Abstract
It is not known to what degree aquaporin-facilitated water uptake differs between root developmental regions and types of root. The aim of this study was to measure aquaporin-dependent water flow in the main types of root and root developmental regions of 14- to 17-d-old barley plants and to identify candidate aquaporins which mediate this flow. Water flow at root level was related to flow at cell and plant level. Plants were grown hydroponically. Hydraulic conductivity of cells and roots was determined with a pressure probe and through exudation, respectively, and whole-plant water flow (transpiration) determined gravimetrically in response to the commonly used aquaporin inhibitor HgCl2. Expression of aquaporins was analysed by real-time PCR and in situ hybridization. Hydraulic conductivity of cortical cells in seminal roots was largest in lateral roots; it was smallest in the fully mature zone and intermediate in the not fully mature ‘transition’ zone along the main root axis. Adventitious roots displayed an even higher (3- to 4-fold) cortical cell hydraulic conductivity in the transition zone. This coincided with 3- to 4-fold higher expression of three aquaporins (HvPIP2;2, HvPIP2;5, HvTIP1:1). These were expressed (also) in cortical tissue. The largest inhibition of water flow (83–95%) in response to HgCl2 was observed in cortical cells. Water flow through roots and plants was reduced less (40–74%). It is concluded that aquaporins contribute substantially to root water uptake in 14- to 17-d-old barley plants. Most water uptake occurs through lateral roots. HvPIP2;5, HvPIP2;2, and HvTIP1;1 are prime candidates to mediate water flow in cortical tissue.
Keywords: Aquaporin inhibitor mercury chloride, barley (Hordeum vulgare), cortical cell, hydraulic conductivity, lateral root
Introduction
Plants are variable hydraulic conductors which use a naturally occurring gradient in energy content of water (water potential) between root and shoot environment to drive the uptake of water and dissolved mineral nutrients. It is in particular the radial, as opposed to axial, resistance to water flow which limits water uptake by roots and supply to the shoot (Frensch and Steudle, 1989; Steudle and Peterson, 1998). The radial resistance can be divided into an apoplastic (cell wall, middle lamella, and intercellular air space) and a cell-to-cell (through plasmodesmata and across membranes) component (Steudle and Peterson, 1998; Steudle, 2000; Knipfer and Fricke, 2010). The cell-to-cell path can involve water transport through aquaporins. The question is not so much whether, but how much, aquaporins contribute to root water uptake (Javot and Maurel, 2003).
Aquaporins belong to the family of major intrinsic proteins (MIPs) and are best known for their ability to facilitate water flow (Fricke and Chaumont, 2006; Hachez et al., 2006a, b; Maurel, 2007; Katsuhara et al., 2008). Water channel activity is typically displayed by those MIPs that reside in the plasma membrane (plasma membrane intrinsic proteins, PIPs) and tonoplast (tonoplast intrinsic proteins, TIPs). Among PIPs, water channel activity is displayed particularly by members of the PIP2 subgroup (Maurel et al., 2008). There exist a number of studies in which the expression of particular PIP or TIP isoforms has been altered through overexpression or knockout of the respective gene. Most of these studies have been carried out on Arabidopsis, maize (Zea mays), tobacco (Nicotiana tabacum), and rice (Oryza sativa) and have provided conflicting evidence, partly confirming and partly not confirming a role in root water transport of the respective MIP (Kaldenhoff et al., 1998; Javot et al., 2003; Katsuhara et al., 2003a; Ma et al., 2004; Schüssler et al., 2008; Beebo et al., 2009; Postaire et al., 2010). Part of the discrepancy between results obtained through transgenic approaches may be explained by the ability of plants to compensate for altered expression of particular aquaporin isoforms. In particular, the complexity of root architecture and of alternative physiological means through which root water uptake is controlled has to be considered (Schreiber et al., 1999; Bramley et al., 2009, Draye et al., 2010). For example, the contribution of different root development regions, types of roots (Graham et al., 1974; Sanderson, 1983), and radial uptake pathways (apoplastic, transcellular) (Steudle, 2000) of water uptake need to be known before trying to identify candidate aquaporins that mediate this flow.
In a recent biophysical study on 14- to 17-d-old hydroponically grown barley plants, it was concluded that a purely apoplastic path of radial water uptake does not exist but that water has to cross membrane(s) (Knipfer and Fricke, 2010). Barley plants of this developmental stage have two types of root, adventitious and seminal. The seminal root system is more developed and provides most of the root surface area. As a result, >90% of water uptake occurs through seminal roots (Knipfer and Fricke, 2011). The aim of the present study was to quantify aquaporin-dependent water flow in the main developmental zones of the two types of root. The commonly used aquaporin inhibitor HgCl2 (see Supplementary Table S1 available at JXB online) was applied and the resulting changes in water flow measured at the level of individual cells, roots, and plant, using cell pressure probe, exudation, and gravimetric transpiration measurements. Candidate aquaporins were identified through real-time (qPCR) expression analyses and in situ hybridization. Water channel function was tested as part of an accompanying study on aquaporins in barley leaves (Besse et al., 2011). The expression of aquaporins and root hydraulic conductance has been shown to vary between day and night-time in several plant species, including barley (Katsuhara et al., 2003b). Since most of the growth and water uptake of barley plants occurred during the daytime, analyses were restricted to the daytime.
Materials and methods
Plant growth
Barley (Hordeum vulgare L. cv. Golf, Svalöf Weibull AB, Svalöf, Sweden) plants were grown on modified Hoagland solution in a growth chamber as described previously (Fricke and Peters, 2002; Knipfer and Fricke, 2010). Plants grew at a day/night length of 16/8 h and temperature of 21/15 °C. Relative humidity was 70% and photosynthetically active radiation 400–500 μmol m−2 s−1. Plants were analysed when they were 14–17 d old. Analyses were carried out 3–7 h into the photoperiod. During this period, transpirational water loss and root water uptake as determined on individual seminal and adventitious roots varied by <27% (not shown).
Root types and developmental zones
The first major roots which appeared during germination of barley seedlings were seminal roots. Adventitious roots, which differ in morphology and anatomy from seminal roots (Fig. 1; see also Knipfer and Fricke, 2011) appeared when plants were 11–13 d old. Barley plants had between six and seven seminal and between two and four adventitious roots.
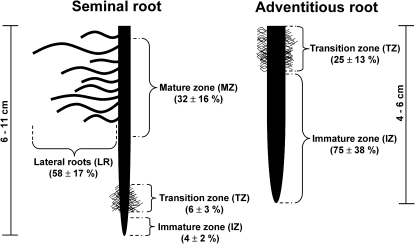
Fig. 1.
Scheme of a seminal and adventitious root of a 14- to 17-d-old barley plant. The main axis of seminal roots can be divided into three developmental zones: an immature zone (tip region) containing the apical meristem and cell elongation zone, an adjacent transition zone, where cells are not elongating any more yet not all tissues are fully mature; and a basal mature zone. Lateral roots emerge from the mature zone and were treated as a separate entity. The less developed adventitious roots contain a longer immature zone. Fully mature tissue, in particular with respect to xylem and endodermis development (Knipfer and Fricke, 2011), is hardly detectable. Most of the root base can be classified as transition zone. Numbers in parentheses give the mean ±SD (n=4 roots) surface area of each zone (as determined according to Knipfer and Fricke (2010, 2011), expressed as a percentage of the total root surface area. Total length of roots is indicated by scale bars.
