Abstract
The behavioral effects of cocaine are affected by gene knockout of the dopamine transporter (DAT), the serotonin transporter (SERT) and the norepinephrine transporter (NET). The relative involvement of each of these transporters varies depending on the particular behavioral response to cocaine considered, as well as on other factors such as genetic background of the subjects. Interestingly, the effects of these gene knockouts on cocaine induced locomotion are quite different from those on reward assessed in the conditioned place preference paradigm. To further explore the role of these genes in the rewarding effects of cocaine, the ability of five daily injections of cocaine to induce conditioned locomotion was assessed in DAT, SERT and NET knockout (KO) mice. Cocaine increased locomotor activity acutely during the initial conditioning session in SERT KO and NET KO, but not DAT KO, mice. Surprisingly, locomotor responses in the cocaine-paired subjects diminished over the 5 conditioning sessions in SERT KO mice, while locomotor responses increased in DAT KO mice, despite the fact that they did not demonstrate any initial locomotor responses to cocaine. Cocaine-induced locomotion was unchanged over the course of conditioning in NET KO mice. In the post-conditioning assessment, conditioned locomotion was not observed in DAT KO mice, and was reduced in SERT KO and NET KO mice. These data reaffirm the central role of dopamine and DAT in the behavioral effects of cocaine. Furthermore, they emphasize the polygenic basis of cocaine mediated behavior and the non-unitary nature of drug reward mechanisms, particularly in the context of previous studies that have shown normal cocaine conditioned place preference in DAT KO mice.
Introduction
Initial transgenic studies of the mechanisms underlying the rewarding effects of cocaine found that deletion of the gene for the dopamine transporter (DAT) alone did not eliminate the rewarding effects of cocaine as assessed in either the conditioned place preference (CPP) or self-administration paradigms (Rocha et al., 1998; Sora et al., 1998). Subsequent studies found that combined elimination of the serotonin transporter (SERT) and DAT eliminated the rewarding effects of cocaine in the conditioned place preference paradigm (Sora et al., 2001). However, the effects of SERT KO are rather complex and can also increase the rewarding effects of cocaine (Sora et al., 1998; Hall et al., 2002; Hall et al., Submitted). This should not be surprising given the diverse effects that pharmacological treatments aimed at specific serotonin receptor subtypes have on drug reward, including both increases and decreases in the rewarding effects of diverse classes of addictive drugs (Carboni et al., 1989; Fadda et al., 1991; Higgins et al., 1992a; Higgins et al., 1992b; Bisaga et al., 1993; Kostowski et al., 1993; Lu et al., 1994; McMillen et al., 1994; Tomkins et al., 1994a; Tomkins et al., 1994b; Rompre et al., 1995; Tomkins et al., 1995; Parsons et al., 1998; Wilson et al., 1998; Fletcher & Korth, 1999; Harrison et al., 1999; Maurel et al., 1999; Tomkins & O'Neill, 2000; Fletcher et al., 2004). Indeed, under some circumstances (e.g. DAT KO mice) the selective serotonin reuptake inhibitor fluoxetine has been shown to have rewarding effects (Hall et al., 2002).
In monoamine transporter knockout mice the rewarding and reinforcing effects of cocaine have been assessed primarily with the conditioned place preference paradigm. The effects of these knockouts in other paradigms have not been extensively characterized, but should not be expected to be necessarily uniform. The different methods used to assess the rewarding properties of drugs of abuse have often been superficially treated as if they are all equivalent measures of a single unitary construct, in part based on early descriptions equating locomotor stimulant effects with drug reward (Wise & Bozarth, 1987), even though the diversity of reward mechanisms has long been recognized (Wise & Leeb, 1993), especially the role of conditioned responses in the maintenance of drug-seeking behavior and sensitization (Post et al., 1981; Stewart, 1983; Post et al., 1987). In fact a critical, though often overlooked, distinction has been made between two factors that contribute to cocaine sensitization, the role of conditioned drug effects and the role of neuropharmacological alterations induced by the repeated exposure to drugs of abuse (Pert et al., 1990). These two factors are sometimes described as context-dependent and context-independent sensitization and have been shown to involve different neurobiological mechanisms (Wise & Leeb, 1993). However, these types of effects involve administration of drugs after repeated treatment and sensitization is evinced by enhanced response to the drug compared to untreated animals or animals treated chronically with saline. However, context-dependent sensitization can be clearly shown to be a conditioned response. The increase in behavioral response in this circumstance is dependent on exposure to the conditioned stimuli and results in conditioned increases in locomotion (e.g. conditioned locomotion) even without any drug treatment. The relative importance of context-dependent and context-independent sensitization for the actual mechanisms underlying addiction is a matter of some debate, and although both are certainly important, it has certainly been argued that alterations in associative processes may play critical roles in addiction (Everitt et al., 2001). However, it is important to note that sensitization to cocaine can be observed independently of conditioned locomotion (Carey & Gui, 1998; Carey & Damianopoulos, 2006). Furthermore, multiple conditioned effects of drugs of abuse can be observed independently of each other, further indicating the non-unitary bases of drug reward and drug seeking behavior. For instance, conditioned locomotor activity can be observed independently from conditioned place preference (Kosten & Miserendino, 1998).
In the initial description of the elimination of the locomotor effects of cocaine in DAT KO mice they were described as “indifferent” to cocaine (Giros et al., 1996), the implication being that lack of locomotor stimulant effects should be equated with elimination of rewarding effects. This was proven to be incorrect (Rocha et al., 1998; Sora et al., 1998), but there often remains a tacit assumption that manipulations that affect one aspect of cocaine-mediated behavior should affect other behaviors in a similar manner. One way to directly address this issue is to evaluate gene knockouts that produce a particular pattern of effects on one cocaine associated behavior, and compare them to the consequences of those gene knockouts on another cocaine-associated behavior. The effects of monoamine transporter knockouts on cocaine conditioned place preference have been well characterized: Cocaine CPP is unaffected in DAT KO mice (Sora et al., 1998), but increased in SERT KO and NET KO mice (Sora et al., 1998; Xu et al., 2000). In addition to producing a place preference cocaine also induces conditioned locomotion (Post et al., 1987), which has not been examined for any of these gene knockouts. Therefore, to further explore the role of these genes in the rewarding effects of cocaine, the ability of repeated injections of cocaine to induce conditioned locomotion was assessed in DAT, SERT and NET KO mice.
