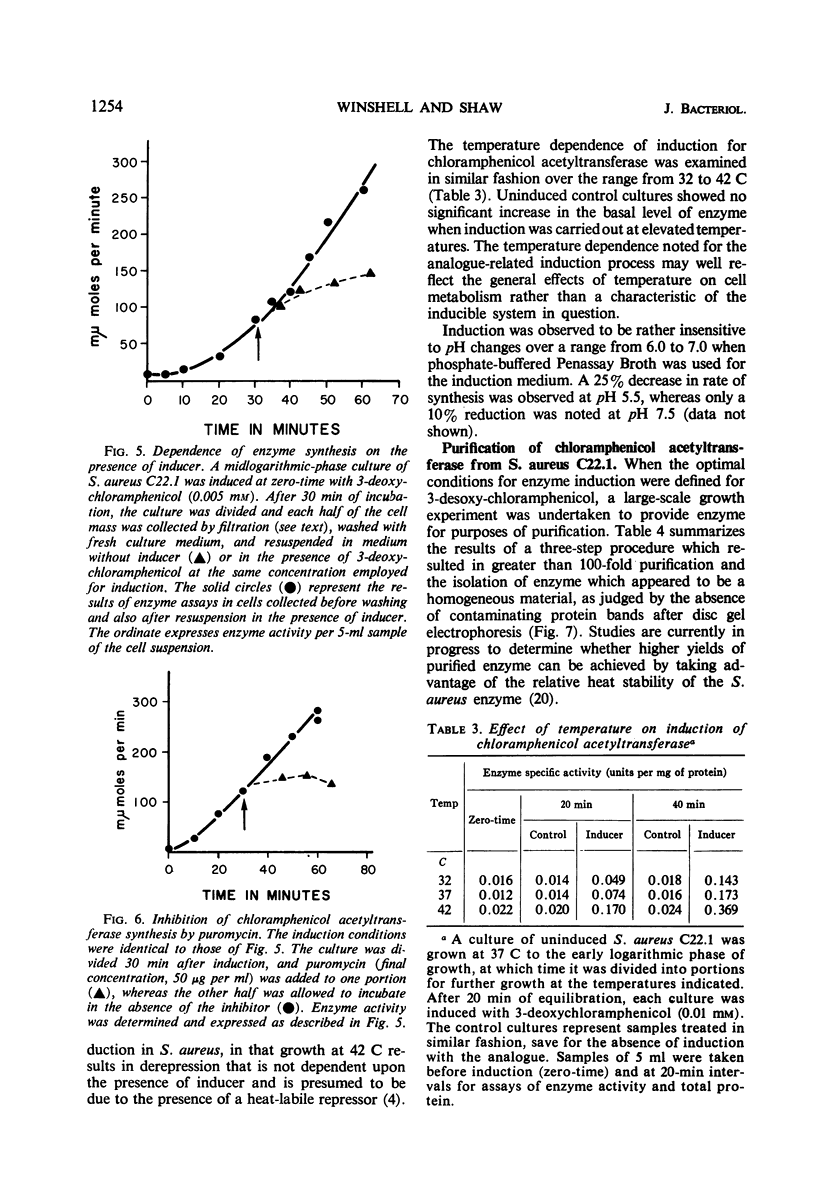
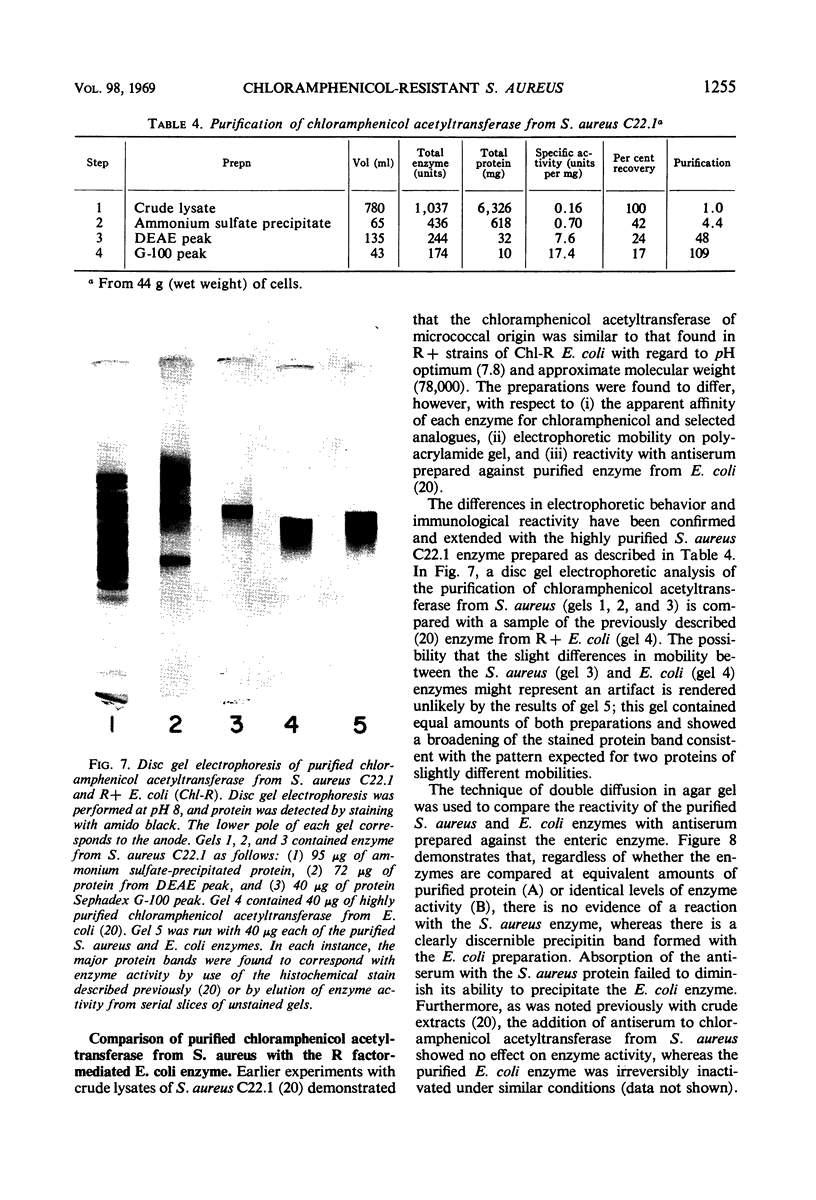
Abstract
Plasmid-mediated chloramphenicol resistance in Staphylococcus aureus has been shown to involve acetylation of chloramphenicol by an enzyme induced by growth in the presence of the antibiotic and certain analogues. Analysis of the kinetics of induction has been complicated by (i) the intrinsic inhibitory effects of chloramphenicol on induced enzyme synthesis and (ii) the rapid disappearance of inducer after synthesis of the acetylating enzyme. The compound related to d-threo chloramphenicol which lacks a C3 hydroxyl substituent (3-deoxychloramphenicol) is a potent inducer of chloramphenicol acetyltransferase but is ineffective as an antibiotic and is not a substrate for the enzyme. The availability of such a “gratuitous” inducer has simplified an analysis of the kinetics of induction of chloramphenicol acetyltransferase. The enzyme from induced bacteria has been purified to homogeneity and has been compared with the analogous enzyme present in E. coli which harbors a resistance transfer factor with the chloramphenicol resistance determinant.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALPERS D. H., TOMKINS G. M. THE ORDER OF INDUCTION AND DEINDUCTION OF THE ENZYMES OF THE LACTOSE OPERON IN E. COLI. Proc Natl Acad Sci U S A. 1965 Apr;53:797–802. doi: 10.1073/pnas.53.4.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock T. D. CHLORAMPHENICOL. Bacteriol Rev. 1961 Mar;25(1):32–48. doi: 10.1128/br.25.1.32-48.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHABBERT Y. A., BAUDENS J. G., GERBAUD G. R. VARIATIONS SOUS L'INFLUENCE DE L'ACRIFLAVINE ET TRANSDUCTION DE LA R'ESISTANCE A LA KANAMYCINE ET AU CHLORAMPH'ENICOL CHEZ LES STAPHYLOCOQUES. Ann Inst Pasteur (Paris) 1964 Nov;107:678–690. [PubMed] [Google Scholar]
