Abstract
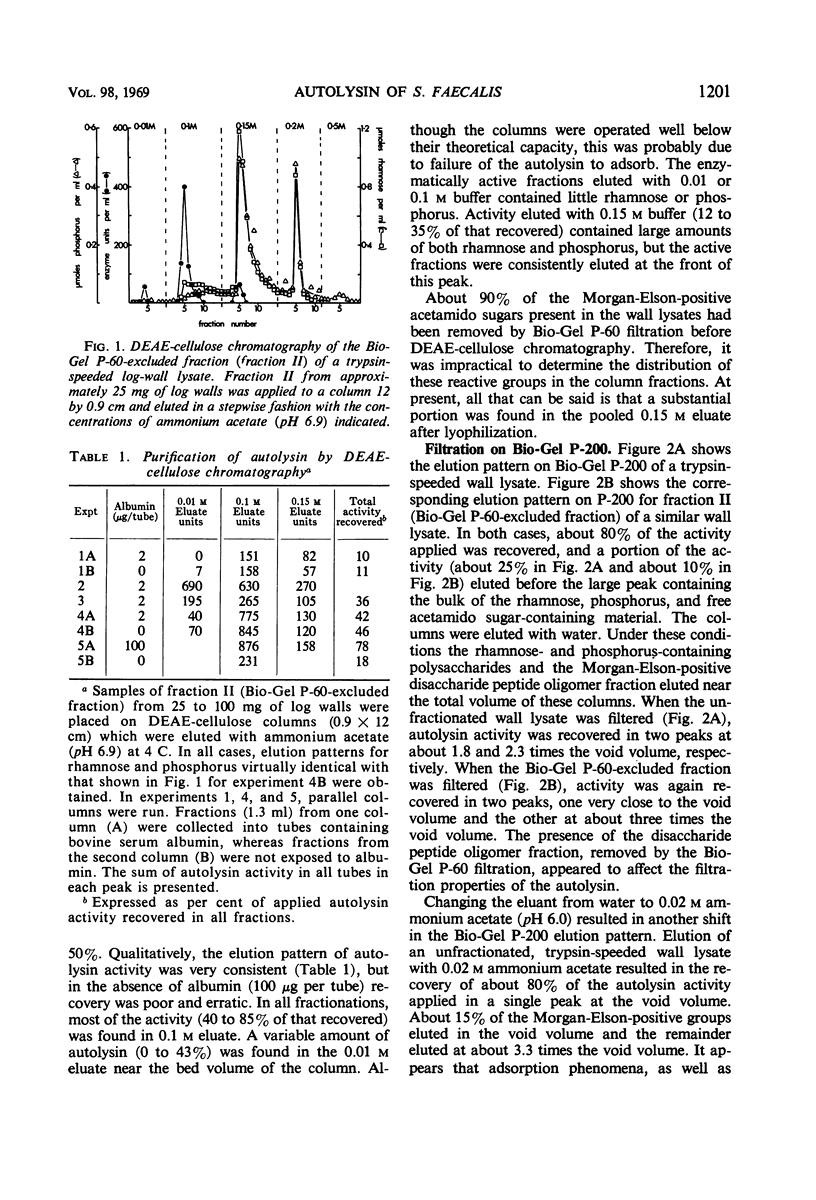
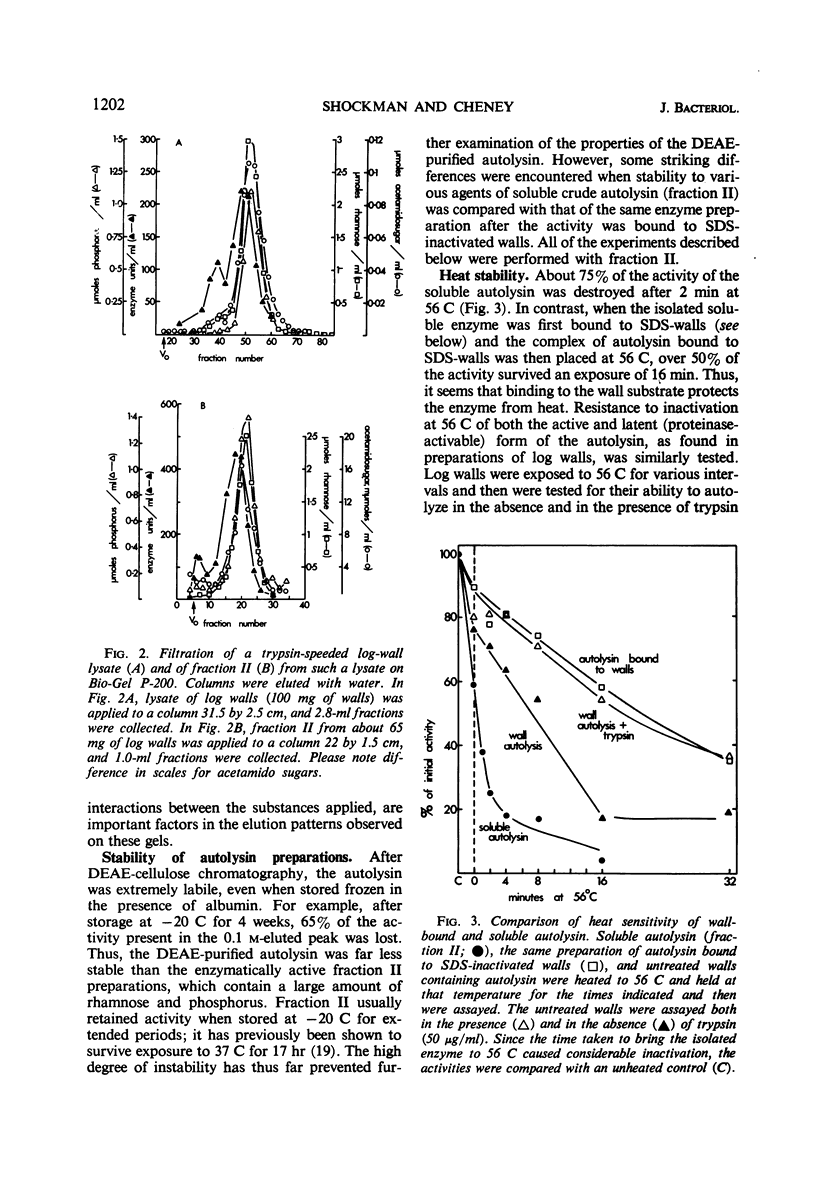
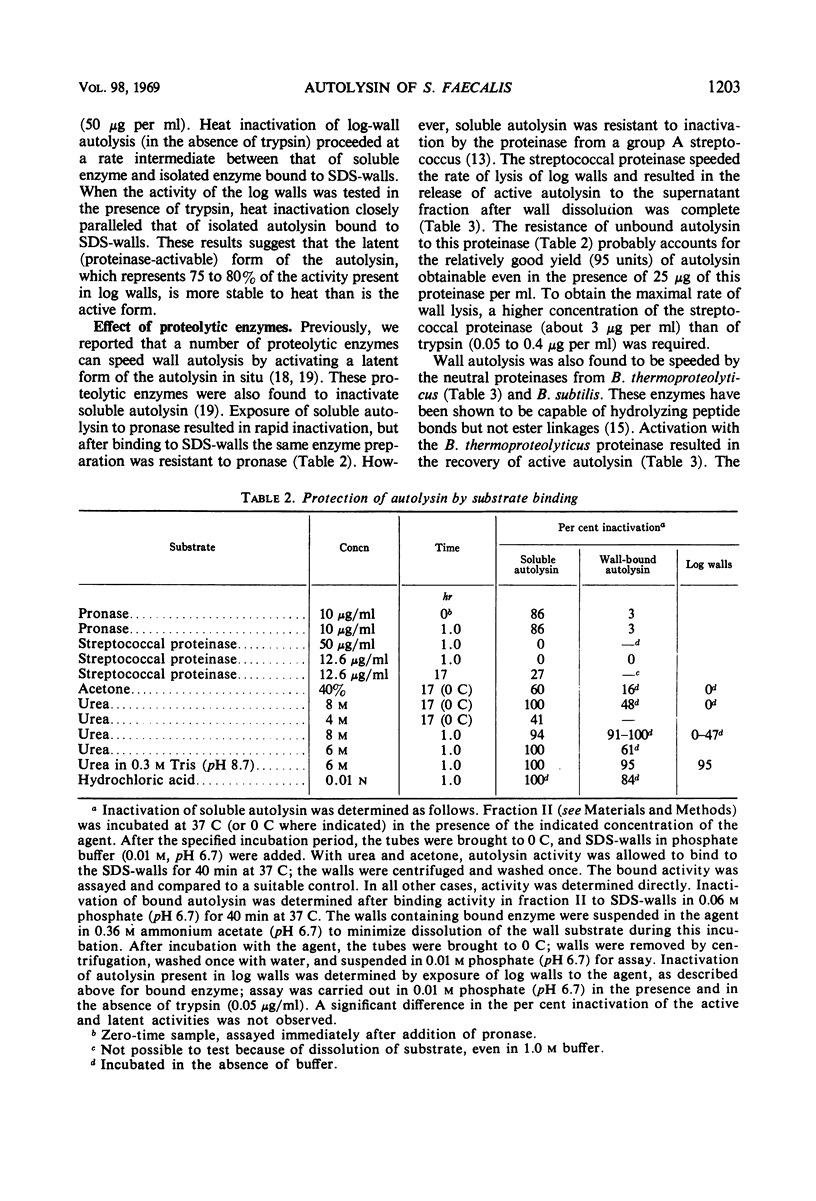
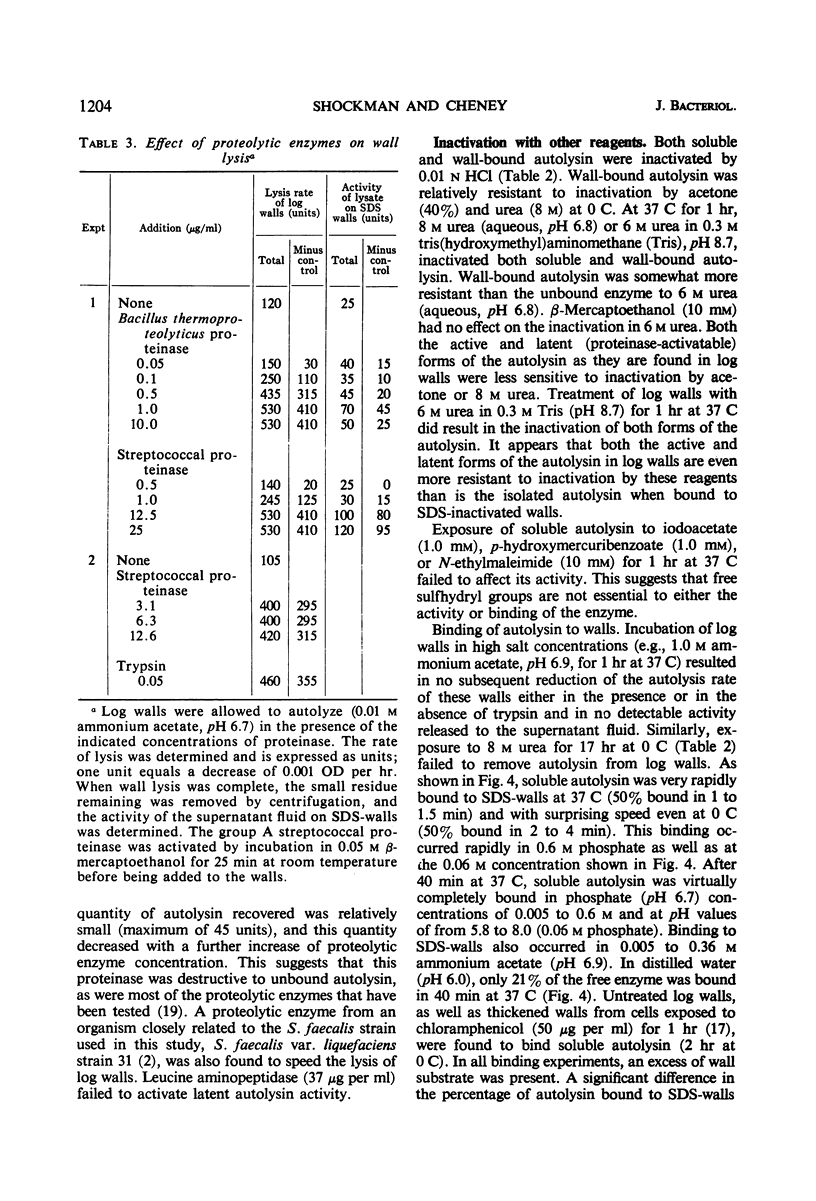
Cell walls from exponential-phase cultures of Streptococcus faecalis ATCC 9790 contain an autolysin (a β-N-acetylmuramide glycanhydrolase, E.C. 3.2.1.17) which has been isolated from trypsin-speeded wall autolysates. The autolysin, which was excluded from Bio-Gel P-60, was further fractionated by diethylaminoethyl (DEAE)-cellulose chromatography or filtration on Bio-Gel P-200. After DEAE-cellulose chromatography, which removed most of the wall polysaccharide, autolysin activity was extremely labile and was rapidly lost at −20 C, even in the presence of albumin. The P-60-excluded enzyme was rapidly bound by walls at both 37 C (50% bound in about 1 min) and 0 C (50% bound in less than 4 min). Wall-bound autolysin could not be removed by 1.0 m ammonium acetate (pH 6.9). Autolysin was also bound by walls that had been extracted with 10% trichloroacetic acid or treated with 0.01 n periodate, suggesting that the nonpeptidoglycan wall polymers are not important for binding. Wall-bound autolysin was more stable than the soluble