Hydraulic properties of root tissues may differ between developmental zones (Hukin et al., 2002). Therefore, to compare hydraulic properties of seminal and adventitious roots, it was necessary to carry out analyses at zones of comparable developmental stage. Adventitious roots contained hardly any fully mature zone; neither did they contain lateral roots at the time of analyses. Since the tip region (see immature zone, IZ; Fig. 1) of the main axis of roots involves little water transport through aquaporins (Hukin et al., 2002), it was decided to compare the not-fully mature zone between seminal and adventitious roots. This zone was referred to as ‘transition zone’ (TZ), since tissues were at the transition between being immature (immature zone, IZ) and fully mature (mature zone, MZ) (Fig. 1). The distinction between zones was based very much on the developmental state of the endodermis, which is supposed to affect radial movement of water and, hence, radial conductivity (IZ, state I endodermis, no Casparian bands, no suberin despositions; TZ, state II–III endodermis with passage cells, Casparian bands, and suberin depositions; MZ, state III endodermis, without passage cells and with Casparian bands, suberin depositions, and secondary wall thickenings; see Knipfer and Fricke, 2011).
Lateral roots accounted for the largest percentage of surface area of seminal roots (Fig. 1). In adventitious roots, 25% and 75% of the surface area was accounted for by the transition and immature zones, respectively (Fig. 1).
Hydraulic measurements
Details of methods, together with calculations, are given in Knipfer and Fricke (2010, 2011) and Supplementary File S1 at JXB online. Transpiration measurements were carried out in the growth chamber; all remaining analyses were carried out in the laboratory. Throughout analyses, the ‘control’ root medium (nutrient solution) was taken from the pot in which the plant had grown during cultivation. Reagents that were tested for an effect on water transport [HgCl2, dithiothreitol (DTT)] were applied in this medium.
Root exudation measurements were performed on entire root systems or individual roots. Individual roots were excised close to the root base, ∼1–2 cm below the root–shoot junction. The length of excised roots ranged from 6 to 11 cm in seminal and 4 to 6 cm in adventitious roots. Seminal roots contained numerous lateral roots, whereas adventitious roots were devoid of lateral roots at the plant developmental stage analysed (see also Fig. 1).
During root exudation, an individual root or entire root system of a plant was attached to a glass capillary and the rise of xylem sap in the capillary recorded at time intervals of 5 min over 1 h. Hydraulic conductivity (in m s−1 MPa−1) was calculated from the linear part of the flow versus time plot and the difference in osmolality between root medium and exudates. Flow rate was related to root surface area, which was determined as detailed previously (Knipfer and Fricke, 2011). Water transport through aquaporins was investigated by application of the aquaporin inhibitor HgCl2. Roots were treated for 5 min with 50 μM HgCl2 and subsequently rinsed with water before being placed back into the root medium (devoid of HgCl2) where exudate flow was measured again. The reversibility of effect of HgCl2 on water uptake was tested by treating roots first in 50 μM HgCl2 and then placing them for 15 min in 5 mM DTT before being analysed.
Cell pressure probe analyses were carried out on roots of intact plants. Exosmotic and endosmotic water flow across the plasma membrane of cells was induced through pressure pulses. The half-time of pressure relaxations was used, together with data on the volume and elastic modulus of cells, to calculate cell hydraulic conductivity (in m s−1 MPa−1). Cells in the four peripheral cortical layers were analysed. There was no obvious difference in variables between these layers, and results were pooled. The average cortical cell surface area determined from cross- and longitudinal sections under a microscope was 9.0±2.3×10−10 m2 in seminal and 14.0±1.8×10−10 m2 in adventitious roots; the average cell volume and length were 2.7±0.7×10−13 m3 and 3.0±0.3×10−4 m in seminal, and 2.4±0.3×10−13 m 3 and 1.8±0.1×10−4 m in adventitious roots. The average cortical cell surface area in lateral roots was 9.5±4.4×10−10 m2, and the average cell volume and length was 0.9±0.5×10−13 m3 and 1.0±0.2×10−4 m, respectively (means ±SD of five root analyses). Cell elastic modulus was determined according to Volkov et al. (2007); calculations are detailed in Supplementary File S1 at JXB online.
To test aquaporin-dependent water transport in cortical cell, cells were first analysed under control conditions. Then, a plant of the same batch as the ‘control’ plant was exposed to 50 μM HgCl2 for 5 min; roots were rinsed shortly, and the plant was transferred back to the nutrient medium devoid of HgCl2 and cortical cells analysed within the following 45 min. To test recovery of Hg-induced reduction in water flow, a new plant was exposed first to 50 μM HgCl2 for 5 min and then to 5 mM DTT for 15 min before being analysed in nutrient medium for up to 45 min. The alternative approach, to analyse cortical cells of the same plant subsequently under control, Hg, and recovery conditions, was not pursued to avoid exposing plants to cumulative physical injury incurred through pressure probing.
Transpiration, whole-plant hydraulics, and leaf water potential
Transpiration rate of plants was determined gravimetrically in the growth chamber (Knipfer and Fricke, 2011). Water transport through aquaporins was tested by exposing plants transiently (5 min) to 50 μM HgCl2 in the nutrient solution before re-measuring transpiration. The reversibility of the effect of HgCl2 on water uptake was tested by treating roots subsequently with 50 μM HgCl2 (5 min) and 5 mM DTT (15 min) prior to analyses in nutrient solution devoid of these reagents. Reagents were applied by draining the existing nutrient solution from the container which held the plant and refilling the container with the respective new (treatment) nutrient solution. This minimized damage to lateral roots. Transpiration was measured for 2 h following treatments and not for only 45 min as was the case for treatments during cell pressure probe analyses. This was done to allow transpiration to recover to a steady level, while minimizing the period for which plants were on the cell pressure probe stage (in a laboraory environment).
Stomatal conductance and net rate of photosynthesis was determined using an infra-red gas analyser (LI-6400; LI-COR, Lincoln, NE, USA).
Leaf water potential was determined as detailed previously (Knipfer and Fricke, 2011). Turgor of between three and four epidermal cells was measured with the cell pressure probe halfway along the fully expanded blade of leaf two, which represented the main transpiring leaf surface of plants. Following completion of turgor analyses, the leaf region was excised and bulk sap was extracted using a centrifugation technique. The sap was analysed for osmotic pressure using picolitre osmometry. The difference between cell turgor and leaf osmotic pressure calculated to leaf water potential [since epidermal cell osmotic pressure is similar to bulk leaf osmotic pressure (Fricke and Peters, 2002)]. Four untreated (control) and four Hg-treated plants were analysed, and results presented as average ±SD.