- COHEN S., SWEENEY H., LEITNER F. TEMPERATURE-SENSITIVE REPRESSION OF STAPHYLOCOCCAL PENICILLINASE. Science. 1965 Aug 20;149(3686):877–879. doi: 10.1126/science.149.3686.877. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- JACOB F., MONOD J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 1961 Jun;3:318–356. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- Kono M., Ogawa K., Mitsuhashi S. Drug resistance of staphylococci. VI. Genetic determinant for chloramphenicol resistance. J Bacteriol. 1968 Mar;95(3):886–892. doi: 10.1128/jb.95.3.886-892.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MIYAMURA S. INACTIVATION OF CHLORAMPHENICOL BY CHLORAMPHENICOL-RESISTANT BACTERIA. J Pharm Sci. 1964 Jun;53:604–607. doi: 10.1002/jps.2600530606. [DOI] [PubMed] [Google Scholar]
- NATHANS D. INHIBITION OF PROTEIN SYNTHESIS BY PUROMYCIN. Fed Proc. 1964 Sep-Oct;23:984–989. [PubMed] [Google Scholar]
- NOVICK R. P., RICHMOND M. H. NATURE AND INTERACTIONS OF THE GENETIC ELEMENTS GOVERNING PENICILLINASE SYNTHESIS IN STAPHYLOCOCCUS AUREUS. J Bacteriol. 1965 Aug;90:467–480. doi: 10.1128/jb.90.2.467-480.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P. Penicillinase plasmids of Staphylococcus aureus. Fed Proc. 1967 Jan-Feb;26(1):29–38. [PubMed] [Google Scholar]
