Abstract
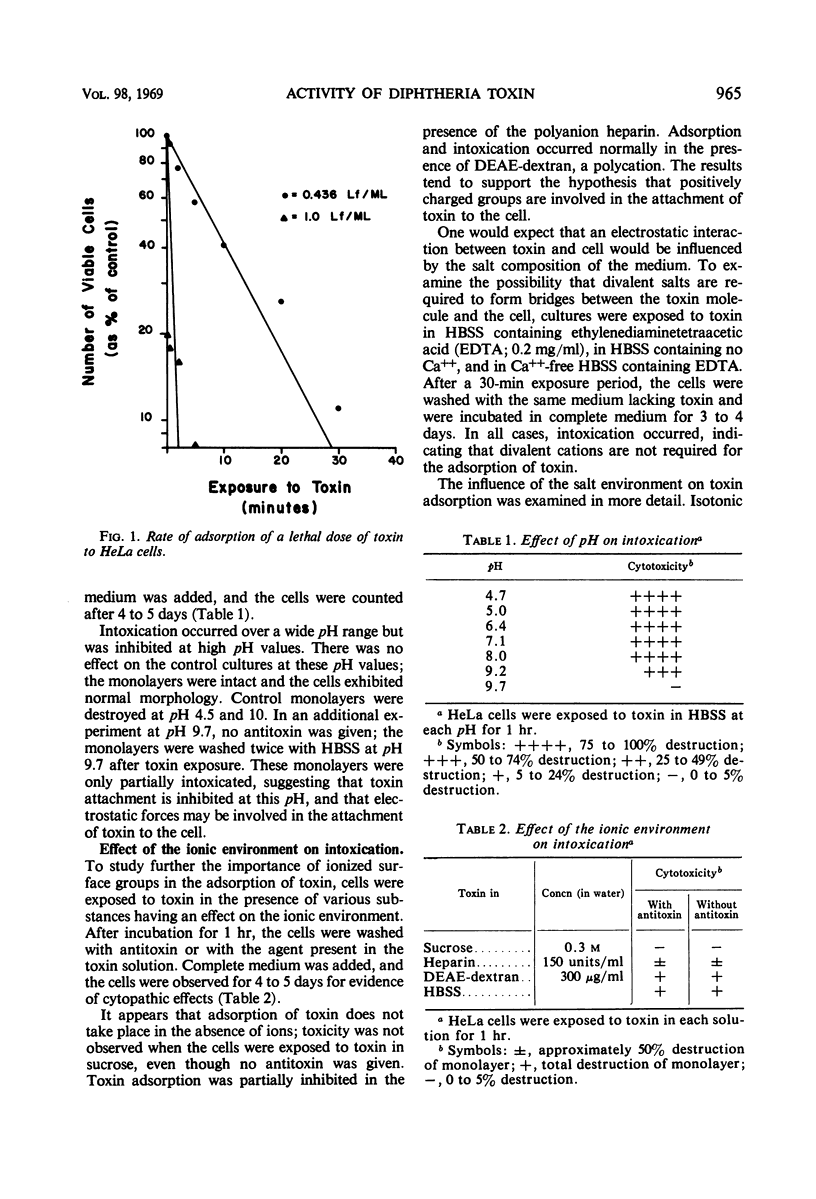
The initial steps in the interaction of diphtheria toxin with HeLa cells were studied. It was demonstrated that lethal doses of toxin are rapidly adsorbed to the cell. The kinetics of uptake, as measured by lethality, indicated that a single toxin molecule is able to cause cell death. Studies on the effect of pH on intoxication showed that adsorption of toxin occurred over a wide pH range but was partially inhibited at high pH values. Experiments to determine the influence of the ionic environment on intoxication indicated that adsorption of toxin did not take place in the absence of salts and was partially inhibited in the presence of a polyanion. The evidence indicates that the initial binding of toxin to the cell is electrostatic in nature, involving positively charged surface groups. Attempts to demonstrate specific receptors for the attachment of toxin to cells were unsuccessful, suggesting that toxin adsorption may be a nonspecific process. The effect of energy inhibitors on intoxication was examined. Sodium fluoride, an inhibitor of glycolysis, almost completely prevented intoxication in HeLa cells, whereas inhibitors of respiration and oxidative phosphorylation had no effect. Sodium fluoride did not prevent adsorption of toxin but appeared to inhibit a later step in the intoxication process, perhaps the transport of toxin to subsurface or intracellular levels.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COLLIER R. J., PAPPENHEIMER A. M., Jr STUDIES ON THE MODE OF ACTION OF DIPHTHERIA TOXIN. II. EFFECT OF TOXIN ON AMINO ACID INCORPORATION IN CELL-FREE SYSTEMS. J Exp Med. 1964 Dec 1;120:1019–1039. doi: 10.1084/jem.120.6.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn Z. A., Parks E. The regulation of pinocytosis in mouse macrophages. II. Factors inducing vesicle formation. J Exp Med. 1967 Feb 1;125(2):213–232. doi: 10.1084/jem.125.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier R. J. Effect of diphtheria toxin on protein synthesis: inactivation of one of the transfer factors. J Mol Biol. 1967 Apr 14;25(1):83–98. doi: 10.1016/0022-2836(67)90280-x. [DOI] [PubMed] [Google Scholar]
- Duncan J. L., Groman N. B. Studies of the activity of diphtheria toxin. I. Poliovirus replication in intoxicated HeLa cells. J Exp Med. 1967 Mar 1;125(3):489–500. doi: 10.1084/jem.125.3.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDBERG E. B., NITOWSKY H. M., COLOWICK S. P. THE ROLE OF GLYCOLYSIS IN THE GROWTH OF TUMOR CELLS. IV. THE BASIS OF GLUCOSE TOXICITY IN OXAMATE-TREATED, CULTURED CELLS. J Biol Chem. 1965 Jul;240:2791–2796. [PubMed] [Google Scholar]
- Gill D. M., Pappenheimer A. M., Jr, Brown R., Kurnick J. T. Studies on the mode of action of diphtheria toxin. VII. Toxin-stimulated hydrolysis of nicotinamide adenine dinucleotide in mammalian cell extracts. J Exp Med. 1969 Jan 1;129(1):1–21. doi: 10.1084/jem.129.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goor R. S., Pappenheimer A. M., Jr, Ames E. Studies on the mode of action of diphtheria toxin. V. Inhibition of peptide bond formation by toxin and NAD in cell-free systems and its reversal by nicotinamide. J Exp Med. 1967 Nov 1;126(5):923–939. doi: 10.1084/jem.126.5.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goor R. S., Pappenheimer A. M., Jr Studies on the mode of action of diphtheria toxin. 3. Site of toxin action in cell-free extracts. J Exp Med. 1967 Nov 1;126(5):899–912. doi: 10.1084/jem.126.5.