Expression analyses of barley aquaporins
Details of the procedures associated with expression analyses of barley aquaporins are provided in the accompanying paper (Besse et al., 2011). Entire roots or root developmental zones were harvested, RNA extracted, cDNA synthesized, and expression analysed using qPCR as detailed previously (Boscari et al., 2009; Besse et al., 2011). Three independent batches of plants were studied and results averaged. Expression of candidate aquaporins was related to the expression of three reference genes (ubiquitin, tubulin, and H+-ATPase) using the ΔCt method (Pfaffl, 2001; Bustin et al., 2005; Boscari et al., 2009). Relative expression of candidate genes was calculated by relating Ct values to Ct values of each housekeeping gene according to,
| (Eqn 1) |
This resulted in three 2–ΔCt values for a particular candidate gene , which were averaged. Average 2–ΔCt values were obtained from three batches of plants , yielding an overall mean 2–ΔCt value (±SD). Sequences of primers used for qPCR are given in Besse et al. (2011).
Tissue distribution of expression of aquaporins was analysed by in situ hybridization (Besse et al., 2011). Three independent batches of plants were analysed, and representative results are shown.
Statistical analyses
Student's t-test (SigmaPlot) and ANOVA (Excel) was used to test for statistical significance of data.
Results
Root hydraulic conductivity
Hydraulic conductivity of individual seminal roots averaged 10.5×10−8 m s−1 MPa−1 (Table 1). Pre-incubation with HgCl2 reduced hydraulic conductivity by 53%, and subsequent addition of DTT recovered conductivity to 87% of the value prior to inhibition (Table 1). Hydraulic conductivity of adventitious roots averaged 4.4×10−8 m s−1 MPa−1; it decreased by 74% in response to HgCl2 and recovered to 66% of its original value in response to DTT. A similar reduction in hydraulic conductivity was observed for entire root systems (recovery not tested). The osmolality of exudate and osmotic force driving exudation was not affected by HgCl2 (not shown).
Table 1.
Root hydraulic conductivity of 14- to 17-d-old barley plants in response to HgCl2 and HgCl2/DTT treatment
| Root type | Hydraulic conductivity |
||
| Control | HgCl2 | HgCl2/DTT | |
| Seminal root | 100a (10.5±2.3) | 47.5±16.0b,e | 86.6±1.7d |
| Adventitious root | 100a (4.4±1.3) | 26.2±2.5c | 65.5±8.6b |
| Entire root system | 100a (17.7±4.6) | 25.6±15.0c,e | –/– |
Conductivity was determined through exudation experiments on individual seminal and adventitious roots or on the entire root system of plants. Exudation was measured in normal growth medium (control) and after transient (5 min) exposure of roots to 50 μM HgCl2, without (HgCl2) or with subsequent recovery (15 min) in 5 mM DTT (‘HgCl2/DTT’). Results are means ±SD of three or four root analyses expressed as a percentage of the control. Hydraulic conductivity (10−8 m s−1 MPa−1) of control plants is given in parenthesis. Different superscripts indicate significant differences (P<0.05); –/–, not tested.
Cell hydraulic conductivity
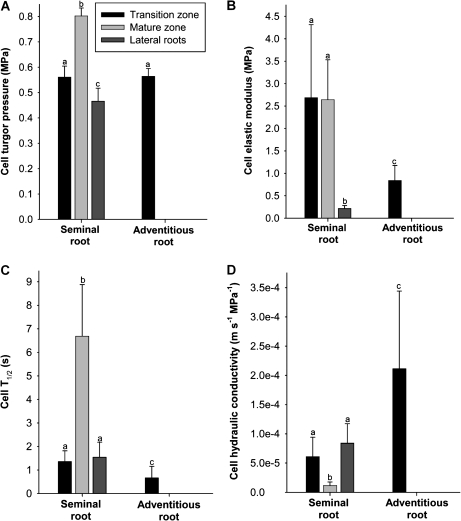
The transition zone, mature zone, and lateral roots were analysed in seminal roots. In the less developed adventitious roots, only the transition zone was analysed. Turgor of cortical cells in the transition zone of both seminal and adventitious roots averaged 0.56 MPa. Turgor in the fully mature zone of seminal roots was significantly higher (0.80 MPa), while turgor in lateral roots was significantly lower (0.47 MPa) (Fig. 2A). Cell elastic modulus was comparable between the transition and fully mature zone in seminal roots (2.6–2.7 MPa) and >10-fold higher than in lateral roots (0.22 MPa). Cortical cells of the transition zone of adventitious roots displayed an intermediate value (0.8 MPa) (Fig. 2B). The half-time of water exchange of cells differed between types of root and developmental zones (Fig. 2C). As a result, cortical cell hydraulic conductivity was 5- to 8-fold higher in lateral roots and in the transition as compared with the fully mature zone of seminal roots. Adventitious roots displayed an even higher cell hydraulic conductivity (Fig. 2D).
Fig. 2.
Hydraulic parameters of root cortical cells of barley. Barley plants were 14–17 d old. The cell pressure probe was used to measure (A) turgor and, together with microscopic determination of cell volume, (B) cell elastic modulus, and (C) half-time of water exchange (Cell T1/2). These data were used to calculate (D) cell hydraulic conductivity. Results are averages ±SD (error bars) of 7–14 cell analyses. Statistical significance of difference is indicated by different letters (P<0.05).
Cortical cell hydraulic conductivity decreased by 83–95% when HgCl2 was added to the root medium (Table 2). The percentage decrease was largest in the transition zone of both types of root. Cell hydraulic conductivity recovered to 92–111% in seminal and 47% in adventitious roots after DTT had been added to the root medium.
Table 2.
Hydraulic conductivity of root cortical cells of barley in response to HgCl2 and HgCl2/DTT treatment
| Root type | Root zone | Conductivity |
||
| Control | HgCl2 | HgCl2/DTT | ||
| Seminal | Transition | 100a (6.1 ± 3.3) | 4.5 ± 0.8b | 91.9 ± 25.1a |
| Mature | 100a (1.2 ± 0.6) | 16.6 ± 3.0c | 111.2 ± 27.3a | |
| Lateral root | 100a (8.4 ± 3.3) | 10.3 ± 3.5c | 106.6 ± 14.7a | |
| Adventitious | Transition | 100a (21.1 ± 13.3) | 5.6 ± 2.0b | 46.9 ± 13.3d |
Conductivity was determined through the cell pressure probe. Cortical cells were analysed in three zones of seminal roots and in the transition zone of adventitious roots. Roots were analysed in normal growth medium (‘control’) and after transient (5 min) exposure to 50 μM HgCl2, without (‘HgCl2’) or with subsequent recovery (15 min) in 5 mM DTT (‘HgCl2/DTT’). Results are means ±SD of 5–14 cell analyses expressed as a percentage of the control. Hydraulic conductivity (10−5 m s−1 MPa−1) of cells of control plants is given in parenthesis; for statistical analysis of these values see Fig. 2D. Different superscripts indicate significant differences between treatments (P<0.05).