enzyme to proteinase digestion, acetone (40%), 8 m urea (at 0 C), or to inactivation at 56 C. Two bacterial neutral proteinases (which do not hydrolyze ester bonds) activated latent wall-bound autolysin, suggesting that activation results from the cleavage of one or more peptide bonds. The group A streptococcal proteinase activated latent autolysin but differed from the other proteinases in that it did not inactivate soluble autolysin. The results suggest that the autolysin is not covalently linked to the wall. The high affinity of the walls for the autolysin appears to be responsible for the firm, not easily reversed binding.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLEIWEIS A. S., ZIMMERMAN L. N. PROPERTIES OF PROTEINASE FROM STREPTOCOCCUS FAECALIS VAR. LIQUEFACIENS. J Bacteriol. 1964 Sep;88:653–659. doi: 10.1128/jb.88.3.653-659.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleiweis A. S., Young F. E., Krause R. M. Cell walls of group D streptococci. II. Chemical studies on the type 1 antigen purified from the autolytic digest of cell walls. J Bacteriol. 1967 Nov;94(5):1381–1387. doi: 10.1128/jb.94.5.1381-1387.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cann J. R., Goad W. B. The theory of transport of interacting systems of biological macromolecules. Adv Enzymol Relat Areas Mol Biol. 1968;30:139–177. doi: 10.1002/9780470122754.ch3. [DOI] [PubMed] [Google Scholar]
- Dianoux A. C., Jollès P. Etude d'un lysozyme pauvre en cystine et en tryptophane: le lysozyme de blanc d'oeuf d'oie. Biochim Biophys Acta. 1967 Apr 11;133(3):472–479. [PubMed] [Google Scholar]
- Ghuysen J. M., Bricas E., Leyh-Bouille M., Lache M., Shockman G. D. The peptide N alpha-(L-alanyl-D-isoglutaminyl)-N epsilon-(D-isoasparaginyl)-L-lysyl-D-alanine and the disaccharide N-acetylglucosaminyl-beta-1,4-N-acetylmuramic acid in cell wall peptidoglycan of Streptococcus faecalis strain ATCC 9790. Biochemistry. 1967 Aug;6(8):2607–2619. doi: 10.1021/bi00860a044. [DOI] [PubMed] [Google Scholar]
- Ghuysen J. M. Use of bacteriolytic enzymes in determination of wall structure and their role in cell metabolism. Bacteriol Rev. 1968 Dec;32(4 Pt 2):425–464. [PMC free article] [PubMed] [Google Scholar]
- Jollès P. Rapports entre la structure et l'activité de quelques lysozymes. Bull Soc Chim Biol (Paris) 1967;49(8):1001–1012. [PubMed] [Google Scholar]