- Okamoto S., Suzuki Y., Mise K., Nakaya R. Occurrence of chloramphenicol-acetylating enzymes in various gram-negative bacilli. J Bacteriol. 1967 Nov;94(5):1616–1622. doi: 10.1128/jb.94.5.1616-1622.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabath L. D., Gerstein D. A., Loder P. B., Finland M. Independent segregation of chloramphenicol resistance in Staphylococcus aureus. Antimicrob Agents Chemother (Bethesda) 1967;7:264–270. [PubMed] [Google Scholar]
- Shaw W. V., Brodsky R. F. Characterization of chloramphenicol acetyltransferase from chloramphenicol-resistant Staphylococcus aureus. J Bacteriol. 1968 Jan;95(1):28–36. doi: 10.1128/jb.95.1.28-36.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw W. V., Brodsky R. F. Chloramphenicol resistance by enzymatic acetylation: comparative aspects. Antimicrob Agents Chemother (Bethesda) 1967;7:257–263. [PubMed] [Google Scholar]
- Shaw W. V. Enzymatic chlorampheicol acetylation and R factor induced antibiotic resistance in Enterobacteriaceae. Antimicrob Agents Chemother (Bethesda) 1966;6:221–226. [PubMed] [Google Scholar]
- Shaw W. V. The enzymatic acetylation of chloramphenicol by extracts of R factor-resistant Escherichia coli. J Biol Chem. 1967 Feb 25;242(4):687–693. [PubMed] [Google Scholar]
- Suzuki Y., Okamoto S., Kono M. Basis of chloramphenicol resistance in naturally isolated resistant staphylococci. J Bacteriol. 1966 Sep;92(3):798–799. doi: 10.1128/jb.92.3.798-799.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y., Okamoto S. The enzymatic acetylation of chloramphenicol by the multiple drug-resistant Escherichia coli carrying R factor. J Biol Chem. 1967 Oct 25;242(20):4722–4730. [PubMed] [Google Scholar]