Transpiration
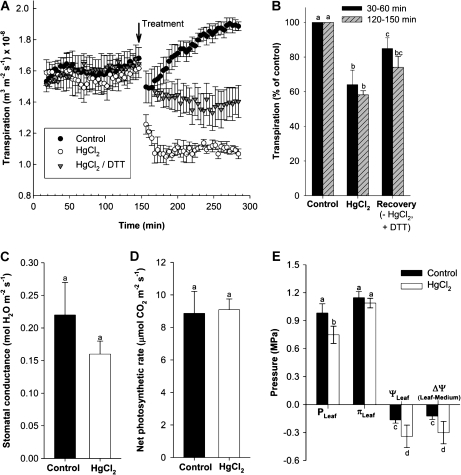
Transpirational water loss was measured continuously and gravimetrically in the growth chamber and related to leaf surface area to calculate transpiration rate. Plants transpired during the day at an average rate of 1.6×10−8 m3 m−2 s−1. The process per se of exchanging nutrient medium, as required to apply treatments, led to an initial decrease in transpiration. Transpiration recovered during the following 2 h to a level slightly higher than the original one [Fig. 3A, control; 1.9×10−8 m3 m−2 s−1 (=100%)]. When HgCl2 was added to the root medium for 5 min, transpiration decreased during the following 30 min by 40% and remained at this level. When roots were first treated with HgCl2 (5 min) and then transferred to medium that was devoid of Hg but contained the reducing agent DTT (15 min treatment), plants transpired at 74% of the level observed in the control (Fig. 3A, B).
Fig. 3.
Daytime transpirational water loss of 14- to 15-d-old barley plants in response to the aquaporin inhibitor HgCl2 and reducing agent DTT. Water loss was measured for >2 h before 50 μM HgCl2 (5 min) was added to the root medium and water loss measured again (HgCl2). In some experiments, plants were exposed, in quick succession, to 50 μM HgCl2 (5 min) and 5 mM DTT (15 min) before being analysed again (HgCl2/DTT). Plants which had normal growth medium exchanged for medium devoid of reagents were used as control. (A) Continuous recording of water loss. Each trace is the average +SD (error bars) of three plant analyses. (B) Mean change in transpiration rate in response to treatment, expressed as a percentage of the control (=100%). Means were calculated from values at 30–60 min and 120–150 min after the treatment shown in (A). (C) Stomatal conductance and (D) net photosynthetic rate in control and Hg-treated plants. Measurements were taken 60–150 min after treatment; DTT recovery was not tested. Values are means ±SD of four plants. (E) Effect of Hg treatment on cell turgor pressure (PLeaf) and leaf osmotic pressure (πLeaf) and water potential (ψLeaf). The resulting gradient in water potential (Δψ) between root medium (–0.04 MPa) and leaf is also shown. Values are given as means ±SD of four leaf analyses. Statistical significance of difference is indicated by different letters (P<0.05).
Exposure of plants to HgCl2 led to a 28% reduction in stomatal conductance as measured 1–2.5 h following Hg treatment. Conductance of Hg-treated plants averaged 0.16±0.02 compared with 0.22±0.05 mol H2O m−2 s−1 in control plants (Fig. 3C). The net rate of photosynthesis was not affected by HgCl2 treatment (Fig. 3D).
Leaf water potential decreased in response to Hg treatment (Fig. 3E). This was due to a decrease in (epidermal) cell turgor from 0.98 MPa in control to 0.75 MPa in Hg-treated plants. The gradient in water potential between root medium (–0.04 MPa; Knipfer and Fricke, 2011) and leaf, which drives water movement between these two compartments, increased 2.5-fold, from –0.12 MPa in control to –0.30 MPa in treated plants. Water flow is the product of driving force and hydraulic conductance; or, hydraulic conductance calculates as flow divided by driving force. Therefore, the changes in transpiration and water potential gradient in response to Hg calculated to a hydraulic conductance of the flow path between root medium and leaf which was (0.6/2.5=0.24) 24% of that in untreated control plants.
Aquaporin expression in roots
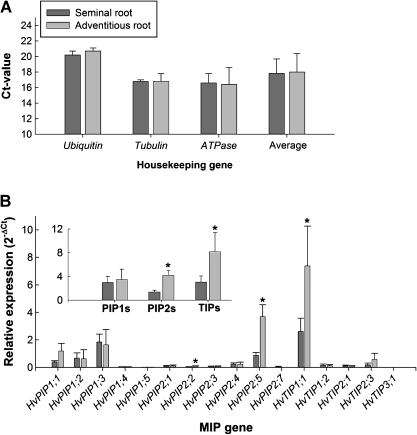
Expression was analysed by real-time PCR (qPCR). Seminal and adventitious roots were always harvested from the same plants. The expression of the, presumably, entire set of barley PIPs (Besse et al., 2011) was determined. In addition, five TIPs were analysed. These TIPs had displayed highest expression or most distinct expression profiles during preliminary experiments. Ubiquitin, tubulin, and H+-ATPase were used as reference genes of expression. The expression of reference genes in adventitious roots was on average 1.01±0.02 (mean ±SD) times that in seminal roots (Fig. 4A), effectively qualifying these genes as suitable references. Three aquaporins (HvPIP2;2, HvPIP2;5, HvTIP1;1) differed significantly in expression between seminal and adventitious roots (Fig. 4B). Expression was 2.5- to 4-fold higher in adventitious roots (P<0.05). HvTIP1;1 was expressed at the highest level of all aquaporins tested. Expression was 10–20 times higher than expression of any other TIP. The second-highest expressed aquaporin was HvPIP2;5. ANOVA analysis (Excel) revealed that there existed significant differences in expression between aquaporins in each type of root and that the two root types differed in aquaporin expression (not shown).
Fig. 4.
qPCR expression analyses of PIPs and TIPs in seminal and adventitious roots of barley. (A) Three genes were used as references of expression (ubiquitin, tubulin, H+-ATPase). (B) Data for aquaporin genes are shown as fold difference in expression [2–(ΔCt)] compared with the mean expression of reference genes (=1.0). Results are means ±SD of three independent experiments. Statistical significance of difference in value in (B) between seminal and adventitious roots is indicated by asterisks (P<0.05).
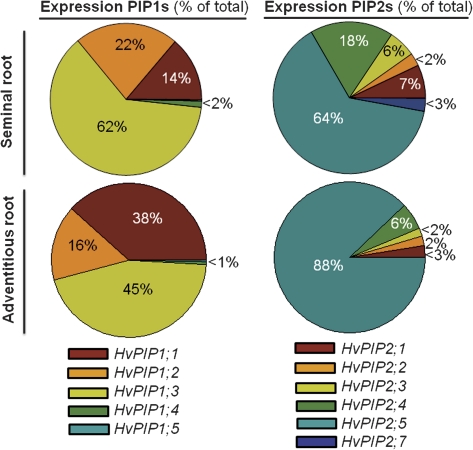
To better illustrate the contribution of each aquaporin to the total expression (=100%) of the respective family, data were presented as pie charts (Fig. 5). In seminal roots, one family member accounted for most of the expression in the PIP1 (HvPIP1;3) and PIP2 (HvPIP2;5) family. The same applied to adventitious roots, except that the contribution of HvPIP1;1 expression was more than twice as high and HvPIP1;3 expression less predominant.
Fig. 5.
Contribution of aquaporin family members to total expression of PIP1s and PIP2s in seminal and adventitious roots of barley. Values are expressed as percentage and are derived from data shown in Fig. 4.
