Abstract
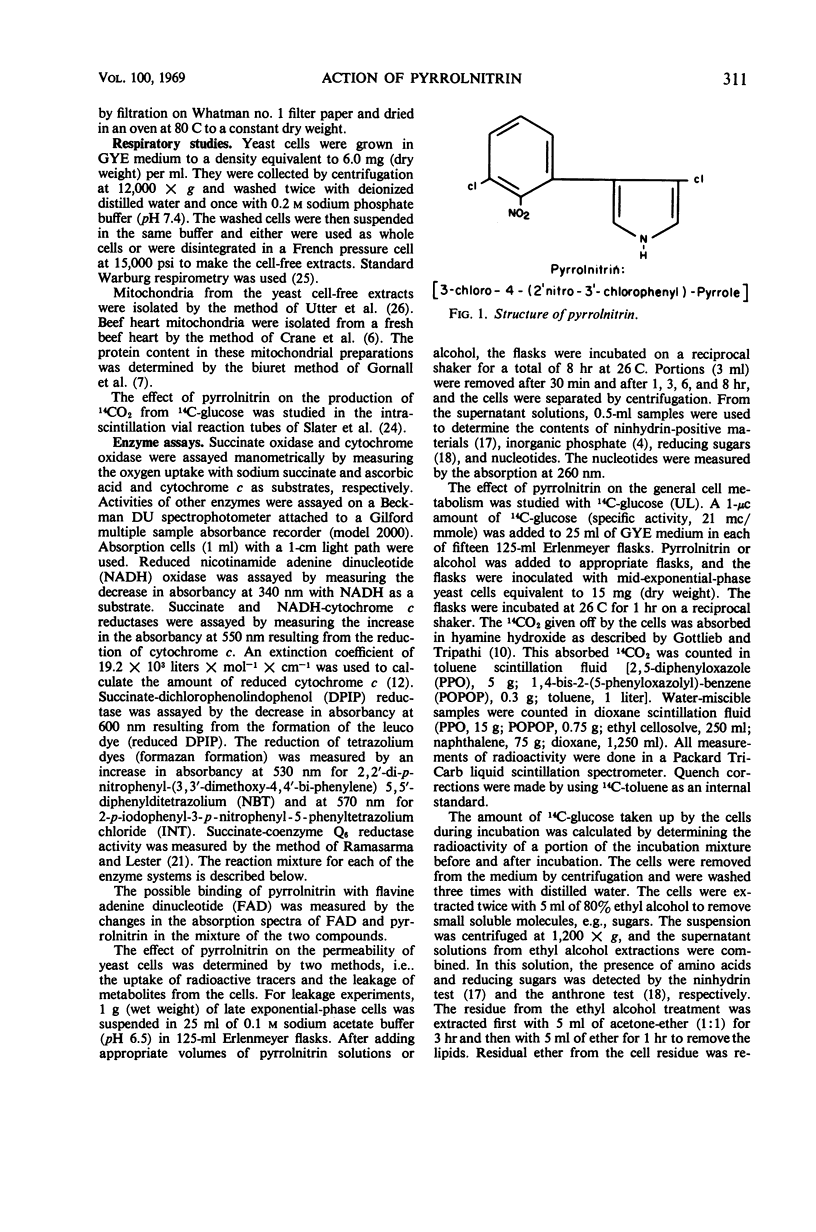
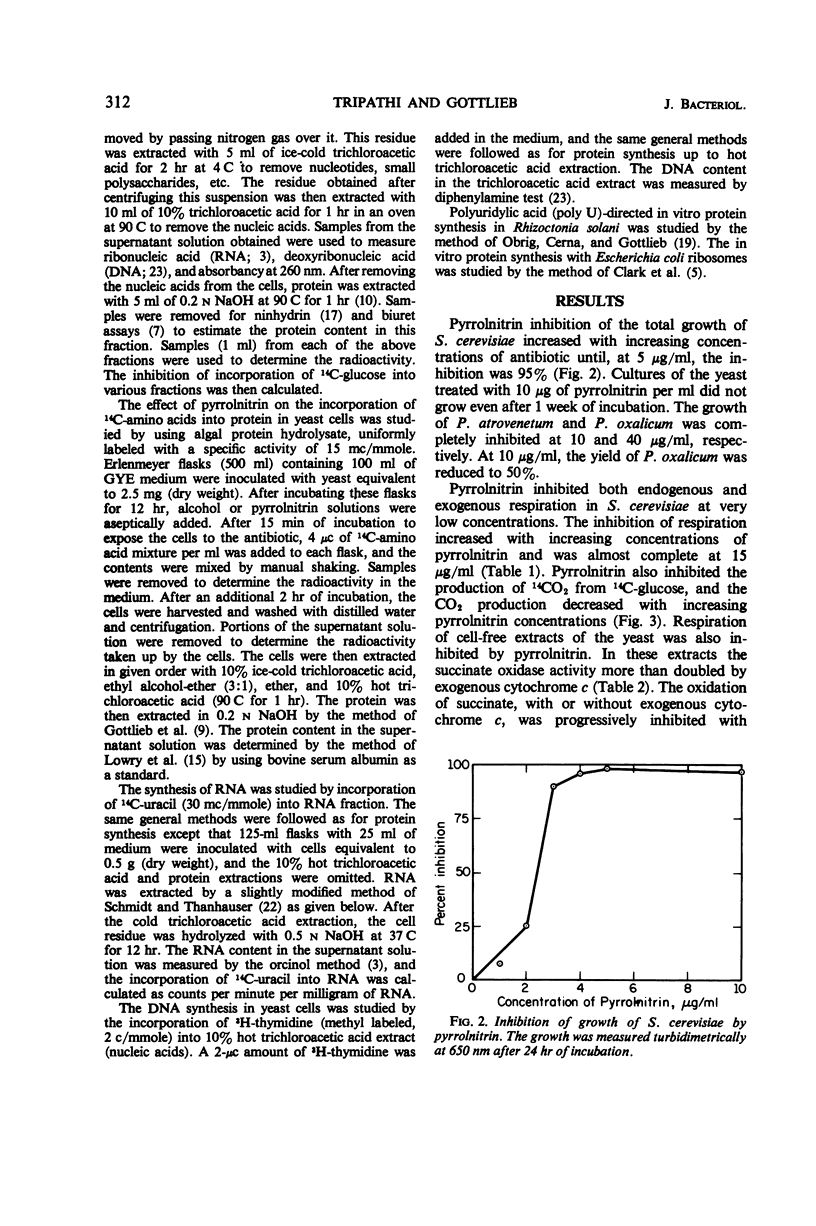
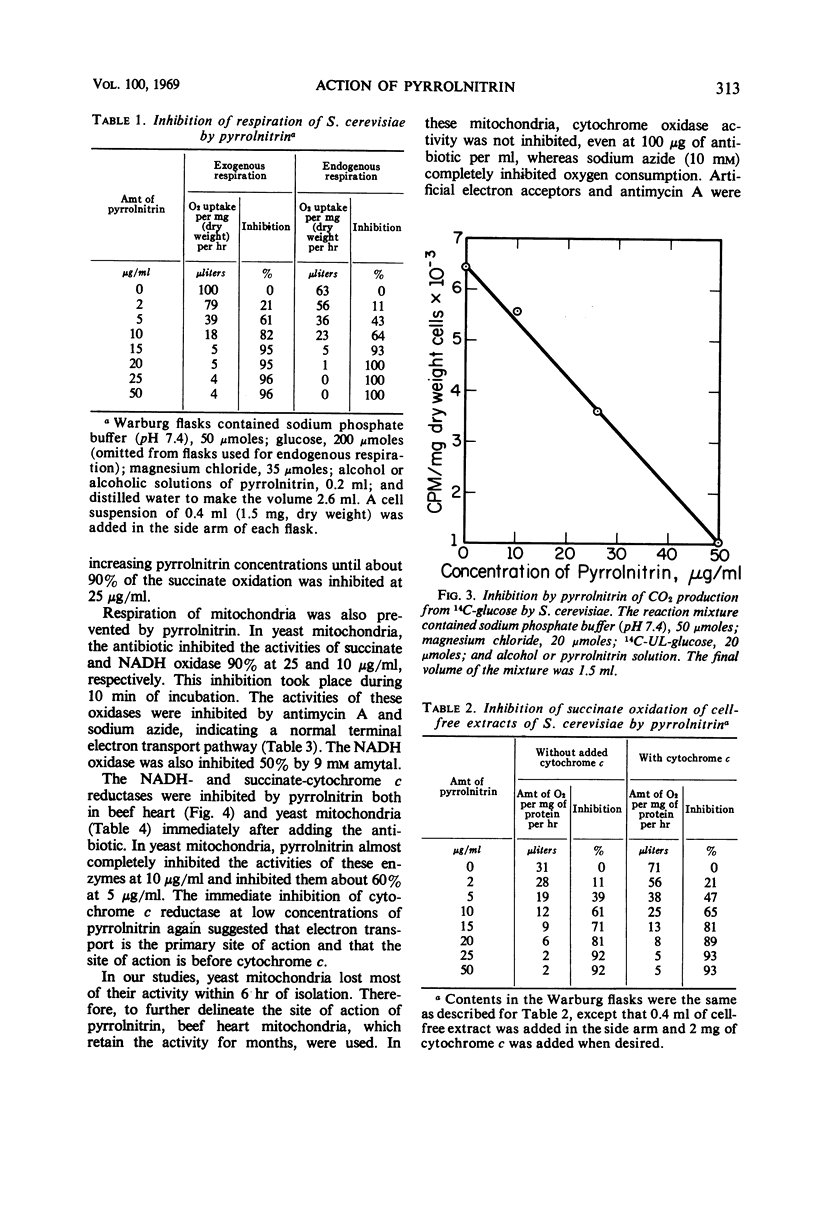
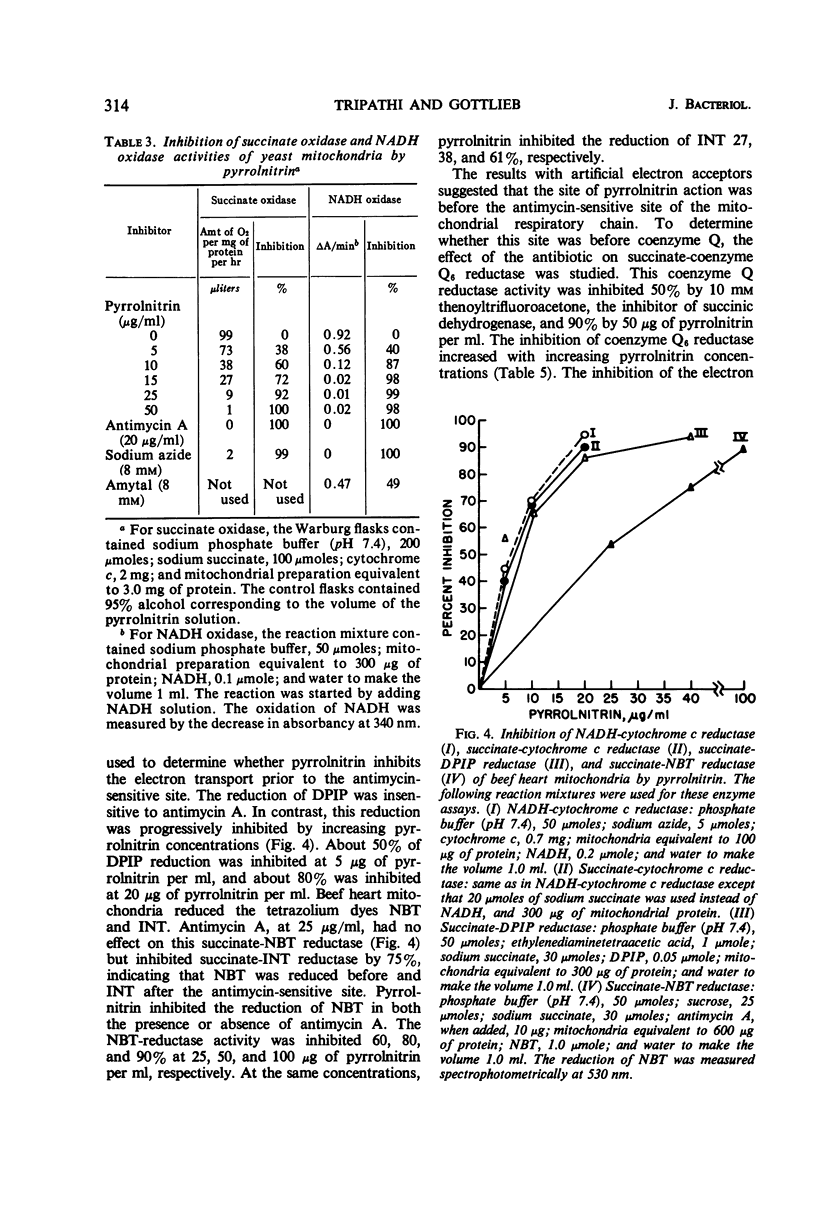
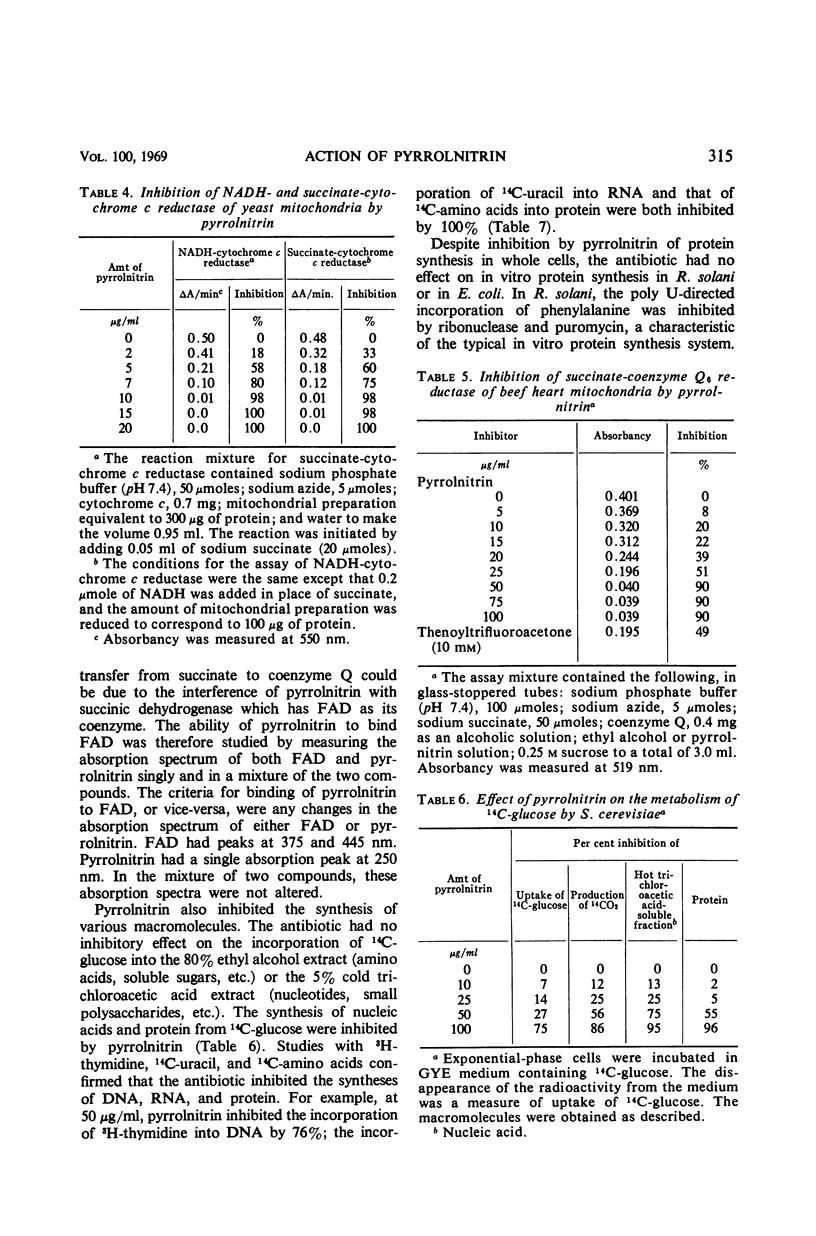
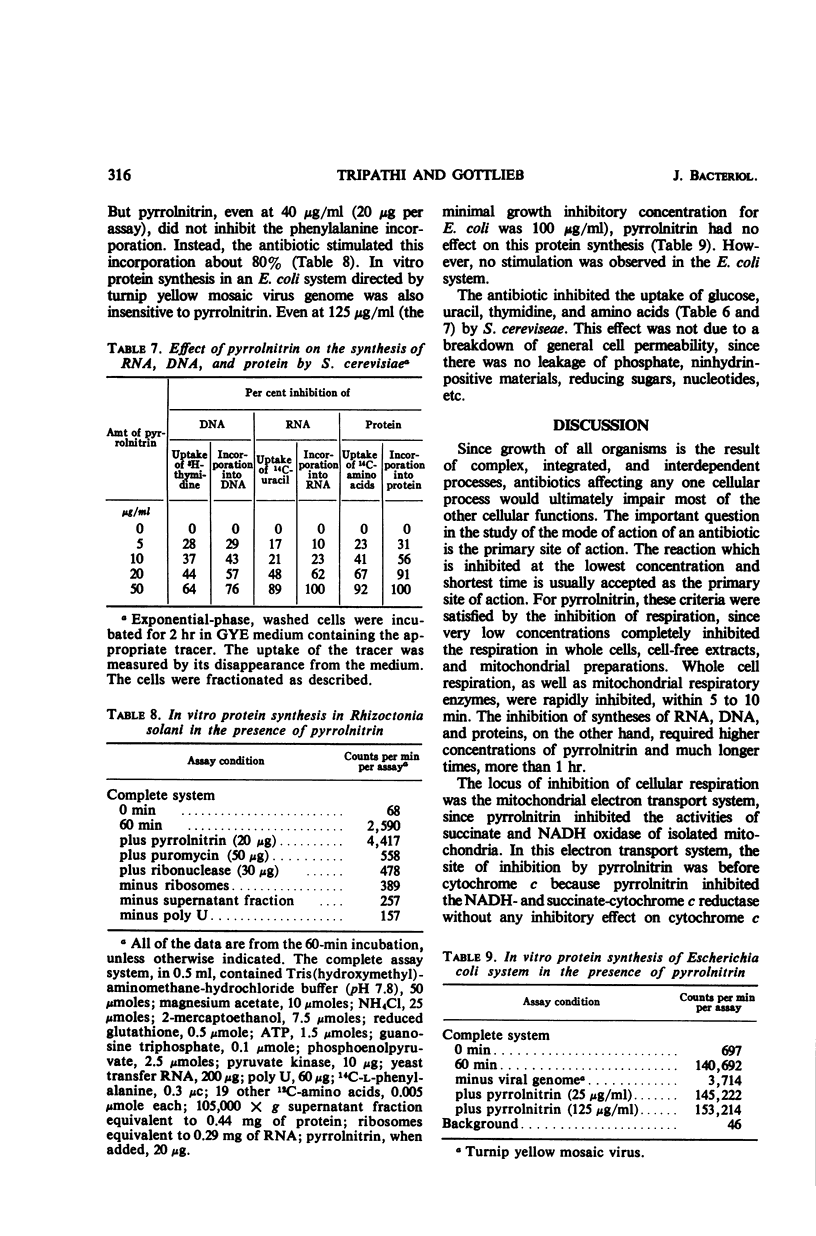
Pyrrolnitrin at 10 μg/ml inhibited the growth of Saccharomyces cerevisiae, Penicillium atrovenetum, and P. oxalicum. The primary site of action of pyrrolnitrin on S. cerevisiae was the terminal electron transport system between succinate or reduced nicotinamide adenine dinucleotide (NADH) and coenzyme Q. At growth inhibitory concentrations, pyrrolnitrin inhibited endogenous and exogenous respiration immediately after its addition to the system. In mitochondrial preparations, the antibiotic