Abstract
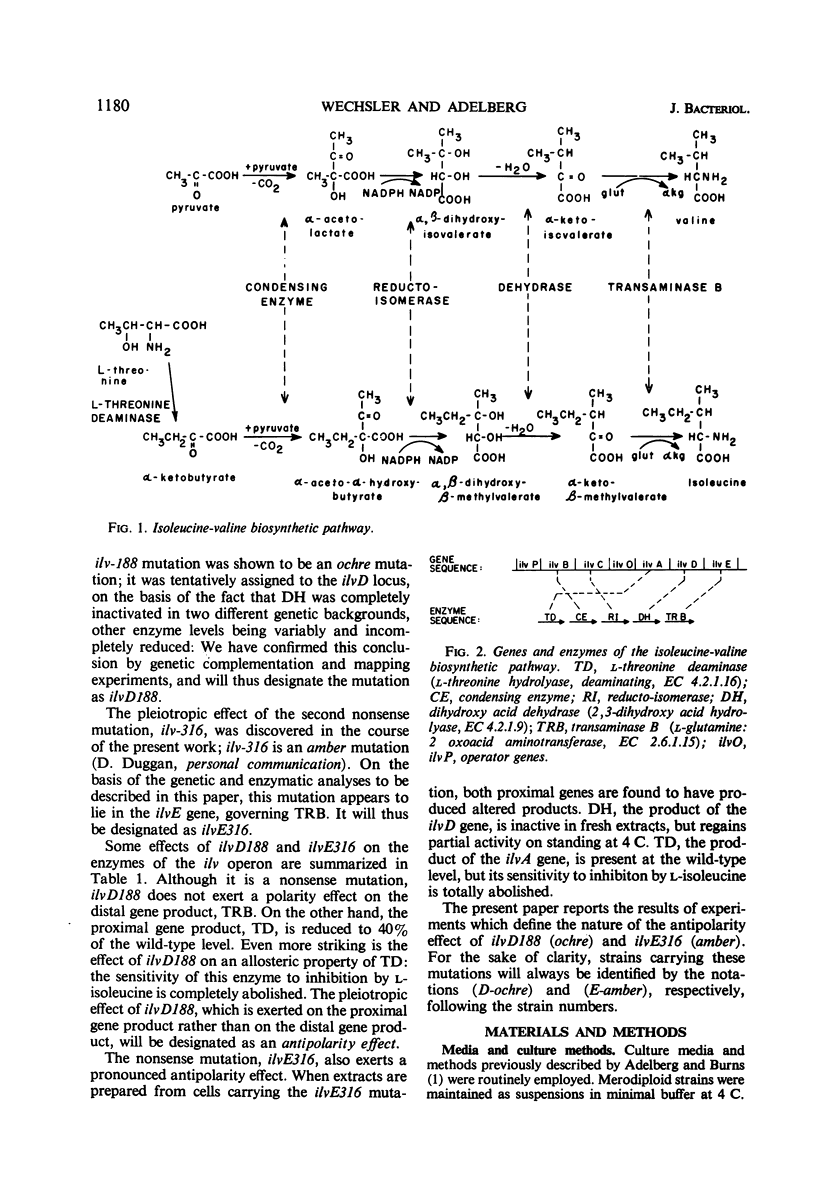
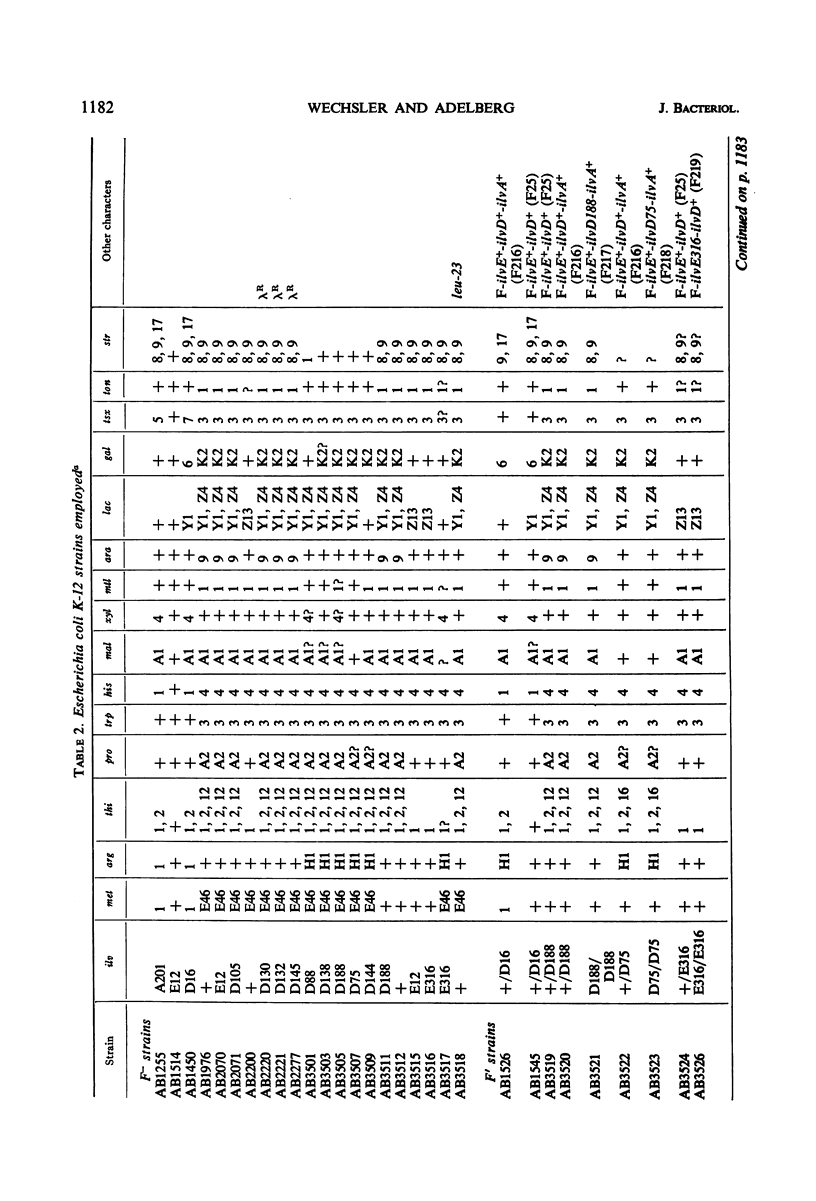
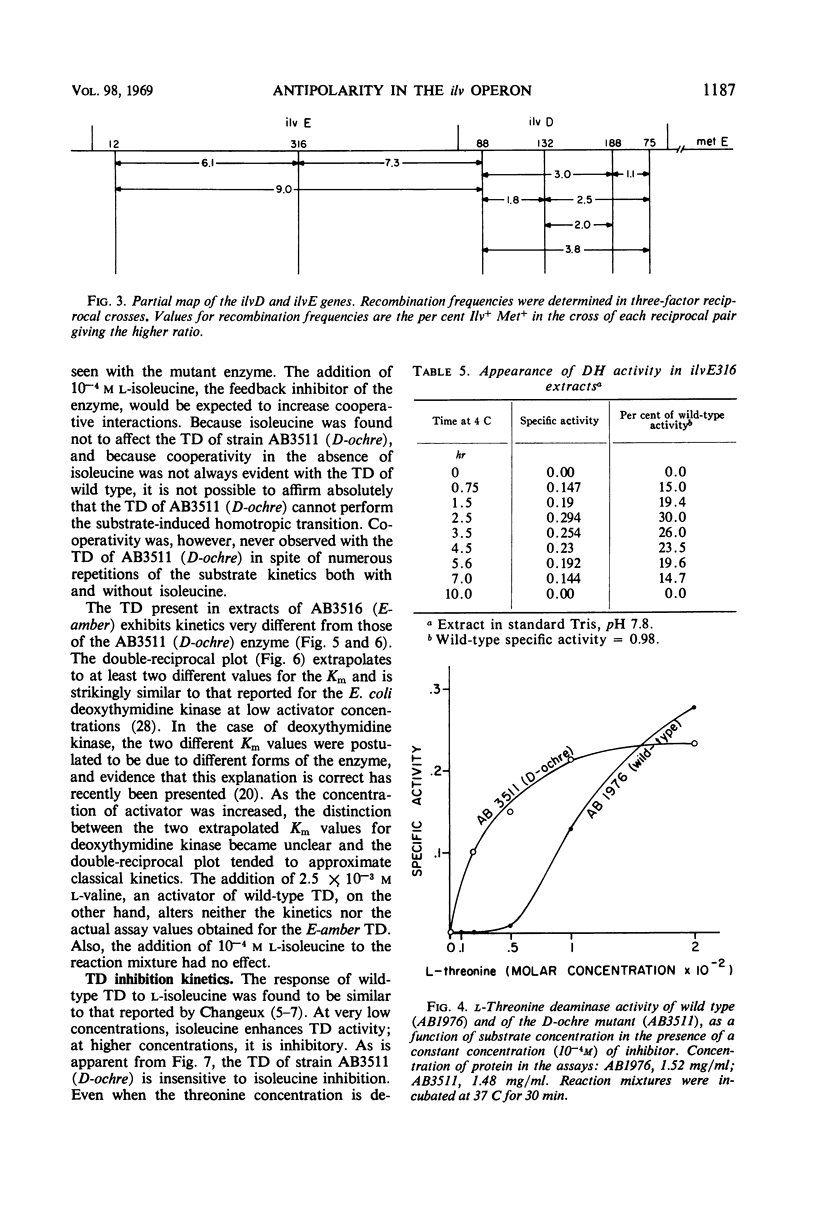
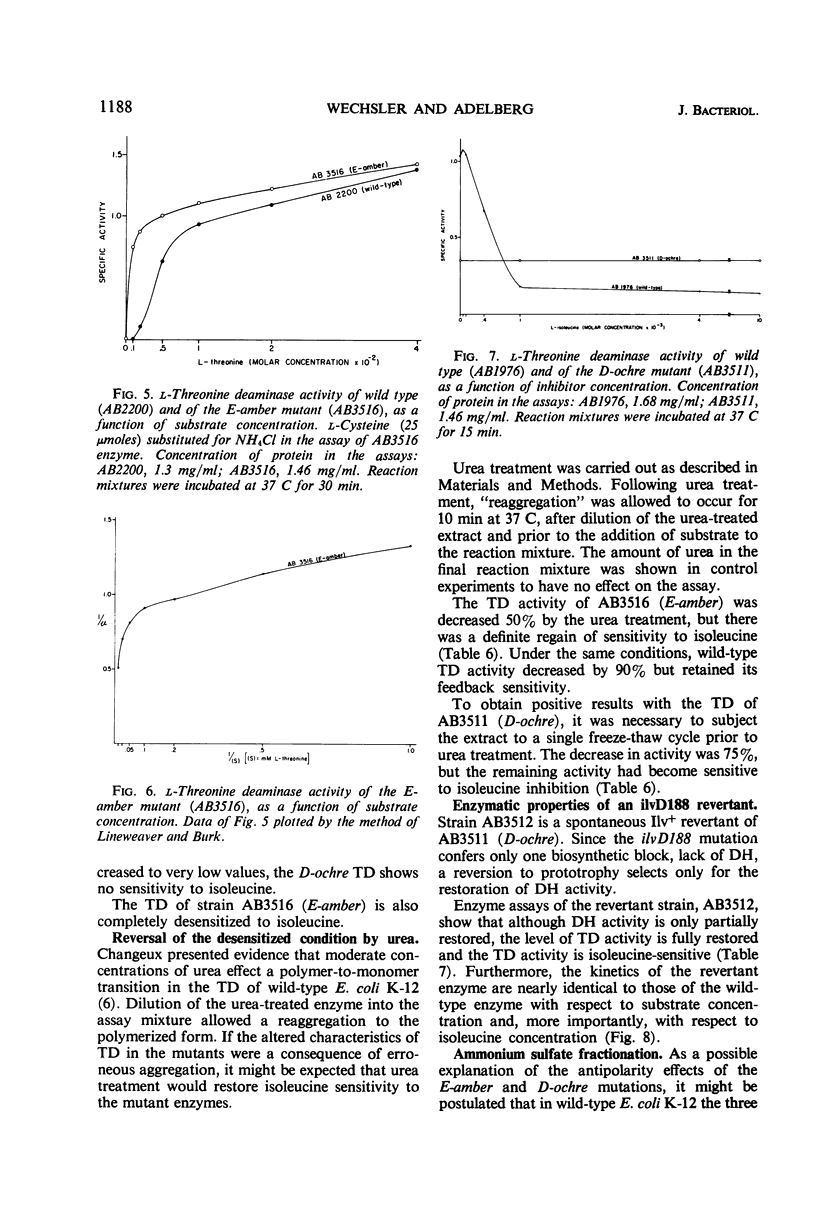
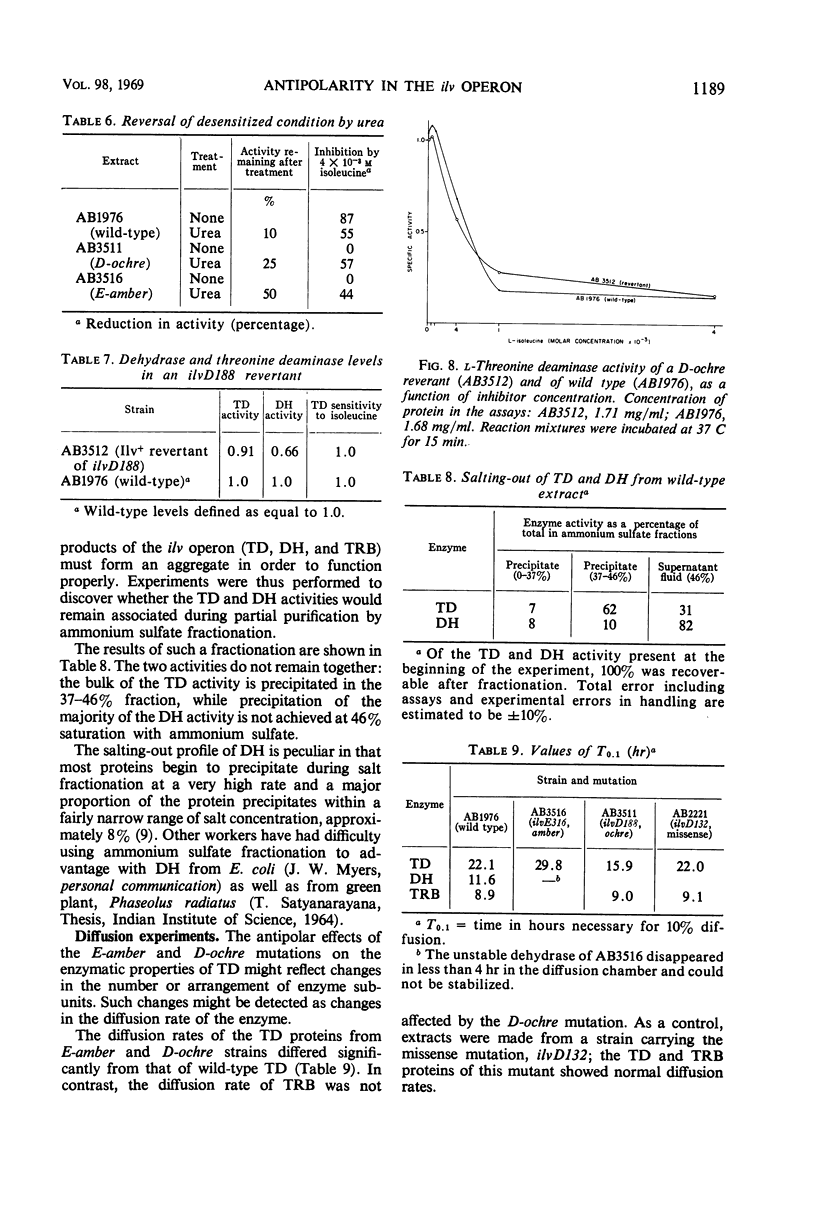
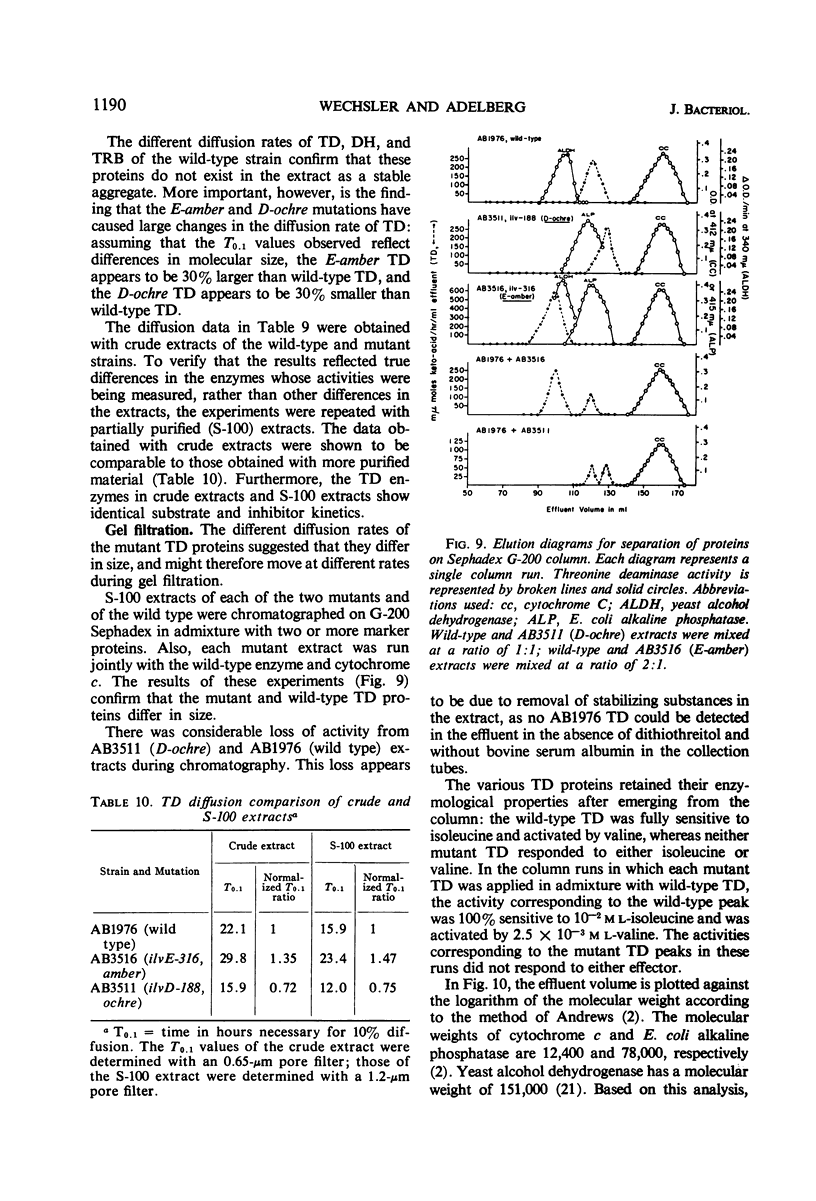
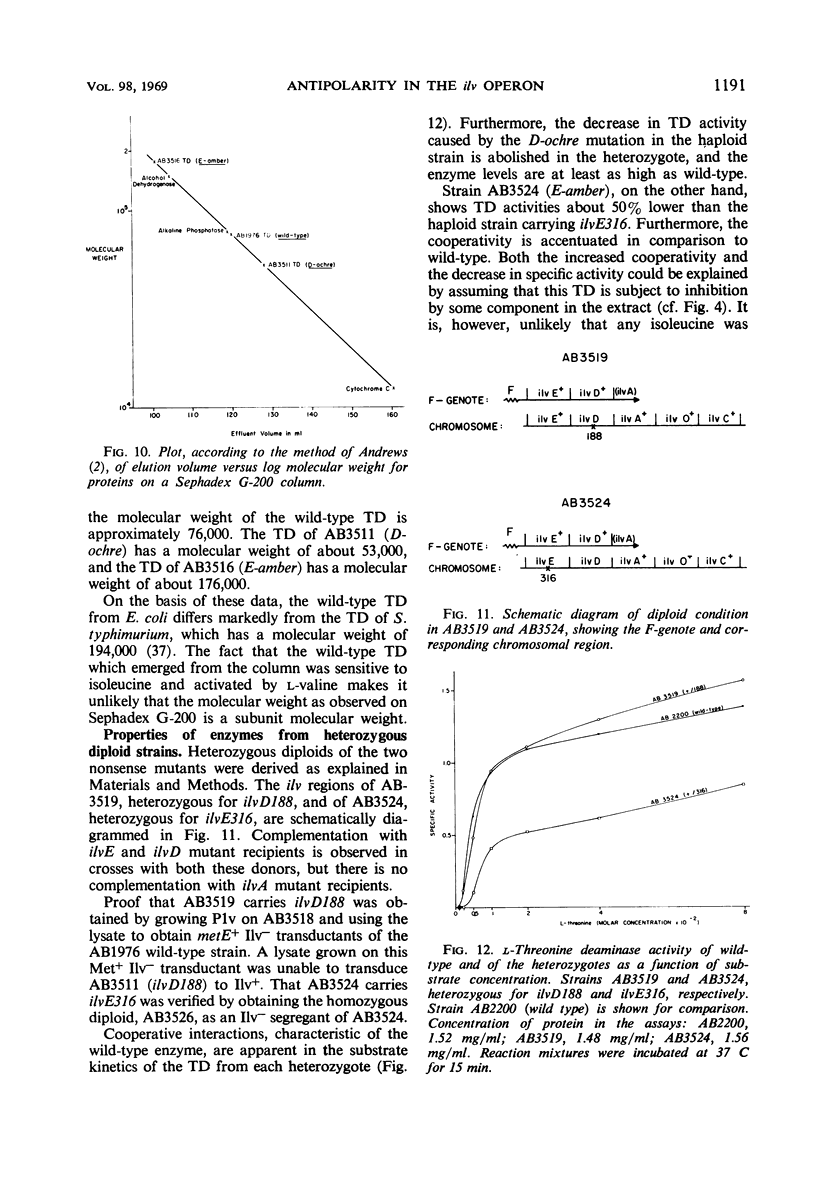
The genes governing three of the enzymes of the isoleucine-valine biosynthetic pathway form the operon: operator-ilvA-ilvD-ilvE. The enzymes are: ilvA, l-threonine deaminase; ilvD, dihydroxy acid dehydrase; and ilvE, transaminase B. A nonsense mutation in the ilvD gene (D-ochre) and a nonsense mutation in the ilvE gene (E-amber) affect the properties of the proximal gene product, l-threonine deaminase (TD), in addition to inactivating the enzymes produced by the genes in which the mutations have occurred. The D-ochre mutation causes TD to move in diffusion and gel filtration experiments as though it were 30% smaller than the wild-type enzyme. The E-amber mutation causes TD to move in