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goor R. S., Pappenheimer A. M., Jr Studies on the mode of action of diphtheria toxin. IV. Specificity of the cofactor (NAD) requirement for toxin action in cell-free systems. J Exp Med. 1967 Nov 1;126(5):913–921. doi: 10.1084/jem.126.5.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLAND J. J., McLAREN L. C. The mammalian cell-virus relationship. II. Adsorption, reception, and eclipse of poliovirus by HeLa cells. J Exp Med. 1959 May 1;109(5):487–504. doi: 10.1084/jem.109.5.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honjo T., Nishizuka Y., Hayaishi O. Diphtheria toxin-dependent adenosine diphosphate ribosylation of aminoacyl transferase II and inhibition of protein synthesis. J Biol Chem. 1968 Jun 25;243(12):3553–3555. [PubMed] [Google Scholar]
- KARNOVSKY M. L. Metabolic basis of phagocytic activity. Physiol Rev. 1962 Jan;42:143–168. doi: 10.1152/physrev.1962.42.1.143. [DOI] [PubMed] [Google Scholar]
- Kim K., Groman N. B. In vitro inhibition of diphtheria toxin action by ammonium salts and amines. J Bacteriol. 1965 Dec;90(6):1552–1556. doi: 10.1128/jb.90.6.1552-1556.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K., Groman N. B. Mode of inhibition of diphtheria toxin by ammonium chloride. J Bacteriol. 1965 Dec;90(6):1557–1562. doi: 10.1128/jb.90.6.1557-1562.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moehring J. M., Moehring T. J. The response of cultured mammalian cells to diphtheria toxin. II. The resistant cell: enhancement of toxin action by poly-L-ornithine. J Exp Med. 1968 Mar 1;127(3):541–554. doi: 10.1084/jem.127.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moehring T. J., Moehring J. M. Response of cultured mammalian cells to diphtheria toxin. 3. Inhibition of protein synthesis studied at the subcellular level. J Bacteriol. 1968 Jul;96(1):61–69. doi: 10.1128/jb.96.1.61-69.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHILIPSON L. THE EARLY INTERACTION OF ANIMAL VIRUSES AND CELLS. Prog Med Virol. 1963;5:43–78. [PubMed] [Google Scholar]
- PLACIDO SOUSA C., EVANS D. G. The action of diphtheria toxin on tissue cultures and its neutralization by antitoxin. Br J Exp Pathol. 1957 Dec;38(6):644–649. [PMC free article] [PubMed] [Google Scholar]
- Pappenheimer A. M., Jr, Brown R. Studies on the mode of action of diphtheria toxin. VI. Site of the action of toxin in living cells. J Exp Med. 1968 Jun 1;127(6):1073–1086. doi: 10.1084/jem.127.6.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRAUSS N., HENDEE E. D. The effect of diphtheria toxin on the metabolism of HeLa cells. J Exp Med. 1959 Feb 1;109(2):145–163. doi: 10.1084/jem.109.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer W. I., Gabliks J., Calitis R. Interference by trypsin in the interaction of staphylococcal enterotoxin B and cell cultures of human embryonic intestine. J Bacteriol. 1967 May;93(5):1489–1492. doi: 10.1128/jb.93.5.1489-1492.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOLMACH L. J. Attachment and penetration of cells by viruses. Adv Virus Res. 1957;4:63–110. doi: 10.1016/s0065-3527(08)60596-5. [DOI] [PubMed] [Google Scholar]
- WU R. Regulatory mechanisms in carbohydrate metabolism. V. Limiting factors of glycolysis in HeLa cells. J Biol Chem. 1959 Nov;234:2806–2810. [PubMed] [Google Scholar]
- ZAJAC I., CROWELL R. L. EFFECT OF ENZYMES ON THE INTERACTION OF ENTEROVIRUSES WITH LIVING HELA CELLS. J Bacteriol. 1965 Mar;89:574–582. doi: 10.1128/jb.89.3.574-582.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]