METHODS
Subjects
DAT (Sora et al., 1998), SERT (Bengel et al., 1998) and NET (Wang et al., 1999) knockout mice have been described previously. These knockout lines were used to create DAT/SERT (Sora et al., 2001) and NET/SERT (Hall et al., 2002) double knockout strains. In the present experiments DAT +/+, DAT +/− and DAT −/− mice were bred from the DAT/SERT line; SERT +/+, SERT +/− and SERT −/− mice were bred from the DAT/SERT line; and NET +/+, NET +/− and NET −/− mice were bred from the NET/SERT line. Male and female mice were used, and were tested at 12 – 18 weeks of age. Mice were bred from double heterozygote (e.g. DAT +/− SERT +/− × DAT +/− SERT +/−) or single heterozygote (e.g. DAT +/− SERT +/+ × DAT +/− SERT +/+) crosses.
Wild-type (+/+), heterozygote KO mice (+/−) and homozygote knockout mice (−/−) were genotyped by PCR, using two internal primers, one targeted at the knockout insertion sequence and one targeted at the WT gene, and one external primer, which generated two products identifying the WT and KO genes. The DAT and SERT transgenic knockout insertion sequences contained a neomycin gene (NEO), while the NET KO contained a green fluorescent protein gene insert (GFP). PCR using TaKaRa DNA polymerase (Takara Bio, Japan) was performed on DNA that was released from tail tip fragments after overnight digestion with Protease K. For DAT genotyping the external primer (5' AGT GTG TGC AGG GCA TGG TGT A 3') and the WT primer (5' TAG GCA CTG CTG ACG ATG ACT G 3') produced a 500 bp band, while the external primer and the NEO primer (5' CTC GTC GTG ACC CAT GGC GAT 3') produced a 600 bp band. For SERT genotyping the external primer (5' GCT CTC AGT CTT GTC TCC ATA AC 3') and the WT primer (5' TGC TGA CTG GAG TAC AGG CTA G 3') produced a 620 bp band, while the external primer and the NEO primer (5' CTC GTC GTG ACC CAT GGC GAT 3') produced an 800 bp band. For NET genotyping the external primer (5' GCT CTG TCC CTG TGC TTC ACG 3') and the WT primer (5' TGA GGC CTA AGC TGG AGC TCG 3') produced a 601 bp band, while the external primer and the GFP primer (5' CGG TGA ACA GCT CCT CGC CC 3') produced a 470 bp band.
Conditioned Locomotion Procedure
Homozygous and heterozygous DAT, NET and SERT KO mice and WT littermate controls were divided into three experimental groups: Paired, Unpaired and Control groups (DAT KO, N=8–12 per genotype per condition; NET KO, N=8–11 per genotype per condition; SERT KO, N=9–18 per genotype per condition). Mice in each group received two injections each day, one before being placed in a locomotor activity chamber and one later in the home cage. Locomotor testing was conducted using an Optovarimax locomotor activity testing apparatus (Columbus Instruments, Columbus, OH, USA) under dark conditions in sound attenuating chambers. Mice in the Paired group received an injection of cocaine HCl (20 mg/kg SC) prior to locomotor testing for 30 minutes. Subjects were then returned to their home cages and 2 hours later they received an injection of saline (10 ml/kg). Mice in the Unpaired group received an injection of saline prior to locomotor testing and an injection of cocaine (20 mg/kg SC) in the home cage. Mice in the control group received saline injections before locomotor testing and in the home cage. This procedure was conducted each day for 5 days; on the day following the final injections, mice were placed in the locomotor activity chambers for 20 minutes without any injections to assess conditioned locomotion.
Statistics
Statistical comparisons were made with analysis of variance (ANOVA) followed by Scheffe's post-hoc analyses using Statview (SAS). Conditioning data were initially analyzed by an overall ANOVA with the between subjects factors of GENOTYPE (+/+, +/− and −/−) and CONDITIONING GROUP (Paired, Unpaired and Control), and the additional within-subjects factor of CONDITIONING TRIAL (Days 1 – 5). Subsequently, the data for each genotype (+/+, +/− and −/−) were analyzed separately with the between-subjects factor of CONDITIONING GROUP (Paired, Unpaired and Control), and the within-subjects factor of CONDITIONING TRIAL (Day 1 – 5). Data from the post-conditioning test were analyzed with the between subjects factors of CONDITIONING GROUP and GENOTYPE (+/+, +/− and −/−). Post hoc comparison's were made with Scheffe's test (p<0.05 significant level).