similar experiments as though it were much larger than the wild-type enzyme. Both mutations completely abolish the sensitivity of TD to l-isoleucine, the normal feedback inhibitor of the wild-type enzyme. The effects of the nonsense mutations on TD can be reversed in three ways: by genetic reversion of the D-ochre mutation; by treatment of the altered enzymes with 3.0 m urea; and by forming a heterozygous diploid, containing the wild-type allele as well as the mutant allele of ilvD or ilvE. The results suggest that the subunits of TD undergo abnormal aggregation in the presence of the partial polypeptides produced by the mutant alleles of ilvD or ilvE; multi-enzyme aggregates in extracts of wild type, however, could not be detected.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADELBERG E. A., BURNS S. N. Genetic variation in the sex factor of Escherichia coli. J Bacteriol. 1960 Mar;79:321–330. doi: 10.1128/jb.79.3.321-330.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauerle R. H., Margolin P. A multifunctional enzyme complex in the tryptophan pathway of Salmonella typhimurium: comparison of polarity and pseudopolarity mutations. Cold Spring Harb Symp Quant Biol. 1966;31:203–214. doi: 10.1101/sqb.1966.031.01.028. [DOI] [PubMed] [Google Scholar]
- Burns R. O., Zarlengo M. H. Threonine deaminase from Salmonella typhimurium. I. Purification and properties. J Biol Chem. 1968 Jan 10;243(1):178–185. [PubMed] [Google Scholar]
- CHANGEUX J. P. The feedback control mechanisms of biosynthetic L-threonine deaminase by L-isoleucine. Cold Spring Harb Symp Quant Biol. 1961;26:313–318. doi: 10.1101/sqb.1961.026.01.037. [DOI] [PubMed] [Google Scholar]
- DIXON M., WEBB E. C. Enzyme fractionation by salting-out: a theoretical note. Adv Protein Chem. 1961;16:197–219. doi: 10.1016/s0065-3233(08)60030-3. [DOI] [PubMed] [Google Scholar]