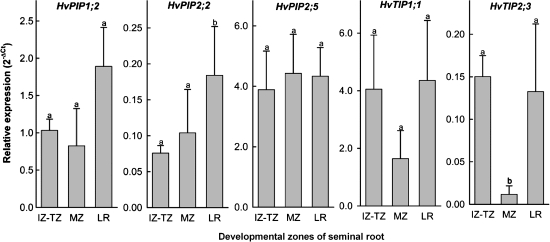
Five aquaporins were tested for differences in expression between root regions in seminal roots. Four aquaporins (HvPIP2;2, HvPIP2;5, HvTIP1;1, HvTIP2;3) had shown water channel activity in an accompanying study, and the fifth aquaporin (HvPIP1;2) had been expressed particularly in root compared with leaf tissue (Besse et al., 2011). HvPIP2;2 and HvTIP2;3 showed significant differences in expression between root zones, including lateral roots (Fig. 6). The expression pattern of both HvTIPs was similar, but was not significant in HvTIP1;1. The abundantly expressed HvPIP2;5 was expressed evenly in seminal roots (Fig. 6).
Fig. 6.
Expression of five aquaporins in different regions of seminal roots of barley. Expression was analysed by qPCR in three regions (combined immature and transition zone, IZ–TZ; mature zone, MZ; lateral roots, LR) and related to the average expression of three reference genes (ubiquitin, tubulin, H+-ATPase). Results are averages ±SD of three experiments. Statistical significance of difference was assessed through a pair-wise t-test and is indicated by different letters (P<0.05). Pair-wise comparison explains why in some cases (e.g. HvPIP2;2) expression differs significantly despite overlapping standard deviations.
Tissue localization of expression
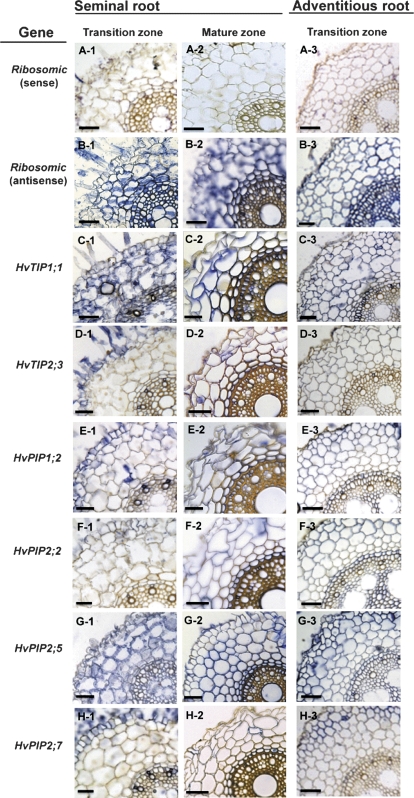
Most of the previous studies on barley aquaporins have focused on the water channel HvPIP2;1 and on its role as CO2 diffusion facilitator in leaves and in the hydraulic response of roots to salinity and day/night changes (Katsuhara et al., 2002, 2003a, b; Hanba et al., 2004). Wei et al. (2007) observed that the barley PIP1 HvPIP1;6, which is identical to HvPIP1;1 and also expressed in roots, displayed some water channel activity. In an accompanying study on barley leaves, HvPIP2;2, HvPIP2;5, HvPIP2;7, HvTIP1;1, and HvTIP2;3 were found to display water channel activity (Besse et al., 2011). Since all of these aquaporins are expressed in roots (Fig. 4), they were selected for an analysis of their tissue localization of expression using in situ hybridization (Fig. 7). In addition, HvPIP1;2 was analysed due to its almost exclusive expression in roots.
Fig. 7.
Tissue localization of expression of aquaporins in seminal and adventitious roots of barley. Expression was analysed by in situ hybridization, in the transition and mature zones. Six candidate aquaporin genes (C–H) and one control gene (ribosomal RNA, A–B) were studied. Messenger RNA was probed through hybridization with antisense RNA and visualized through peroxidase-based staining (blue colour). Non-specific staining was tested through hybridization with sense RNA [shown representatively for ribosomal RNA in (B)]. Three batches of plants were analysed, with qualitatively similar results. Scale bar 50 μm (seminal roots) and 70 μm (adventitious roots).
There seemed to be two patterns of expression. HvTIP1;1, HvPIP1;2, HvPIP2;2, and HvPIP2;5 were expressed almost ubiquitously in all major root tissues, including epidermis, cortex, endodermis, and stele. Expression in the cortex was generally most pronounced, and HvPIP2;5 and HvTIP1;1 produced the strongest signal of all aquaporin isoforms tested. The tissue pattern of expression of HvTIP2;3 and HvPIP2;7 differed from that of the other aquaporins, in that expression appeared to be most prominent in the epidermis, with comparatively little expression in cortex, endodermis, and stele. For a particular aquaporin isoform, the tissue pattern of expression did not differ between seminal and adventitious root (transition zone). There was a tendency towards stronger expression in the endodermis and stele of adventitious compared with seminal roots.
Discussion
Roots as ‘miners’ and ‘recipients’
Roots have evolved to optimize their function as biological miners in a soil environment which is patchy and unpredictable in the supply of resources and where diffusional resistances can rate-limit the availability of these resources. Tip-localized growth and a branched root system are best examples of such adaptations. In accordance with this, earlier studies on barley roots showed that the highest rate of water uptake occurred in a region 4–5 cm behind the tip of roots and that lateral roots contributed between one-quarter and two-thirds to root water uptake (Graham et al., 1974; Sanderson, 1983). The present study supports these findings and emphasizes the importance of lateral roots. The study also points to membranes, involving aquaporins in general and specific aquaporin isoforms in particular, as sites through which water uptake is controlled. It has to be remembered though, that the present, as previous, studies were carried out on plants grown in hydroponics. In contrast to soil, hydroponics provides a highly convective and comparably uniform root environment; roots are not so much miners as recipients which receive nutrients and water ‘on a plate’. For example, the previous observation that removal of almost the entire root systems reduces transpiration rate little in barley plants grown hydroponically (Knipfer and Fricke, 2011) may not hold in a soil environment. Despite these differences, roots of hydroponically grown plants maintain a spatial distribution of water uptake along the main axis and an involvement of laterals as predicted for roots in soil. Whether the hydraulic properties and aquaporin isoforms of root regions described here also apply to roots in a soil environment cannot be said with certainty. However, the results demonstrate properties of roots through which water uptake in a soil environment could be controlled.
Root hydraulic properties were measured through exudation, where water flow was driven through osmotic forces. Cell hydraulic conductivity was determined through application of hydrostatic pressure pulses. These caused a gradient in water potential between the inside and the outside of the cell, which in turn drove water flow. Notwithstanding the possibility that osmotic and hydrostatic forces can yield different hydraulic conductivities at root level (for review, see Steudle, 2000), both types of analysis can be compared for the purpose of the present study, since water flow in each (exudation, cell pressure probe) was driven by gradients in water potential.