RESULTS
Locomotion during conditioning trials in DAT KO mice
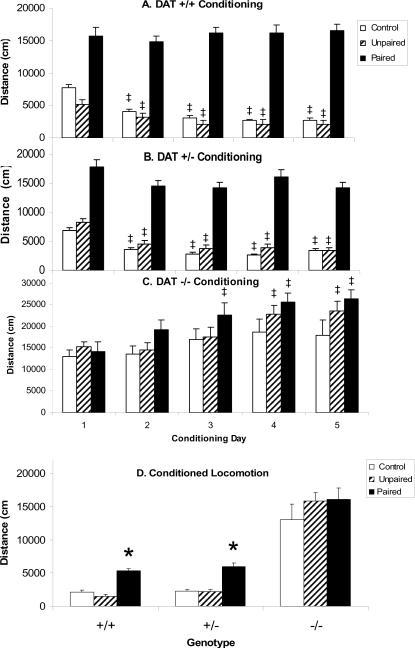
During the conditioning trials mice receiving injections of cocaine prior to testing (Paired Group) were significantly more active than mice treated with saline prior to testing (Unpaired and Control groups) as reflected by an overall significant effect of CONDITIONING GROUP (F[2,87]=66.2, P=0.0001; Figs. 1A–C). DAT −/− mice were significantly more active under all conditions compared to DAT +/− and DAT +/+ mice as reflected by a significant effect of GENOTYPE (F[2,87]=71.0, P=0.0001), but did not exhibit increases in locomotor activity after acute cocaine administration so that there was also a significant GENOTYPE × CONDITIONING GROUP interaction (F[4,87]=4.9, P=0.0013). Over the course of the conditioning trials locomotor activity decreased in DAT +/+ and DAT +/− saline treated subjects, but not DAT +/+ and DAT +/−cocaine treated subjects so that the relative magnitude of the cocaine effect increased over trials. In DAT −/− mice a different pattern of effects was observed. Unlike DAT +/+ and DAT +/− mice the activity of Control DAT −/− mice did not decrease. Furthermore, although there was no initial difference in locomotor activity between conditioning groups, over trials the activity of the cocaine treated groups (Paired and Unpaired) increased. Note that only the Paired subjects received cocaine prior to this locomotor test, the Unpaired subjects were injected with saline. Thus, in the ANOVA there were significant effects of CONDITIONING TRIAL (F[4,348]=8.1, P=0.0001), CONDITIONING TRIAL × CONDITIONING GROUP (F[8,348]=6.0, P=<0.0001), CONDITIONING TRIAL × GENOTYPE (F[8,348]=30.5, P=0.0001), and CONDITIONING TRIAL × CONDITIONING GROUP × GENOTYPE (F[16, 348]=2.0, P=0.012). To further clarify the nature of these effects individual ANOVA were performed on the data from each genotype.
Figure 1. Conditioned locomotion in DAT KO mice.
Locomotor activity during conditioning sessions in DAT +/+ (A), DAT +/− (B) and DAT −/− (C) mice from each of the conditioning groups (Paired, Unpaired and Control) expressed in terms of distance traveled. Conditioned locomotion (D) in all groups. *Significant difference from Control conditioning group based on Scheffe's post hoc comparison (p<0.05). ‡Significant difference from Trial 1 based on Scheffe's post hoc comparison (p<0.05). Data are represented as mean ± the standard error of the mean.
DAT +/+ mice treated with cocaine prior to locomotor testing (Paired group) were significantly more active than mice treated with saline (Unpaired and Control groups) throughout all five conditioning trials (Fig. 1A; CONDITIONING GROUP: F[2,31]=99.6, P=0.0001). Over the course of the 5 conditioning trials the activity of mice in the Unpaired and Control groups decreased, but the activity of mice in the Paired group was unchanged compared to day 1 so that the relative difference between saline injected and cocaine injected animals was greater in later trials. Thus, there was a significant interaction between CONDITIONING GROUP and CONDITIONING TRIAL (F[8,124]=5.6, P=0.0001). Post hoc Scheffe's comparisons demonstrated significantly reduced locomotion in acute saline treated groups (Unpaired and Control) for conditioning trials 2–5 compared to the first conditioning trial, but no differences between trials in the acute cocaine treated group (Paired).
A somewhat similar pattern was observed in DAT +/− mice (Fig. 1B), where there was a significant effect of CONDITIONING GROUP (F[2,31]=147.6, P=0.0001), but not a significant interaction between CONDITIONING GROUP and CONDITIONING TRIAL (F[8,124]=1.4, NS). In addition to decreases in locomotion in the Unpaired and Control groups, there was also a slight decrease in the activity of Paired subjects over trials. Post hoc one way ANOVA for each conditioning group revealed significant effects of CONDITIONING TRIAL in all three conditioning groups. Post hoc Scheffe's comparisons demonstrated significantly reduced locomotion in acute saline treated groups (Unpaired and Control) for conditioning trials 2–5 compared to trial 1. In the Paired group the reduction in locomotion was much smaller than in Unpaired and Control subjects so that no individual comparisons were significant even though there was an overall effect in the ANOVA.
In contrast to the pattern of effects observed in DAT +/+ and DAT −/− mice, a completely different pattern was observed in DAT −/−. As has been observed previously, cocaine did not increase locomotor activity in DAT −/− mice (Fig. 1C), although locomotion was substantially higher than the activity observed in DAT +/+ and DAT +/− mice (compare saline treated subjects in Figs. 1A−1C). Nonetheless, there was an increase in locomotion on the second and subsequent days in cocaine treated subjects (Paired group compared to the Control group). This increase in locomotion however was not limited to mice in the Paired group; the activity of mice in the unpaired group also increased over conditioning trials. Although there was not a significant overall effect of CONDITIONING GROUP (F[2,25]=2.6, NS), there was a significant effect of CONDITIONING TRIAL (F[4,100]=21.4, P=0.0001) and a significant interaction between CONDITIONING GROUP and CONDITIONING TRIAL (F[8,100]=2.6, P=0.013). In separate 1 way ANOVA performed on each conditioning group no effect of CONDITIONING TRIAL was found in Control subjects (F[4,28]=1.5, NS), but significant effects were observed in both Paired (F[4,36]=18.9, P=0.0001) and Unpaired (F[4,36]=9.2, P=0.0001) groups. Post hoc comparisons of activity versus the first testing day demonstrated significant increases in both Paired subjects on trials 3–5 compared to trial 1, and on trials 4–5 compared to trial 1 in Unpaired mice (Scheffe's post hoc comparisons).
Conditioned locomotion in DAT KO mice
In the post-conditioning test DAT +/+ and DAT +/− mice demonstrated a typical pattern consistent with conditioned locomotion (Fig. 1D): increased locomotor activity during the postconditioning test in Paired mice compared to both Unpaired and Control mice. This test was conducted without any drug injection so it only reflects the ability of the conditioned associations of the environment to evoke locomotion. DAT −/− mice were much more active than DAT +/+ and DAT +/− mice independent of conditioning group. Thus, there were significant effects of both CONDITIONING GROUP (F[2,87]=12.7, P=0.0001) and GENOTYPE (F[2,87]=126.5, P=0.0001). In post hoc Scheffe's comparisons in DAT +/+ and DAT +/− Paired subjects were significantly more active than either Unpaired or Control subjects. Locomotor activity during the post-conditioning test was slightly greater in both Paired and Unpaired DAT −/− mice, compared to Control subjects, but neither comparison was significant.