- Jollès P. Relationship between chemical structure and biological activity of hen egg-white lysozyme and lysozymes of different species. Proc R Soc Lond B Biol Sci. 1967 Apr 18;167(1009):350–364. doi: 10.1098/rspb.1967.0033. [DOI] [PubMed] [Google Scholar]
- Jollès P., Saint Blancard J., Charlemagne D., Dianoux A. C., Jollès J., Le Baron J. L. Comparative behavior of six different lysozymes in the presence of an inhibitor. Biochim Biophys Acta. 1968 Feb 5;151(2):532–534. doi: 10.1016/0005-2744(68)90123-x. [DOI] [PubMed] [Google Scholar]
- LIU T. Y., ELLIOTT S. D. STREPTOCOCCAL PROTEINASE: THE ZYMOGEN TO ENZYME TRANSFROMATION. J Biol Chem. 1965 Mar;240:1138–1142. [PubMed] [Google Scholar]
- LOWRY O. H., ROBERTS N. R., LEINER K. Y., WU M. L., FARR A. L. The quantitative histochemistry of brain. I. Chemical methods. J Biol Chem. 1954 Mar;207(1):1–17. [PubMed] [Google Scholar]
- Morihara K. The specificities of various neutral and alkaline proteinases from microorganisms. Biochem Biophys Res Commun. 1967 Mar 21;26(6):656–661. doi: 10.1016/s0006-291x(67)80122-0. [DOI] [PubMed] [Google Scholar]
- Racker E. Resolution and reconstitution of the inner mitochondrial membrane. Fed Proc. 1967 Sep;26(5):1335–1340. [PubMed] [Google Scholar]