Root hydraulic properties in relation to root development and flow paths
Aquaporin activity can control root water uptake only if water does not move exclusively along a highly conductive apoplastic pathway between root medium and stele. The main barriers of such a pathway are the endo- and exodermis (Steudle and Peterson, 1998; Steudle, 2000). An exodermis was not apparent in the barley roots analysed as judged from microscopic inspection and staining (Supplementary Fig. S1 at JXB online; see also Lehmann et al., 2000). The endodermis constituted the only significant apoplastic barrier. Radial hydraulic conductivity was similar in seminal and adventitious roots, despite major differences in endodermis development (Knipfer and Fricke, 2011; seminal roots more developed). This finding suggests that a purely apoplastic pathway of water movement does not exist along the main axis of roots of 14- to 17-d-old barley plants, as recently concluded on theoretical grounds and measurements of radial root reflection coefficients (Knipfer and Fricke, 2010). Bramley et al. (2009) and Fritz et al. (2010) reached the same conclusion for the closely related wheat and maize, respectively. It is possible that some water uptake occurred through a purely apoplastic pathway in lateral roots, where the endodermis was not fully developed. This would explain why the aquaporin inhibitor HgCl2 reduced water flow in entire seminal roots less than in individual cortical cells. Faiyue et al. (2010) recently concluded that apoplastic bypass flow of Na+ (and water) in salt-stressed rice (O. sativa) occurred through lateral roots.
Tempting as it may be to consider significant movement of water along a purely apoplastic path in lateral roots, it should not be overlooked that the hydraulic conductivity of cortical cells was the highest in lateral roots of all seminal root regions analysed. Lateral roots also had the lowest number of cortical cell layers, and the hydraulic conductivity of cortical cells was inhibited by 90% in response to HgCl2, in a fully reversible manner (Table 2). Using data on cell hydraulic conductivity and cortex layer number, it can be calculated that the radial conductance of lateral roots was well in excess of the conductance required to sustain water uptake by seminal roots along a transmembrane pathway (not shown). Lateral roots accounted for almost two-thirds of the surface area of seminal roots (Fig. 1), which provide 92% of the water uptake of 13- to 17-d-old barley plants (Knipfer and Fricke, 2011). Together, the data point to lateral roots and to aquaporins being the key to understanding the hydraulic response of roots.
The involvement of aquaporins in root water uptake is further supported by the present observation that cortical cell hydraulic conductivity decreased in response to HgCl2 in the transition zone of seminal and adventitious roots, and in lateral roots, to a value close to the value observed in non-inhibited cortical cells of the mature zone of seminal roots. The reduction (>90%) in hydraulic conductivity in response to HgCl2 reported here for barley root cortical cells is slightly larger than values reported for Arabidopsis (∼73%, Jang et al., 2007), wheat (∼70–80%, Bramley et al., 2009), and maize (60–70%, Hukin et al., 2002; Ehlert et al., 2009).
Aquaporin-dependent water uptake and transpiration
Hydraulic and transpiration analyses of Hg-treated plants showed that inhibition of root aquaporins can have a real effect on whole-plant transpiration and leaf physiology. The reduction in root hydraulic conductivity as calculated from transpiration and water potential measurements on intact plants (76%) was almost identical to the reduction measured on isolated, entire root systems (74%). Addition of Hg to the root medium caused a decrease in water supply to the shoot, which in turn led to a reduction in leaf cell turgor and water potential by ∼0.2 MPa. A steady reduction in transpiration rate was achieved through a decrease in stomatal conductance, the latter presenting the by far highest resistance to water movement along the root medium–plant–atmosphere continuum (Knipfer and Fricke, 2011). It is not clear why leaf water potential did not decrease further to compensate (through increased driving force) fully the reduction in root hydraulic conductivity. Some regulatory mechanism, possibly involving root-derived abscisic acid (Wan and Zwiazek, 1999) must have set in Hg-treated plants as leaf water potential decreased to –0.34 MPa, leading to partial stomatal closure. In contrast to transpiration, net photosynthetic rate was not affected by addition of HgCl2 to the root medium. Plants must have had increased water use efficiency and were unlikely to suffer from any toxic effect of Hg which might have been transported to leaves with the transpiration stream.
Incomplete inhibition of water transport at root level by HgCl2 as observed in the present study and others (e.g. Tazawa et al., 1997, 2001; Bramley et al., 2009) may result from insensitivity to HgCl2 of some aquaporin homologues (Daniels et al., 1994) or reflect simple diffusion of water through the phospholipid bilayer and pressure-driven symplastic flow (Pickard, 2003). Addition of the reducing agent DTT recovered hydraulic conductivity close to the original value in seminal roots but not in adventitious roots. This suggests that in adventitious roots effects of Hg on cortical cells were for some reason less specific.
Candidate aquaporins to facilitate water uptake in barley
Compared with seminal roots, adventitious roots had a three-fold higher cortical cell hydraulic conductivity and total expression of PIP2s and TIPs. The latter was due to higher expression of three aquaporins, HvPIP2;2, HvPIP2;5, and HvTIP1;1, all of which display water channel activity (Besse et al., 2011). These aquaporins were expressed in the epidermis, cortex, endodermis, and stele of the transition zone of adventitious roots. HvPIP2;5, and HvTIP1;1 were the highest-expressed aquaporins tested. In seminal roots, HvTIP1;1 was expressed lowest in the mature zone, and this coincided with the lowest cortical cell hydraulic conductivity in this root region. Based on measurements of osmotic water permeability of isolated membranes (Maurel et al., 1993), PIPs, in particular PIP2s, are the most likely candidates limiting cell hydraulic conductivity measured with the pressure probe. Together, this renders HvTIP1;1 and particularly HvPIP2;5 prime candidates to facilitate the higher hydraulic conductivity of cortical cells in adventitious roots.
Sequence comparison between barley and maize PIPs shows that ZmPIP2;1 and ZmPIP2;2 share highest sequence identity with HvPIP2;5. ZmPIP2;1 is among the highest-expressed PIPs in maize roots and the tissue localization of protein changes during root development from a predominant location in the stele and endodermis to a location in the cortex and epidermis (Hachez et al., 2006a). Such a change in tissue localization was not observed for HvPIP2;5 in the present study, where expression was analysed. HvPIP2;5 was expressed in cortical tissue in both the transition and mature zones. Sakurai et al. (2008), using immunocytochemistry, observed for rice roots that candidate aquaporins occurred predominantly in the endodermis and stele, with some protein in the rhizodermis and very little in cortex. The difference in results between the present study and the studies by Hachez et al. (2006a) and Sakurai et al. (2008) may reflect that aquaporin gene and protein abundance do not correlate in time and space.
TIP1;1 isoforms are generally the most abundantly expressed members of the TIP family of aquaporins (e.g. Alexandersson et al., 2005; Sakurai et al., 2005) and share the highest sequence identity with TIP1;1 isoforms across plant species tested. The ubiquitous and abundant expression of HvTIP1;1 in barley roots suggests that this aquaporin is a ‘housekeeping’ type of aquaporin, which provides a ‘baseline’ level tonoplast hydraulic conductance to guarantee rapid osmotic equilibration between vacuole and cytosol (Maurel et al., 1993). A complete loss of (water channel) function of HvTIP1;1 is not expected to cause a phenotype in barley (see also Schüssler et al., 2008 for Arabidopsis), as another TIP (HvTIP2;3) which shows water channel activity (Besse et al., 2011) is expressed in roots. It remains to be shown why TIPs which show water channel activity are co-expressed abundantly in root cells.
Supplementary data
Supplementary data are available at JXB online.
File S1. Methodological details of hydraulic measurements.
Table S1. Summary of a selection of studies that examined the effect of mercury chloride (HgCl2) as an aquaporin inhibitor on cell-, root-, and whole-plant hydraulics.