Locomotion during conditioning trials in NET KO mice
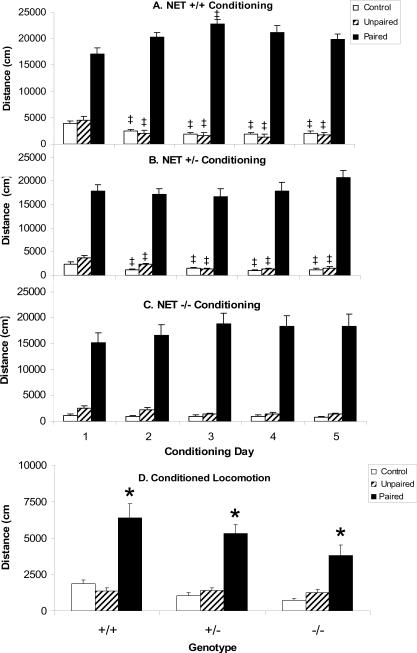
During the conditioning trials mice receiving injections of cocaine prior to testing (Paired Group) were significantly more active than mice treated with saline prior to testing (Unpaired and Control groups) as reflected by an overall significant effect of CONDITIONING GROUP (F[2,81]=373.6, P=0.0001; Figs. 2A–C). Over the course of the conditioning trials locomotor activity decreased in saline treated subjects, but not in cocaine treated subjects, so that there was a significant CONDITIONING TRIAL × CONDITIONING GROUP interaction (F[8,324]=6.7, P=0.0001). There was no effect of GENOTYPE (F[2,81]=2.7, NS), nor any significant interactions with genotype: CONDITIONING GROUP × GENOTYPE (F[4,81]=0.5, NS), CONDITIONING TRIAL × GENOTYPE (F[8,324]=1.6, NS), and CONDITIONING TRIAL × CONDITIONING GROUP × GENOTYPE (F[16, 324]=1.6, NS). Thus, for all genotype individual post hoc ANOVA identified only the effects of CONDITIONING TRIAL, CONDITIONING GROUP, and their interaction.
Figure 2. Conditioned locomotion in NET KO mice.
Locomotor activity during conditioning sessions in NET +/+ (A), NET +/− (B) and NET −/− (C) mice from each of the conditioning groups (Paired, Unpaired and Control) expressed in terms of distance traveled. Conditioned locomotion (D) in all groups. *Significant difference from Control conditioning group based on Scheffe's post hoc comparison (p<0.05). ‡Significant difference from Trial 1 based on Scheffe's post hoc comparison (p<0.05). Data are represented as mean ± the standard error of the mean.
NET +/+ mice treated with cocaine before testing (Paired group) were significantly more active than mice treated with saline (Unpaired and Control groups) over all conditioning trials (Fig. 2A; F[2,25]=128.5, P=0.0001). Over the course of the 5 conditioning trials the activity of saline-treated mice in the Unpaired and Control groups decreased, but the activity of mice in the paired group actually increased compared to day 1. These differential changes over conditioning trials resulted in a significant interaction between CONDITIONING GROUP and CONDITIONING TRIAL in the ANOVA (F[8,100]=9.6, P=0.0001). Post hoc 1-way ANOVA for each conditioning group in NET +/+ mice revealed a significant effect of CONDITIONING TRIAL in Control mice (F[4,36]=17.1, P=0.0001), Unpaired mice (F[4,36]=14.8, P=0.0001) and Paired mice (F[4,28]=4.2, P=0.0082). In both Control and Unpaired NET +/+ mice locomotor activity scores in trials 2–5 were all significantly lower than trial 1 (P<0.05 Scheffe's comparison). In Paired NET +/+ mice only trial 3 was significantly greater than trial 1 (p<0.05 Scheffe's comparison), but in no cases were decreases in activity observed in relation to trial 1.
A similar pattern was observed in NET +/− mice (Fig. 2B), where there was a significant effect of CONDITIONING GROUP (F[2,28]=141.1, P=0.0001), CONDITIONING TRIAL (F[4,112]=3.1, P=0.019) and a significant interaction between CONDITIONING GROUP and CONDITIONING TRIAL (F[8,112]=2.9, P=0.0060). Post hoc 1-way ANOVA for each conditioning group in NET +/− mice revealed a significant effect of CONDITIONING TRIAL in Control mice (F[4,36]=6.6, P=0.0004), Unpaired mice (F[4,36]=12.2, P=0.0001) but not Paired mice (F[4,40]=2.3, NS). In Control NET +/− mice locomotor activity was significantly reduced in conditioning trials 2, 4 and 5 compared to trial 1 (P<0.05, Scheffe's comparison), while in and Unpaired NET +/− mice locomotor activity was significantly reduced in trials 2–5 compared to trial 1 (P<0.05, Scheffe's comparison). In Paired NET +/− mice no decreases in activity were observed.
In NET −/− mice administration of cocaine produced increases in locomotion across all conditioning trials but activity in NET −/− mice changed less across conditioning trials than activity in NET +/+ and NET +/− mice (Fig. 2C). Thus, there was a significant effect of CONDITIONING GROUP (F[2,28]=108.4, P=0.0001), but not CONDITIONING TRIAL (F[4,112]=0.3, NS), nor was there a significant interaction between CONDITIONING GROUP and CONDITIONING TRIAL (F[8,112]=1.3, NS).
Conditioned locomotion in NET KO mice
NET +/+, NET +/− and NET −/− mice demonstrated the typical pattern consistent with conditioned locomotion (Fig. 2D) as shown by a significant effect of CONDITIONING GROUP in the ANOVA (F[2,81]=73.0, P=0.0001). In addition, activity was slightly reduced in NET KO mice independent of conditioning group. Thus, there was a significant effect of GENOTYPE (F[2,81]=5.9, P=0.0041) in the ANOVA, but not a significant GENOTYPE × CONDITIONING GROUP interaction (F[4,81]=1.9, NS).