- Demerec M., Adelberg E. A., Clark A. J., Hartman P. E. A proposal for a uniform nomenclature in bacterial genetics. Genetics. 1966 Jul;54(1):61–76. doi: 10.1093/genetics/54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggertsson G., Adelberg E. A. Map positions and specificities of suppressor mutations in Escherichia coli K-12. Genetics. 1965 Aug;52(2):319–340. doi: 10.1093/genetics/52.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREUNDLICH M., BURNS R. O., UMBARGER H. E. Control of isoleucine, valine, and leucine biosynthesis. I. Multivalent repression. Proc Natl Acad Sci U S A. 1962 Oct 15;48:1804–1808. doi: 10.1073/pnas.48.10.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUCHS E., GORIN G. Diffusion of protein molecules through membranes of controlled pore size. Biochem Biophys Res Commun. 1961 Jun 28;5:196–202. doi: 10.1016/0006-291x(61)90109-7. [DOI] [PubMed] [Google Scholar]
- Giles N. H., Case M. E., Partridge C. W., Ahmed S. I. A gene cluster in Nuerospora crassa coding for an aggregate of five aromatic synthetic enzymes. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1453–1460. doi: 10.1073/pnas.58.4.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer H., Cennamo C., Boll M. Product activation of yeast threonine dehydratase by ammonia. Biochem Biophys Res Commun. 1964;14:487–492. doi: 10.1016/0006-291x(64)90256-6. [DOI] [PubMed] [Google Scholar]
- Ikeda H., Tomizawa J. I. Transducing fragments in generalized transduction by phage P1. I. Molecular origin of the fragments. J Mol Biol. 1965 Nov;14(1):85–109. doi: 10.1016/s0022-2836(65)80232-7. [DOI] [PubMed] [Google Scholar]
- Iwatsuki N., Okazaki R. Mechanism of regulation of deoxythymidine kinase of Escherichia coli. I. Effect of regulatory deoxynucleotides on the state of aggregation of the enzyme. J Mol Biol. 1967 Oct 14;29(1):139–154. doi: 10.1016/0022-2836(67)90186-6. [DOI] [PubMed] [Google Scholar]
- KAGI J. H., VALLEE B. L. The role of zinc in alcohol dehydrogenase. V. The effect of metal-binding agents on thestructure of the yeast alcohol dehydrogenase molecule. J Biol Chem. 1960 Nov;235:3188–3192. [PubMed] [Google Scholar]
