Abstract
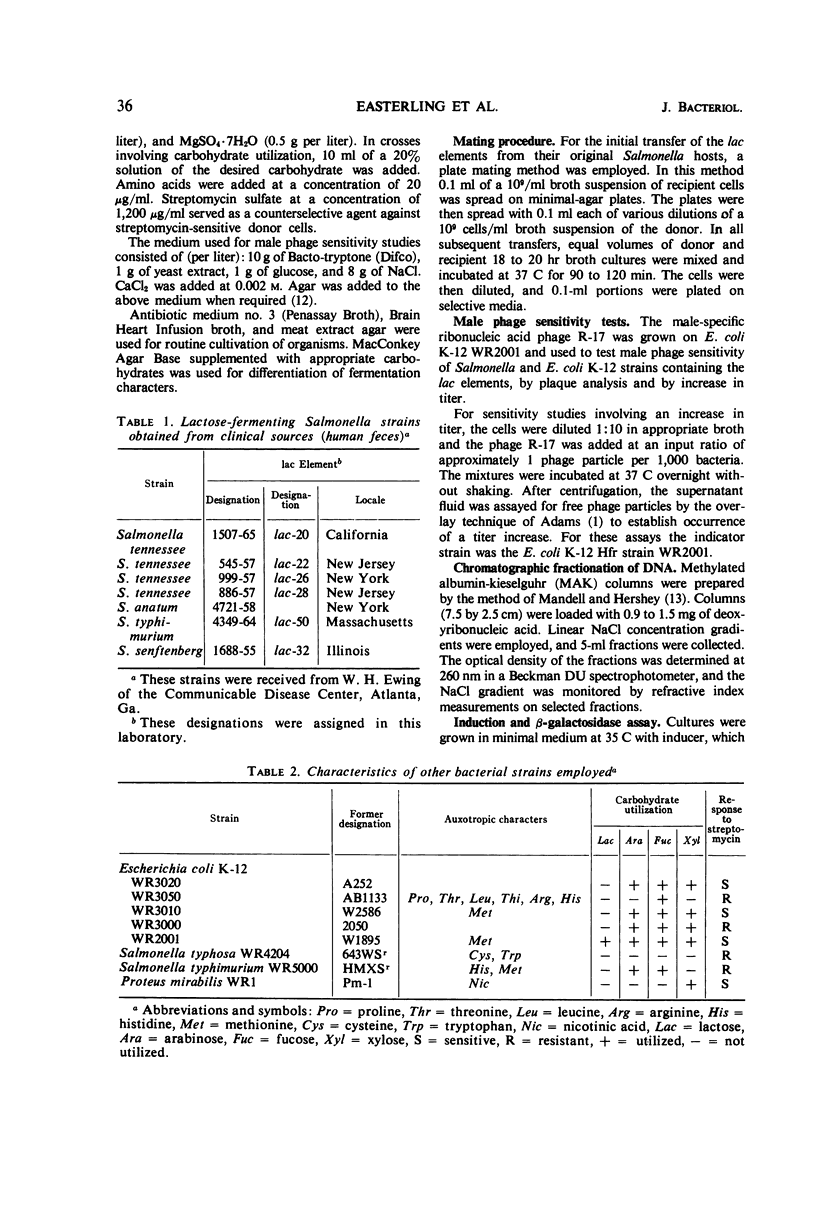
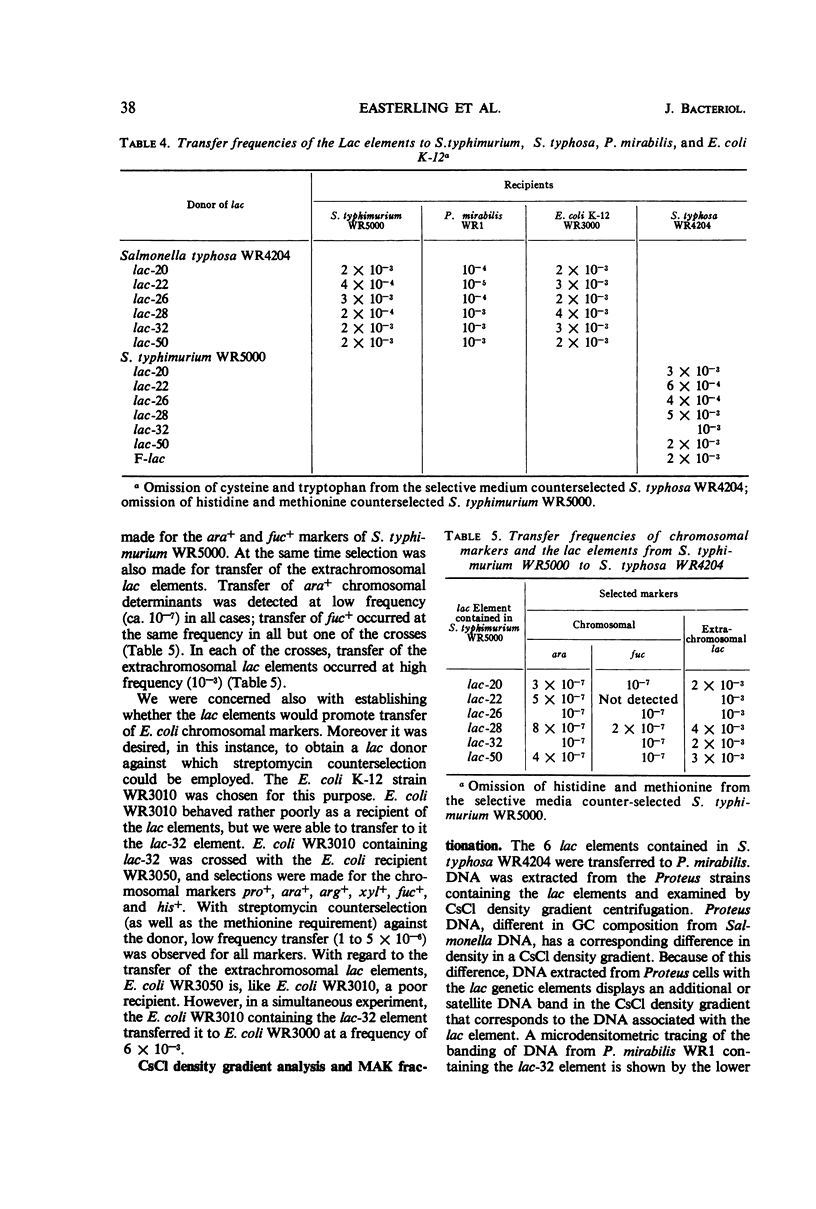
Six of seven lactose-fermenting (lac+) Salmonella strains obtained from clinical sources were found to be capable of transferring the lac+ property by conjugation to Salmonella typhosa WR4204. All of the six S. typhosa strains which received the lac+ property transferred it in turn to S. typhimurium WR5000 at the high frequencies typical of extrachromosomal F-merogenotes. These six lac elements were also transmissible from S. typhosa WR4204 to Proteus mirabilis and to some strains of Escherichia coli K-12; moreover, they were capable of promoting low frequency transfer of chromosomal genes from S. typhimurium WR5000 to S. typhosa WR4204. One of these lac elements was shown also to be capable of promoting low frequency chromosome transfer in E. coli K-12. E. coli K-12 strains harboring these lac elements exhibited sensitivity to the male specific phage R-17. Sensitivity to R-17 was not detected in Salmonella strains containing the elements. Examination of the lac elements in P. mirabilis by cesium chloride density gradient centrifugation showed that each element had a guanine plus cytosine content of 50%. The sizes of the elements varied from 0.8 to 3% of the total Proteus deoxyribonucleic acid. The amount of β-galactosidase produced by induced and uninduced cultures of S. typhimurium WR5000 and S. typhosa WR4204 containing the lac elements was lower than that produced by these strains with the F-lac episome. The heat sensitivity of β-galactosidase produced by the lac elements in their original Salmonella hosts indicated that the enzyme made by these strains differs from E. coli β-galactosidase.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADELBERG E. A., BURNS S. N. Genetic variation in the sex factor of Escherichia coli. J Bacteriol. 