Locomotion during conditioning trials SERT KO mice
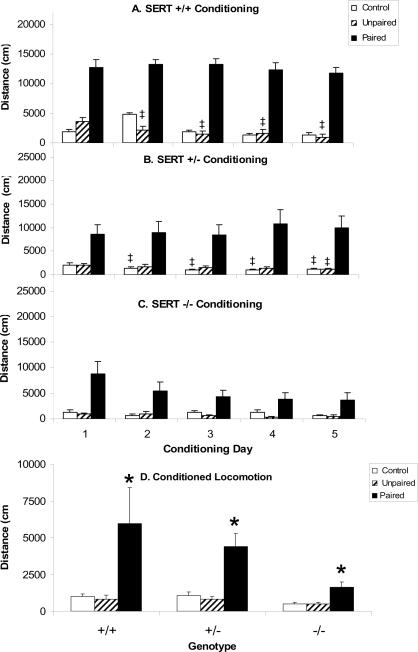
During the conditioning trials mice receiving cocaine prior to testing were significantly more active than mice treated with saline as reflected by an significant effect of CONDITIONING GROUP (F[2,104]=51.9, P=0.0001; Figs. 3A–C). SERT −/− mice were significantly less active under all conditions compared to SERT +/− and SERT +/+ mice as reflected by a significant effect of GENOTYPE (F[2,104]=5.1, P=0.0078). The GENOTYPE × CONDITIONING GROUP interaction was not significant overall (F[4,104]=1.9, NS), but there were differences between groups that emerged over repeated conditioning trials resulting in a significant GENOTYPE × CONDITIONING GROUP × CONDITIONING TRIAL interaction (F[16,416]=2.0, P=0.012). Over the course of the conditioning trials locomotor activity decreased in saline treated subjects of all genotypes. Locomotor activity did not decrease in SERT +/+ or SERT +/− acute cocaine treated subjects so that the relative magnitude of the cocaine effect increased over trials, but the magnitude of locomotion in the SERT −/− mice treated with cocaine decreased so that the magnitude of the cocaine effect did not change over conditioning trials.
Figure 3. Conditioned locomotion in SERT KO mice.
Locomotor activity during conditioning sessions in SERT +/+ (A), SERT +/− (B) and SERT −/− (C) mice from each of the conditioning groups (Paired, Unpaired and Control) expressed in terms of distance traveled. Conditioned locomotion (D) in all groups. *Significant difference from Control conditioning group based on Scheffe's post hoc comparison (p<0.05). ‡Significant difference from Trial 1 based on Scheffe's post hoc comparison (p<0.05). Data are represented as mean ± the standard error of the mean.
SERT +/+ mice treated with cocaine prior to locomotor testing (Paired group) were significantly more active than mice treated with saline (Unpaired and Control groups) on the first and subsequent days (Fig. 3A). There was a significant effect of CONDITIONING GROUP (F[2,27]=22.5, P=0.0001), but the interaction between CONDITIONING GROUP and CONDITIONING TRIAL was not significant (F[8,108]=0.4, NS). Decreased locomotion across trials was observed in both saline treated groups as confirmed in 1-way ANOVA for the Unpaired (F[4,44]=14.8, P=0.0001) and Control (F[4,32]=3.9, P=0.011) groups. In post hoc Scheffe's comparisons in the Control group there were no individual trials that were significantly different from trial 1, but in the Unpaired group trials 2–5 were all significantly lower than trial one. There was no change in locomotion over trials in the Paired group (F[4,32]=0.1, NS).
A similar pattern was observed in SERT +/− mice (Fig. 3B), where there was a significant effect of CONDITIONING GROUP (F[2,40]=14.1, P=0.0001), but not a significant interaction between CONDITIONING GROUP and CONDITIONING TRIAL (F[8,160]=1.5, NS). Again, decreased locomotion across trials was observed in both saline treated groups as confirmed in 1-way ANOVA for the Unpaired (F[4,40]=4.0, P=0.084) and Control (F[4,52]=9.2, P=0.0001) groups. In post hoc Scheffe's comparisons for the Control group trials 2–5 were significantly lower than trial one. For the Unpaired group only trial 5 was significantly lower than trial 1. There was no change in locomotion over trials in the Paired group (F[4,68]=1.3, NS).
In SERT −/− mice a different pattern of effects emerged. Administration of cocaine produced increases in locomotion on all conditioning trials (Fig. 2C), as shown by a significant effect of CONDITIONING GROUP (F[2,37]=33.7, P=0.0001). Locomotion decreased across trials, as shown by a significant overall effect of CONDITIONING TRIAL (F[4,148]=5.6, P=0.003), but this effect was due primarily to reductions in locomotion in the cocaine treated group. Thus, there was a significant interaction between CONDITIONING GROUP and CONDITIONING TRIAL (F[8,148]=3.3, P=0.0017). Individual post hoc 1-way ANOVA revealed a significant effect of CONDITIONING TRIAL in Paired SERT −/− mice (F[4,40]=3.2, P=0.023), but not Unpaired (F[4,68]=1.3, NS) or Control (F[4,40]=2.1, NS) SERT −/− mice.
Conditioned locomotion in SERT KO mice
SERT +/+, SERT +/− and SERT −/− mice demonstrated the typical pattern consistent with conditioned locomotion (Fig. 3D), as demonstrated by a significant overall effect of CONDITIONING GROUP (F[2,104]=19.7, P=0.0001). In addition, SERT −/− mice had reduced locomotion independent of conditioning group as demonstrated by a significant overall effect of GENOTYPE (F[2,104]=4.7, P=0.011). This reduction in locomotor activity in SERT −/− mice compared to SERT +/+ mice was somewhat greater in Paired subjects than in Unpaired or Control subjects. The CONDITIONING GROUP × GENOTYPE interaction was just statistically significant (F[4,104]=2.5, P=0.050). Nonetheless, for all genotypes Paired mice had significantly greater activity than Unpaired or Control mice (p<0.05, Scheffe's post hoc comparison).
