentially growing E. coli cells with HgCl2 led to the unmasking of ribonuclease I, which resulted in the extensive degradation of cellular RNA. The activation of ribonuclease by HgCl2 in the isolated particulate fraction of E. coli K-12 which occurred in vitro suggested the presence of an Hg-sensitive inhibitor for ribonuclease I.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allwood M. C., Russell A. D. Thermally induced ribonucleic acid degradation and leakage of substances from the metabolic pool in Staphylococcus aureus. J Bacteriol. 1968 Feb;95(2):345–349. doi: 10.1128/jb.95.2.345-349.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BILLEN D. Modification of the release of cellular constituents by irradiated Escherichia coli. Arch Biochem Biophys. 1957 Apr;67(2):333–340. doi: 10.1016/0003-9861(57)90288-6. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesteland R. F. Isolation and characterization of ribonuclease I mutants of Escherichia coli. J Mol Biol. 1966 Mar;16(1):67–84. doi: 10.1016/s0022-2836(66)80263-2. [DOI] [PubMed] [Google Scholar]
- Jackson R. W., DeMoss J. A. Effects of toluene on Escherichia coli. J Bacteriol. 1965 Nov;90(5):1420–1425. doi: 10.1128/jb.90.5.1420-1425.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KINSKY S. C. Alterations in the permeability of Neurospora crassa due to polyene antibiotics. J Bacteriol. 1961 Dec;82:889–897. doi: 10.1128/jb.82.6.889-897.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leive L. Studies on the permeability change produced in coliform bacteria by ethylenediaminetetraacetate. J Biol Chem. 1968 May 10;243(9):2373–2380. [PubMed] [Google Scholar]
- MARKHAM R., SMITH J. D. The structure of ribonucleic acids. II. The smaller products of ribonuclease digestion. Biochem J. 1952 Dec;52(4):558–565. doi: 10.1042/bj0520558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEU H. C., HEPPEL L. A. THE RELEASE OF RIBONUCLEASE INTO THE MEDIUM WHEN ESCHERICHIA COLI CELLS ARE CONVERTED TO SPEROPLASTS. J Biol Chem. 1964 Nov;239:3893–3900. [PubMed] [Google Scholar]
- Nose K., Mizuno D., Ozeki H. Degradation of ribosomal RNA from Escherichia coli induced by colicine E2. Biochim Biophys Acta. 1966 Jun 22;119(3):636–638. doi: 10.1016/0005-2787(66)90142-0. [DOI] [PubMed] [Google Scholar]
- Nozawa R., Horiuchi T., Mizuno D. Degradation of ribosomal RNA in a temperature-sensitive Escherichia coli. Arch Biochem Biophys. 1967 Feb;118(2):402–409. doi: 10.1016/0003-9861(67)90367-0. [DOI] [PubMed] [Google Scholar]
- ROTH J. S. Ribonuclease. IX. Further studies on ribonuclease inhibitor. Biochim Biophys Acta. 1962 Dec 31;61:903–915. doi: 10.1016/0926-6550(62)90007-5. [DOI] [PubMed] [Google Scholar]
- ROTH J. S. Ribonuclease. V. Studies on the properties and distribution of ribonuclease inhibitor in the rat. Biochim Biophys Acta. 1956 Jul;21(1):34–43. doi: 10.1016/0006-3002(56)90091-9. [DOI] [PubMed] [Google Scholar]
- ROTH J. S. Ribonuclease. VII. Partial purification and characterization of a ribonuclease inhibitor in rat liver supernatant fraction. J Biol Chem. 1958 Apr;231(2):1085–1095. [PubMed] [Google Scholar]
- ROTH J. S. Ribonuclease. VIII. Studies on the inactive ribonuclease in the supernatant fraction of rat liver. J Biol Chem. 1958 Apr;231(2):1097–1105. [PubMed] [Google Scholar]
- WADE H. E. The autodegradation of ribonucleoprotein in Escherichia coli. Biochem J. 1961 Mar;78:457–472. doi: 10.1042/bj0780457. [DOI] [PMC free article] [PubMed] [Google Scholar]