Figure S1. Cross-sections of seminal and adventitious roots of hydroponically grown barley plants highlighting the absence of an exodermis.
Acknowledgments
The authors thank Brendan and Eugene for provision of additional laboratory space. This project was funded through a PhD fellowship from IRCSET (Irish Research Council for Science, Engineering and Technology, to T.K) and a research grant from SFI (Science Foundation Ireland, grant no 07/RFP/EEEOBF277), to W.F. Thanks to Geneviève Conejero (PHIV-CIRAD Montpellier) for help with in situ hybridization and Dr Saul Otero (University College Dublin, Ireland) for help with stomatal conductance measurements. Special thanks also to two anonymous referees for some very helpful comments on an earlier version of the manuscript.
Glossary
Abbreviations
- DTT
dithiothreitol
- MIP
major intrinsic protein
- PIP
plasma membrane intrinsic protein
- TIP
tonoplast intrinsic protein
References
- Alexandersson E, Fraysse L, Sjövall-Larsen S, Gustavsson S, Fellert M, Karlsson M, Johanson U, Kjellbom P. Whole gene family expression and drought stress regulation of aquaporins. Plant Molecular Biology. 2005;59:469–484. doi: 10.1007/s11103-005-0352-1. [DOI] [PubMed] [Google Scholar]
- Beebo A, Thomas D, Der C, Sanchez L, Leborgne-Castel N, Marty F, Schoefs B, Bouhidel K. Life with and without AtTIP1;1, an Arabidopsis aquaporin preferentially localized in the apposing tonoplasts of adjacent vacuoles. Plant Molecular Biology. 2009;70:193–209. doi: 10.1007/s11103-009-9465-2. [DOI] [PubMed] [Google Scholar]
- Besse M, Knipfer T, Miller T, Verdeil J-L, Jahn T, Fricke W. Developmental control of aquaporin expression in barley (Hordeum vulgare L.) leaves. Journal of Experimental Botany, 2011 doi: 10.1093/jxb/err175. submitted as accompanying paper. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscari A, Clement M, Volkov V, Golldack D, Hybiak J, Miller AJ, Amtmann A, Fricke W. Potassium channels in barley: cloning, functional characterization and expression analyses in relation to leaf growth and development. Plant, Cell and Environment. 2009;32:1761–1777. doi: 10.1111/j.1365-3040.2009.02033.x. [DOI] [PubMed] [Google Scholar]
- Bramley H, Turner NC, Turner DW, Tyerman SD. Roles of morphology, anatomy, and aquaporins in determining contrasting hydraulic behavior of roots. Plant Physiology. 2009;150:348–364. doi: 10.1104/pp.108.134098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustin SA, Benes V, Nolan T, Pfaffl MW. Quantitative real-time RT-PCR – a perspective. Journal of Molecular Endocrinology. 2005;34:597–601. doi: 10.1677/jme.1.01755. [DOI] [PubMed] [Google Scholar]
- Daniels MJ, Mirkov TE, Chrispeels MJ. The plasma membrane of Arabidopsis thaliana contains a mercury-insensitive aquqaporin that is a homolog of the tonoplast water channel protein TIP. Plant Physiology. 1994;106:1325–1333. doi: 10.1104/pp.106.4.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draye X, Kim Y, Lobet G, Javaux M. Model-assisted integration of physiological and environmental constraints affecting the dynamic and spatial patterns of root water uptake from soils. Journal of Experimental Botany. 2010;61:2145–2155. doi: 10.1093/jxb/erq077. [DOI] [PubMed] [Google Scholar]
- Ehlert C, Maurel C, Tardieu F, Simonneau T. Aquaporin-mediated reduction in maize root hydraulic conductivity impacts cell turgor and leaf elongation even without changing transpiration. Plant Physiology. 2009;150:1093–1104. doi: 10.1104/pp.108.131458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faiyue B, Al-Azzawi MJ, Flowers TJ. The role of lateral roots in bypass flow in rice (Oryza sativa L.) Plant, Cell and Environment. 2010;33:702–716. doi: 10.1111/j.1365-3040.2009.02078.x. [DOI] [PubMed] [Google Scholar]
- Frensch J, Steudle E. Axial and radial hydraulic resistance to roots of maize (Zea mays L.) Plant Physiology. 1989;91:719–726. doi: 10.1104/pp.91.2.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricke W, Chaumont F. Solute and water relations of growing leaf cells. In: Verbelen J-P, Vissenberg K, editors. The expanding cell. 2006. Plant Cell Monographs 5. pp. 7–32. Springer, Berlin. [Google Scholar]
- Fricke W, Peters WS. The biophysics of leaf growth in salt-stressed barley: a study at the cell level. Plant Physiology. 2002;129:1–15. doi: 10.1104/pp.001164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz M, Lorenzen S, Popova M, Ehwald R. Transient and permanent changes of xylem sap exudation by root systems of Zea mays after application of hydrostatic and osmotic forces. Functional Plant Biology. 2010;37:813–827. [Google Scholar]
- Graham JP, Clarkson DT, Sanderson J. Water uptake by roots of marrow and barley plants. Agricultural Research Council Letcombe Laboratory Annual Report. 1974;1973:9–12. [Google Scholar]
- Hachez C, Moshelion M, Zelazny E, Cavez D, Chaumont F. Localization and quantification of plasma membrane aquaporin expression in maize primary roots: a clue to understanding their role as cellular plumbers. Plant Molecular Biology. 2006a;62:305–323. doi: 10.1007/s11103-006-9022-1. [DOI] [PubMed] [Google Scholar]
- Hachez C, Zelazny E, Chaumont F. Modulating the expression of aquaporin genes in planta: a key to understand their physiological functions? Biochimica et Biophysica Acta. 2006b;1758:1142–1156. doi: 10.1016/j.bbamem.2006.02.017. [DOI] [PubMed] [Google Scholar]
- Hanba YT, Shibasaka M, Hayakawa T, Kasamo K, Terashima I, Katsuhara M. Overexpression of the barley aquaporin HvPIP2;1 increases internal CO2 conductance and CO2 assimilation in the leaves of transgenic rice plants. Plant and Cell Physiology. 2004;45:521–529. doi: 10.1093/pcp/pch070. [DOI] [PubMed] [Google Scholar]
- Hukin D, Doering-Saad C, Thomas CR, Pritchard J. Sensitivity of cell hydraulic conductivity to mercury is coincident with symplasmic isolation and expression of plasmalemma aquaporin genes in growing maize roots. Planta. 2002;215:1047–1056. doi: 10.1007/s00425-002-0841-2. [DOI] [PubMed] [Google Scholar]
- Jang JY, Lee SH, Rhee JY, Chung GC, Ahn SJ, Kang H. Transgenic Arabidopsis and tobacco plants overexpressing an aquaporin respond differently to various abiotic stresses. Plant Molecular Biology. 2007;64:621–632. doi: 10.1007/s11103-007-9181-8. [DOI] [PubMed] [Google Scholar]