DISCUSSION
The main conclusion that may be drawn from these experiments is that the ability of cocaine to produce conditioned locomotion is dependent on the dopamine transporter, but not the norepinephrine or serotonin transporters. This is consistent with a dopamine lesion study which found the 6-OHDA induced lesions of the nucleus accumbens attenuated amphetamine conditioned locomotion (Gold et al., 1988). In addition, differences in context-independent sensitization and context-dependent sensitization were found in DAT KO, SERT KO and NET KO mice during the conditioning phase of the experiment. These are discussed in below in detail but further emphasize the non-unitary structure of drug reward mechanisms, the polygenic basis of drug reward mechanisms, and the involvement of all three of these neurotransmitters in cocaine mediated behavior, albeit to a different degree and in different circumstances.
The role of conditioned responses in drug-seeking behavior has long been recognized, including the role of conditioned responses in cocaine sensitization (Post et al., 1981; Stewart, 1983; Post et al., 1987). Different underlying mechanisms are known to be involved in context-dependent sensitization and context-independent sensitization (Wise & Leeb, 1993), in particular, but this same argument can be applied to numerous cocaine induced behaviors including acute locomotor responses, conditioned locomotion and conditioned place preference as well. For instance, differential sensitivity to cocaine sensitization across inbred strains of mice is not simply the result of differential acute sensitivity (Elmer et al., 1996). Different types of drug exposure experiences that enhance cocaine responses clearly have a different basis, including those relating to repeated drug exposure alone and those involving associative mechanisms. Enhanced responses after repeated cocaine treatments has both context-dependent and context-independent components, which can be dissociated, but specific conditioned responses can be further dissociated, including conditioned locomotion (Carey & Gui, 1998; Carey & Damianopoulos, 2006), which is not correlated with sensitization to cocaine (Hotsenpiller & Wolf, 2002; Tirelli et al., 2003) and persists for a longer time (Tirelli et al., 2005). Furthermore, conditioned responses can be dissociated from each other, including conditioned locomotion and conditioned place preference (Kosten & Miserendino, 1998).
Since many effects of cocaine and other psychostimulants have been thought to involve primarily dopaminergic mechanisms (Wise & Bozarth, 1987), much research has emphasized the importance of dopamine in these effects. This includes the first publication in DAT KO mice, in which these mice were described as “indifferent” to cocaine because they failed to exhibit locomotor stimulant responses after acute treatment (Giros et al., 1996). The presumption here was that all cocaine effects, including rewarding effects, could be represented in a unitary fashion by cocaine-stimulated locomotion. This was found to be incorrect by the demonstration that DAT KO mice can exhibit both cocaine conditioned place preference and cocaine self-administration (Rocha et al., 1998; Sora et al., 1998), although more recent evidence clearly demonstrates that the ability of cocaine to act as a reinforcer is substantially degraded in DAT KO mice (Thomsen et al., 2009). These and further studies also demonstrated the ability of gene knockouts of other cocaine targets (e.g. SERT and NET) to modulate the rewarding effects of cocaine (Sora et al., 1998; Sora et al., 2001; Hall et al., 2002). Since there appears to be a somewhat differential involvement of these systems in different cocaine effects, the present experiments examined another paradigm that examines cocaine conditioned responses.
Although the primary aim of the present study was to examine cocaine conditioned locomotion, analysis of behavior during the five conditioning sessions also allowed the examination of context-dependent sensitization of the acute locomotor stimulant effects of cocaine to some extent. Context-dependent sensitization could be observed during the conditioning trials but context-independent sensitization, in the unpaired subjects, obviously could not. DAT knockout eliminated cocaine conditioned locomotion, as might be expected since there was no initial locomotor stimulant response to cocaine in DAT −/− mice, but in addition locomotor activity increased slightly across conditioning trials in cocaine treated DAT −/− mice, regardless of whether or not cocaine was paired with the testing environment. In addition, it was apparent that the activity of Control DAT −/− did not decrease across trials, indicating impaired between-session habituation. A previous study found that sensitization of cocaine induced locomotion was eliminated in both DAT +/− and DAT −/− mice (Mead et al., 2002). However, the methods used in that experiment to examine cocaine sensitization are difficult to compare to the present findings or to the literature: in that study cocaine was administered intravenously after an extended period of habituation that almost normalized activity between the DAT −/− and DAT +/+ mice. Extended habituation would substantially affect the ability of the environment to act as a conditioned stimulus. In addition, the temporal differences between s.c. and i.v. drug administration would also affect the ability of different types of stimuli to act as reinforcers. Finally, the experimental conditions appeared to affect the acute locomotor stimulant effects of cocaine as well; in that study acute locomotor stimulant effects of cocaine were eliminated in DAT +/− mice, which was not observed in previous studies (Giros et al., 1996; Sora et al., 1998; Sora et al., 2001). The length of drug treatment may be another factor influencing sensitization in DAT knockout mice as a recent study found that methamphetamine sensitization was not attenuated in DAT +/− mice, but its development was delayed (Fukushima et al., 2007).
The mechanism underlying those remaining cocaine effects in DAT KO mice has been a matter of some speculation. Despite the fact that acute locomotor stimulatory effects are eliminated in DAT KO mice, cocaine still retains the ability to increase extracellular levels of dopamine, at least in some brain areas (Mateo et al., 2004b; Shen et al., 2004). There is some evidence that the locus of this effect may be different in DAT KO mice than that in wild-type mice. Local infusions of cocaine in either the dorsal or ventral striatum fail to increase extracellular dopamine levels (Mateo et al., 2004b; Shen et al., 2004) nor does cocaine affect DA clearance in striatal slices (Mateo et al., 2004a). Although there has been some suggestion that reuptake by NET or SERT, in the absence of DAT, might account for the effects of cocaine, neither desipramine nor fluoxetine affect DA clearance in striatal slices (Mateo et al., 2004a). However, peripheral injections of SERT blockers do increase extracellular DA in the striatum (Mateo et al., 2004b; Shen et al., 2004), an effect that is not observed in WT mice. The locus of the cocaine effect might involve SERT in the VTA where local injections of cocaine or fluoxetine lead to increased release of dopamine in the nucleus accumbens (Mateo et al., 2004b). This is consistent with the ability of combined DAT-SERT knockouts to eliminate cocaine conditioned place preference (Sora et al., 2001), and for fluoxetine to produce conditioned place preference in DAT KO mice (Hall et al., 2002).