inhibited succinate oxidase, NADH oxidase, succinate-cytochrome c reductase, NADH-cytochrome c reductase, and succinate-coenzyme Q6 reductase. In addition, pyrrolnitrin inhibited the antimycin-insensitive reduction of dichlorophenolindophenol and of the tetrazolium dye 2,2′-di-p-nitrophenyl-(3,3′-dimethoxy-4,4′-bi-phenylene)5,5′-diphenylditetrazolium. The reduction of another tetrazolium dye, 2-p-iodophenyl-3-p-nitrophenyl-5-phenyltetrazolium chloride, that was antimycin-sensitive, was also inhibited by pyrrolnitrin. The antibiotic had no effect on the activity of cytochrome oxidase, and it did not appear to bind with flavine adenine dinucleotide, the coenzyme of succinic dehydrogenase. In whole cells of S. cerevisiae, pyrrolnitrin inhibited the incorporation of 14C-glucose into nucleic acids and proteins. It also inhibited the incorporation of 14C-uracil, 3H-thymidine, and 14C-amino acids into ribonucleic acid, deoxyribonucleic acid, and protein, respectively. The in vitro protein synthesis in Rhizoctonia solani and Escherichia coli was not affected by pyrrolnitrin. Pyrrolnitrin also inhibited the uptake of radioactive tracers, but there was no general damage to the cell membranes that would result in an increased leakage of cell metabolites. Apparently, pyrrolnitrin inhibits fungal growth by inhibiting the respiratory electron transport system.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arima K., Imanaka H., Kousaka M., Fukuda A., Tamura G. Studies on