- KOIKE M., REED L. J., CARROLL W. R. alpha-Keto acid dehydrogenation complexes. IV. Resolution and reconstitution of the Escherichia coli pyruvate dehydrogenation complex. J Biol Chem. 1963 Jan;238:30–39. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- METZLER D. E., SNELL E. E. Deamination of serine. I. Catalytic deamination of serine and cysteine by pyridoxal and metal salts. J Biol Chem. 1952 Sep;198(1):353–361. [PubMed] [Google Scholar]
- MYERS J. W. Dihydroxy acid dehydrase: an enzyme involved in the biosynthesis of isoleucine and valine. J Biol Chem. 1961 May;236:1414–1418. [PubMed] [Google Scholar]
- NOVICK A., SZILARD L. Description of the chemostat. Science. 1950 Dec 15;112(2920):715–716. doi: 10.1126/science.112.2920.715. [DOI] [PubMed] [Google Scholar]
- Newton W. A., Beckwith J. R., Zipser D., Brenner S. Nonsense mutants and polarity in the lac operon of Escherichia coli. J Mol Biol. 1965 Nov;14(1):290–296. doi: 10.1016/s0022-2836(65)80250-9. [DOI] [PubMed] [Google Scholar]
- OKAZAKI R., KORNBERG A. DEOXYTHYMIDINE KINASE OF ESCHERICHIA COLI. II. KINETICS AND FEEDBACK CONTROL. J Biol Chem. 1964 Jan;239:275–284. [PubMed] [Google Scholar]
- RAMAKRISHNAN T., ADELBERG E. A. REGULATORY MECHANISMS IN THE BIOSYNTHESIS OF ISOLEUCINE AND VALINE. 3. MAP ORDER OF THE STRUCTURAL GENES AND OPERATOR GENES. J Bacteriol. 1965 Mar;89:661–664. doi: 10.1128/jb.89.3.661-664.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAMAKRISHNAN T., ADELBERG E. A. REGULATORY MECHANISMS IN THE BIOSYNTHESIS OF ISOLEUCINE AND VALINE. I. GENETIC DEREPRESSION OF ENZYME FORMATION. J Bacteriol. 1964 Mar;87:566–573. doi: 10.1128/jb.87.3.566-573.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAMAKRISHNAN T., ADELBERG E. A. REGULATORY MECHANISMS IN THE BIOSYNTHESIS OF ISOLEUCINE AND VALINE. II. IDENTIFICATION OF TWO OPERATOR GENES. J Bacteriol. 1965 Mar;89:654–660. doi: 10.1128/jb.89.3.654-660.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Revised linkage map of Escherichia coli. Bacteriol Rev. 1967 Dec;31(4):332–353. doi: 10.1128/br.31.4.332-353.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee B. L., Hoch F. L. ZINC, A COMPONENT OF YEAST ALCOHOL DEHYDROGENASE. Proc Natl Acad Sci U S A. 1955 Jun 15;41(6):327–338. doi: 10.1073/pnas.41.6.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAGNER R. P., BERGQUIST A. Synthesis of valine and isoleucine in the presence of a particulate cell fraction of Neurospora. Proc Natl Acad Sci U S A. 1963 Jun;49:892–897. doi: 10.1073/pnas.49.6.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANOFSKY C. The tryptophan synthetase system. Bacteriol Rev. 1960 Jun;24(2):221–245. doi: 10.1128/br.24.2.221-245.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarlengo M. H., Robinson G. W., Burns R. O. Threonine deaminase from Salmonella typhimurium. II. The subunit structure. J Biol Chem. 1968 Jan 10;243(1):186–191. [PubMed] [Google Scholar]