Since cocaine retains its ability to elevate extracellular dopamine in, albeit via different mechanisms than in WT mice, and only in some brain regions, it might be suspected that cocaine sensitization may still be possible in DAT KO mice. Indeed the present study suggests that context-independent sensitization may be enhanced, while at the same time conditioned locomotion is eliminated. Because of the profound changes in dopamine clearance in DAT KO mice (Giros et al., 1996; Jones et al., 1998) there may be substantial alterations in spatiotemporal aspects of dopamine transmission between wiring (local synaptic) and volume transmission (Gonon et al., 2000), which may include the influence of dopamine on glutamate function. In addition to elevating extracellular levels of dopamine, cocaine also increases glutamate levels (Smith et al., 1995) an effect that is increased in animals that have developed context dependent cocaine sensitization (Pierce et al., 1996; Reid & Berger, 1996; Kalivas & Duffy, 1998). These changes are associated with increased sensitivity of dopaminergic neurons to glutamatergic stimulation (White et al., 1995; Zhang et al., 1997), and are associated with changes in glutamate receptor subunit expression in the NAC and VTA (Churchill et al., 1999). Sensitization of the glutamate response to cocaine has been found to result from context dependent, but not independent, sensitization (Bell et al., 2000) and the development, but not expression, of context-dependent sensitization can be blocked by AMPA antagonists (Li et al., 1997), and NMDA antagonists (Damianopoulos & Carey, 1995; Cervo & Samanin, 1996; Kim et al., 1996). Conditioned activity is associated with increases in nucleus accumbens glutamate and can be attenuated by AMPA antagonists (Cervo & Samanin, 1996; Hotsenpiller et al., 2001) and NMDA antagonists (Cervo & Samanin, 1996). Both NMDA and AMPA antagonists block the development of context independent sensitization as well (Li et al., 1999). Expression of a mutant NMDA receptor with impaired Ca++ flux in cells containing dopamine D1 receptors (DRD1) prevents the development of context-dependent cocaine sensitization and cocaine conditioned place preference (Heusner & Palmiter, 2005). Convergent DRD1-NMDA stimulation has been suggested to play a critical role in the development of context-dependent sensitization (Valjent et al., 2005). The observed role of glutamate and glutamatedopamine interactions in these phenomena are dependent in part upon experimental parameters and are not entirely clear by any means. However, because of the profound alterations in the dynamics of dopamine release in DAT KO mice it would appear likely that glutamatergic mechanisms would also be affected, although perhaps in such a way as to differentially affect context independent sensitization and conditioned locomotion. This possibility has not been investigated to any great degree, although glutamate manipulations do affect baseline hyperactivity in DAT KO mice (Gainetdinov et al., 2001).
Other evidence indicates that cocaine enhances glutamatergic inputs to midbrain dopamine neurons in a manner dependent on both DRD1 and glutamate AMPA receptors (Dong et al., 2004). Part of the evidence for this interaction involved the elimination of these effects in GLURA knockout mice. Elimination of this gene also blocked both conditioned locomotion and conditioned place preference, without affecting acute locomotor responses to cocaine (Dong et al., 2004). This study implicates potential neuroadaptations in glutamatergic afferents to midbrain dopamine neurons in the effects of context on conditioned responses that enhance drug-seeking behavior. Changes in synaptic spine density are observed in the nucleus accumbens core in response to a cocaine treatment regimen that induced context-dependent sensitization (Li et al., 2004). Interestingly, the same dose regimen produced neither behavioral or morphological changes when administered in the home cage, but higher doses that induced context-independent sensitization were able to increase spine density in the nucleus accumbens core. Increased spine densities were also observed in the nucleus accumbens shell, but were observed even after repeated context-independent treatment with low doses of cocaine that did not produce sensitization. Increased spine densities were also observed in the medial prefrontal cortex under both conditions, but the increases were greater after context-dependent sensitization. These data would suggest that experimental parameters have a substantial effect on the morphological consequences of cocaine treatment, which are highly dependent on experimental parameters and differ substantially across brain regions. These changes are likely to underlie changes in glutamate responsiveness to cocaine and various forms of cocaine conditioning and cocaine sensitization.
Such differential effects are necessary to explain the difference in context independent responses in DAT KO mice and conditioned locomotion. The anatomical locus most critical to conditioned locomotion appears to be different from that involved in the acute locomotor stimulant properties of cocaine. Quinolinic acid induced lesions of the amygdala have no effect on the acute locomotor effects of cocaine, but block the development of conditioned locomotion (Brown & Fibiger, 1993). This brain region has not been investigated in DAT KO mice. Pairing of novel contextual cues with cocaine produces greater sensitization than pairing with discrete stimuli (Crombag et al., 2000), although conditioning to discrete stimuli is also observed in terms of both context dependent sensitization and conditioned locomotion (Panlilio & Schindler, 1997). Interestingly, in that study the discrete stimuli that were used to produce conditioned locomotion also acted as conditioned reinforcers in a subsequent operant circumstance in which lever processing produced presentation of the conditioned stimuli. It has been recently shown that there is a substantial overlap between striatal neurons activated by acute cocaine (e.g. c-fos) and those that are activated by chronic cocaine (e.g. FOSB), but that the number of activated neurons is a small percentage of the overall number of striatal neurons and that each environment may induce a distinct subset, or ensemble, of striatal neurons (Mattson et al., 2008). These subjects were not tested for conditioned locomotion (e.g. the effect of re-exposure to the conditioned environment without any injections), but nonetheless subjects that were returned to the cocaine-paired environment and injected with saline showed substantial elevations in c-fos and a substantial overlap with FOSB; this activation probably represents the effect of the environmental context on the neuronal ensemble that drives locomotor behavior in this circumstance, and is likely related to the changes in synaptic morphology associated with chronic cocaine treatments discussed above.