pyrrolnitrin, a new antibiotic. I. Isolation and properties of pyrrolnitrin. J Antibiot (Tokyo) 1965 Sep;18(5):201–204. [PubMed] [Google Scholar]
- CRANE F. L., GLENN J. L., GREEN D. E. Studies on the electron transfer system. IV. The electron transfer particle. Biochim Biophys Acta. 1956 Dec;22(3):475–487. doi: 10.1016/0006-3002(56)90058-0. [DOI] [PubMed] [Google Scholar]
- Clark J. M., Jr, Chang A. Y., Spiegelman S., Reichmann M. E. The in vitro translation of a monocistronic message. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1193–1197. doi: 10.1073/pnas.54.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb D. Antibiotics and cell metabolism. Hindustan Antibiot Bull. 1967 Nov;10(2):123–134. [PubMed] [Google Scholar]
- Inoue Y., Gottlieb D. Mechanism of action of flavensomycin on Penicillium oxalicum. Antimicrob Agents Chemother (Bethesda) 1966;6:470–479. [PubMed] [Google Scholar]
- LESTER R. L., SMITH A. L. Studies on the electron transport system. 28. The mode of reduction of tetrazolium salts by beef heart mitochondria; role of coenzyme Q and other lipids. Biochim Biophys Acta. 1961 Mar 4;47:475–496. doi: 10.1016/0006-3002(61)90543-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lively D. H., Gorman M., Haney M. E., Mabe J. A. Metabolism of tryptophans by Pseudomonas aureofaciens. I. Biosynthesis of pyrrolnitrin. Antimicrob Agents Chemother (Bethesda) 1966;6:462–469. [PubMed] [Google Scholar]
- Morris D. L. Quantitative Determination of Carbohydrates With Dreywood's Anthrone Reagent. Science. 1948 Mar 5;107(2775):254–255. doi: 10.1126/science.107.2775.254. [DOI] [PubMed] [Google Scholar]
- Nose M., Arima K. On the mode of action of a new antifungal antibiotic, pyrrolnitrin. J Antibiot (Tokyo) 1969 Apr;22(4):135–143. doi: 10.7164/antibiotics.22.135. [DOI] [PubMed] [Google Scholar]
- RAMASARMA T., LESTER R. L. Studies on the electron transport system. XXIV. The reduction and oxidation of exogenous coenzyme Q. J Biol Chem. 1960 Nov;235:3309–3314. [PubMed] [Google Scholar]
- UTTER M. F., KEECH D. B., NOSSAL P. M. Oxidative phosphorylation by subcellular particles from yeast. Biochem J. 1958 Mar;68(3):431–440. doi: 10.1042/bj0680431. [DOI] [PMC free article] [PubMed] [Google Scholar]