1960 Mar;79:321–330. doi: 10.1128/jb.79.3.321-330.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRINTON C. C., Jr, GEMSKI P., Jr, CARNAHAN J. A NEW TYPE OF BACTERIAL PILUS GENETICALLY CONTROLLED BY THE FERTILITY FACTOR OF E. COLI K 12 AND ITS ROLE IN CHROMOSOME TRANSFER. Proc Natl Acad Sci U S A. 1964 Sep;52:776–783. doi: 10.1073/pnas.52.3.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron L. S., Carey W. F., Spilman W. M. CHARACTERISTICS OF A HIGH FREQUENCY OF RECOMBINATION (HFR) STRAIN OF SALMONELLA TYPHOSA COMPATIBLE WITH SALMONELLA, SHIGELLA, AND ESCHERICHIA SPECIES. Proc Natl Acad Sci U S A. 1959 Dec;45(12):1752–1757. doi: 10.1073/pnas.45.12.1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton C. C., Jr The structure, function, synthesis and genetic control of bacterial pili and a molecular model for DNA and RNA transport in gram negative bacteria. Trans N Y Acad Sci. 1965 Jun;27(8):1003–1054. doi: 10.1111/j.2164-0947.1965.tb02342.x. [DOI] [PubMed] [Google Scholar]
- Clowes R. C., Moody E. E. Chromosomal transfer from "recombination-deficient" strains of Escherichia coli K-12. Genetics. 1966 Apr;53(4):717–726. doi: 10.1093/genetics/53.4.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FALKOW S., CITARELLA R. V. MOLECULAR HOMOLOGY OF F-MEROGENOTE DNA. J Mol Biol. 1965 May;12:138–151. doi: 10.1016/s0022-2836(65)80288-1. [DOI] [PubMed] [Google Scholar]
- FALKOW S., WOHLHIETER J. A., CITARELLA R. V., BARON L. S. TRANSFER OF EPISOMIC ELEMENTS TO PROTEUS. I. TRANSFER OF F-LINKED CHROMOSOMAL DETERMINANTS. J Bacteriol. 1964 Jan;87:209–219. doi: 10.1128/jb.87.1.209-219.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkow S., Baron L. S. EPISOMIC ELEMENT IN A STRAIN OF SALMONELLA TYPHOSA. J Bacteriol. 1962 Sep;84(3):581–589. doi: 10.1128/jb.84.3.581-589.