- Shockman G. D., Pooley H. M., Thompson J. S. Autolytic enzyme system of Streptococcus faecalis. 3. Localization of the autolysin at the sites of cell wall synthesis. J Bacteriol. 1967 Nov;94(5):1525–1530. doi: 10.1128/jb.94.5.1525-1530.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D. Symposium on the fine structure and replication of bacteria and their parts. IV. Unbalanced cell-wall synthesis: autolysis and cell-wall thickening. Bacteriol Rev. 1965 Sep;29(3):345–358. doi: 10.1128/br.29.3.345-358.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D., Thompson J. S., Conover M. J. The autolytic enzyme system of Streptococcus faecalis. II. Partial characterization of the autolysin and its substrate. Biochemistry. 1967 Apr;6(4):1054–1065. doi: 10.1021/bi00856a014. [DOI] [PubMed] [Google Scholar]
- TOENNIES G., BAKAY B., SHOCKMAN G. D. Bacterial composition and growth phase. J Biol Chem. 1959 Dec;234:3269–3275. [PubMed] [Google Scholar]
- Young F. E. Fractionation and partial characterization of the products of autolysis of cell walls of Bacillus subtilis. J Bacteriol. 1966 Oct;92(4):839–846. doi: 10.1128/jb.92.4.839-846.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]