- Javot H, Lauvergeat V, Santoni V, et al. Role of a single aquaporin isoform in root water uptake. The Plant Cell. 2003;15:509–522. doi: 10.1105/tpc.008888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javot H, Maurel C. The role of aquaporins in root water uptake. Annals of Botany. 2003;90:301–313. doi: 10.1093/aob/mcf199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaldenhoff R, Grote K, Zhu JJ, Zimmermann U. Significance of plasmalemna aquaporins for water transport in Arabidopsis thaliana. The Plant Journal. 1998;14:121–128. doi: 10.1046/j.1365-313x.1998.00111.x. [DOI] [PubMed] [Google Scholar]
- Katsuhara M, Akiyama Y, Koshio K, Shibasaka M, Kasamo K. Functional analysis of water channels in barley roots. Plant and Cell Physiology. 2002;43:885–893. doi: 10.1093/pcp/pcf102. [DOI] [PubMed] [Google Scholar]
- Katsuhara M, Hanba Y, Shiratake K, Maeshima M. Expanding roles of plant aquaporins in plasma membranes and cell organelles. Functional Plant Biology. 2008;35:1–14. doi: 10.1071/FP07130. [DOI] [PubMed] [Google Scholar]
- Katsuhara M, Koshio K, Shibasaka M, Hayashi Y, Hayakawa T, Kasamo K. Over-expression of a barley aquaporin increased the shoot/root ratio and raised salt sensitivity in transgenic rice plants. Plant and Cell Physiology. 2003a;44:1378–1383. doi: 10.1093/pcp/pcg167. [DOI] [PubMed] [Google Scholar]
- Katsuhara M, Koshio K, Shibasaka M, Kasamo K. Expression of an aquaporin at night in relation to the growth and root water permeability in barley seedlings. Soil Science and Plant Nutrition. 2003b;49:883–888. [Google Scholar]
- Knipfer T, Fricke W. Root pressure and a solute reflection coefficient close to unity exclude a purely apoplastic pathway of radial water transport in barley (Hordeum vulgare) New Phytologist. 2010;187:159–170. doi: 10.1111/j.1469-8137.2010.03240.x. [DOI] [PubMed] [Google Scholar]
- Knipfer T, Fricke W. Water uptake of seminal and adventitious roots in relation to whole-plant water flow in barley (Hordeum vulgare L.) Journal of Experimental Botany. 2011;62:717–733. doi: 10.1093/jxb/erq312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann H, Stelzer R, Holzamer S, Kunz U, Gierth M. Analytical electron microscopical investigations on apoplastic pathways of lanthanum transport in barley roots. Planta. 2000;211:816–822. doi: 10.1007/s004250000346. [DOI] [PubMed] [Google Scholar]
- Ma S, Quist TM, Ulanov A, Joly R, Bohnert HJ. Loss of TIP1;1 aquaporin in Arabidopsis leads to cell and plant death. The Plant Journal. 2004;40:845–859. doi: 10.1111/j.1365-313X.2004.02265.x. [DOI] [PubMed] [Google Scholar]
- Maurel C. Plant aquaporins: novel functions and regulation properties. FEBS Letters. 2007;581:2227–2236. doi: 10.1016/j.febslet.2007.03.021. [DOI] [PubMed] [Google Scholar]
- Maurel C, Reizer J, Schroeder JI, Chrispeels MJ. The vacuolar membrane protein gamma-TIP creates water specific channels in Xenopus oocytes. EMBO Journal. 1993;12:2241–2247. doi: 10.1002/j.1460-2075.1993.tb05877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurel C, Verdoucq L, Luu DT, Santoni V. Plant aquaporins: membrane channels with multiple integrated functions. Annual Review of Plant Biology. 2008;59:595–624. doi: 10.1146/annurev.arplant.59.032607.092734. [DOI] [PubMed] [Google Scholar]
- Pfaffl M. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Research. 2001;29:2002–2007. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickard WF. The riddle of root pressure. I. Putting Maxwell's demon to rest. Functional Plant Biology. 2003;30:121–134. doi: 10.1071/FP02035. [DOI] [PubMed] [Google Scholar]
- Postaire O, Tournaire-Roux C, Grondin A, Boursiac Y, Morillon R, Schäffner AR, Maurel C. A PIP1 aquaporin contributes to hydrostatic pressure-induced water transport in both the root and rosette of Arabidopsis. Plant Physiology. 2010;152:1418–1430. doi: 10.1104/pp.109.145326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai J, Ahamed A, Murai M, Maeshima M, Uemura M. Tissue and cell-specific localization of rice aquaporins and their water transport activities. Plant and Cell Physiology. 2008;49:30–39. doi: 10.1093/pcp/pcm162. [DOI] [PubMed] [Google Scholar]
- Sakurai J, Ishikawa F, Yamaguchi T, Uemura M, Maeshima M. Identification of 33 aquaporin genes and analysis of their expression and function. Plant and Cell Physiology. 2005;46:1568–1577. doi: 10.1093/pcp/pci172. [DOI] [PubMed] [Google Scholar]
- Sanderson J. Water uptake by different regions of the barley root. Pathways of radial flow in relation to development of the endodermis. Journal of Experimental Botany. 1983;34:240–253. [Google Scholar]
- Schreiber L, Hartmann K, Skrabs M, Zeier J. Apoplastic barriers in roots: chemical composition of endodermal and hypodermal cell walls. Journal of Experimental Botany. 1999;50:1267–1280. [Google Scholar]
- Schüssler MD, Alexandersson E, Bienert GP, Kichey T, Laursen KH, Johanson U, Kjellbom P, Schjoerring JK, Jahn TP. The effects of the loss of TIP1;1 and TIP1; 2 aquaporins in Arabidopsis thaliana. The Plant Journal. 2008;56:756–767. doi: 10.1111/j.1365-313X.2008.03632.x. [DOI] [PubMed] [Google Scholar]
- Steudle E. Water uptake by plant roots: an integration of views. Plant and Soil. 2000;226:46–56. [Google Scholar]
- Steudle E, Peterson CA. How does water get through roots? Journal of Experimental Botany. 1998;49:775–788. [Google Scholar]
- Tazawa M, Ohkuma E, Shibasaka M, Nakashima S. Mercurial-sensitive water transport in barley roots. Journal of Plant Research. 1997;110:435–442. [Google Scholar]
- Tazawa M, Sutou E, Shibasaka M. Onion root water transport sensitive to water channel and K+ channel inhibitors. Plant and Cell Physiology. 2001;42:28–36. doi: 10.1093/pcp/pce004. [DOI] [PubMed] [Google Scholar]
- Volkov V, Hachez C, Moshelion M, Draye X, Chaumont F, Fricke W. Water permeability differs between growing and non-growing barley leaf tissues. Journal of Experimental Botany. 2007;58:377–390. doi: 10.1093/jxb/erl203. [DOI] [PubMed] [Google Scholar]
- Wan XC, Zwiazek JJ. Mercury chloride effects on root water transport in aspen seedlings. Plant Physiology. 1999;121:939–946. doi: 10.1104/pp.121.3.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Alexandersson E, Golldack D, Miller AJ, Kjellbom PO, Fricke W. HvPIP1;6 a barley (Hordeum vulgare L.) plasma membrane water channel particularly expressed in growing compared with non-growing leaf tissues. Plant and Cell Physiology. 2007;48:1132–1147. doi: 10.1093/pcp/pcm083. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.