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freifelder D. R., Freifelder D. Studies on Escherichia coli sex factors. II. Some physical properties of F'Lac and F DNA. J Mol Biol. 1968 Feb 28;32(1):25–35. doi: 10.1016/0022-2836(68)90142-3. [DOI] [PubMed] [Google Scholar]
- LEDERBERG J. The beta-d-galactosidase of Escherichia coli, strain K-12. J Bacteriol. 1950 Oct;60(4):381–392. doi: 10.1128/jb.60.4.381-392.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOEB T., ZINDER N. D. A bacteriophage containing RNA. Proc Natl Acad Sci U S A. 1961 Mar 15;47:282–289. doi: 10.1073/pnas.47.3.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANDELL J. D., HERSHEY A. D. A fractionating column for analysis of nucleic acids. Anal Biochem. 1960 Jun;1:66–77. doi: 10.1016/0003-2697(60)90020-8. [DOI] [PubMed] [Google Scholar]
- Meselson M., Stahl F. W., Vinograd J. EQUILIBRIUM SEDIMENTATION OF MACROMOLECULES IN DENSITY GRADIENTS. Proc Natl Acad Sci U S A. 1957 Jul 15;43(7):581–588. doi: 10.1073/pnas.43.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARANCHYCH W., GRAHAM A. F. Isolation and properties of an RNA-containing bacteriophage. J Cell Comp Physiol. 1962 Dec;60:199–208. doi: 10.1002/jcp.1030600303. [DOI] [PubMed] [Google Scholar]
- REVEL H. R., LURIA S. E., ROTMAN B. Biosynthesis of B-D-galactosidase controlled by phage-carried genes. I. Induced beta-D-galactosidase biosynthesis after transduction of gene z-plus by phage. Proc Natl Acad Sci U S A. 1961 Dec 15;47:1956–1967. doi: 10.1073/pnas.47.12.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- WOHLHIETER J. A., FALKOW S., CITARELLA R. V., BARON L. S. CHARACTERIZATION OF DNA FROM A PROTEUS STRAIN HARBORING AN EPISOME. J Mol Biol. 1964 Aug;9:576–588. doi: 10.1016/s0022-2836(64)80228-x. [DOI] [PubMed] [Google Scholar]