Although there has been accumulating evidence that serotonin and norepinephrine may modulate cocaine reward, and that SERT and NET may have a role in cocaine reward under some circumstances the present experiments suggest that these effects are limited for conditioned locomotion. Both SERT KO and NET KO mice demonstrated conditioned locomotion. Although the effects appeared to be reduced, this decrease was not significant in NET KO mice and marginally significant in SERT KO mice. These effects may be the result of other factors, such as the reduced locomotion observed here in SERT KO and NET KO mice. Reduced locomotion has been described in SERT KO mice previously (Kalueff et al., 2007a; Kalueff et al., 2007b). Generally speaking in the paradigm utilized in these studies context-dependent sensitization was not observed (e.g. increased locomotion in paired subjects). Because of the habituation of activity across trials in saline-treated subjects, the relative magnitude of cocaine effects in Paired mice was greater in trial 5 than in trial one, which might be taken to indicate sensitization. With this in mind, the activity of paired SERT KO mice decreased across trials, similarly to saline treated subjects, which may indicate an impairment of context dependent sensitization or even tolerance to the locomotor stimulant effects of cocaine. However, as this study was not designed to primarily examine context dependent sensitization this conclusion must be tentatively placed forward until this phenomenon can be examined in a more appropriate paradigm. There is some evidence that stimulation of dorsal raphé 5-HT1A receptors potentiates cocaine induced locomotion, cocaine induced dopamine release and cocaine induced glutamate release (Szumlinski et al., 2004). There are substantial reductions in these receptors observed in SERT KO mice (Fabre et al., 2000) as well as other neuroadaptations (Mathews et al., 2004).
In conclusion, it would seem that the primary mechanism by which cocaine produces conditioned locomotion is via actions at the dopamine transporter. Although there is evidence that both SERT and NET gene knockout modulate cocaine-mediated behavior during conditioning, these differences do not profoundly affect the ability of cocaine to produce conditioned locomotion. Furthermore, in DAT KO mice context-independent sensitization of cocaine induced locomotion is observed, that is, the sensitization occurs in DAT −/− mice treated with repeated cocaine in the same or a different environment. This occurs under conditions that do not produce sensitization in other animals, and likely reflects substantial alterations in dopamine-glutamate interactions that occur in response to cocaine administration and that change in response to repeated cocaine administration. Further investigation of the function of glutamate in DAT KO mice may help illuminate the behavioral differences observed in these mice as well as those dopamine-glutamate interactions that are critical in these phenomena.
Acknowledgments
The authors acknowledge financial support from the NIH-IRP (NIDA), DHHS, and substantial assistance with the care and housing of our mice from the Charles River animal care staff, in particular the extensive assistance of our Charles River Breeder/Geneticist, Kriss Knestaut.
REFERENCES
- Bell K, Duffy P, Kalivas PW. Context-specific enhancement of glutamate transmission by cocaine. Neuropsychopharmacology. 2000;23:335–344. doi: 10.1016/S0893-133X(00)00100-7. [DOI] [PubMed] [Google Scholar]
- Bengel D, Murphy DL, Andrews AM, Wichems CH, Feltner D, Heils A, Mossner R, Westphal H, Lesch KP. Altered brain serotonin homeostasis and locomotor insensitivity to 3, 4- methylenedioxymethamphetamine (“Ecstasy”) in serotonin transporter- deficient mice. Mol Pharmacol. 1998;53:649–655. doi: 10.1124/mol.53.4.649. [DOI] [PubMed] [Google Scholar]
- Bisaga A, Sikora J, Kostowski W. The effect of drugs interacting with serotonergic 5HT3 and 5HT4 receptors on morphine place conditioning. Pol J Pharmacol. 1993;45:513–519. [PubMed] [Google Scholar]
- Brown EE, Fibiger HC. Differential effects of excitotoxic lesions of the amygdala on cocaine-induced conditioned locomotion and conditioned place preference. Psychopharmacology (Berl) 1993;113:123–130. doi: 10.1007/BF02244344. [DOI] [PubMed] [Google Scholar]
- Carboni E, Acquas E, Leone P, Di Chiara G. 5HT3 receptor antagonists block morphine- and nicotine- but not amphetamine-induced reward. Psychopharmacology. 1989;97:175–178. doi: 10.1007/BF00442245. [DOI] [PubMed] [Google Scholar]
- Carey RJ, Damianopoulos EN. Cocaine conditioning and sensitization: the habituation factor. Pharmacol Biochem Behav. 2006;84:128–133. doi: 10.1016/j.pbb.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Carey RJ, Gui J. Cocaine conditioning and cocaine sensitization: what is the relationship? Behav Brain Res. 1998;92:67–76. doi: 10.1016/s0166-4328(97)00126-5. [DOI] [PubMed] [Google Scholar]
- Cervo L, Samanin R. Effects of dopaminergic and glutamatergic receptor antagonists on the establishment and expression of conditioned locomotion to cocaine in rats. Brain Res. 1996;731:31–38. doi: 10.1016/0006-8993(96)00455-6. [DOI] [PubMed] [Google Scholar]
- Churchill L, Swanson CJ, Urbina M, Kalivas PW. Repeated cocaine alters glutamate receptor subunit levels in the nucleus accumbens and ventral tegmental area of rats that develop behavioral sensitization. J Neurochem. 1999;72:2397–2403. doi: 10.1046/j.1471-4159.1999.0722397.x. [DOI] [PubMed] [Google Scholar]
- Crombag HS, Badiani A, Maren S, Robinson TE. The role of contextual versus discrete drug-associated cues in promoting the induction of psychomotor sensitization to intravenous amphetamine. Behav Brain Res. 2000;116:1–22. doi: 10.1016/s0166-4328(00)00243-6. [DOI] [PubMed] [Google Scholar]
- Damianopoulos EN, Carey RJ. Evidence for N-methyl-D-aspartate receptor mediation of cocaine induced corticosterone release and cocaine conditioned stimulant effects. Behav Brain Res. 1995;68:219–228. doi: 10.1016/0166-4328(94)00175-f. [DOI] [PubMed] [Google Scholar]
- Dong Y, Saal D, Thomas M, Faust R, Bonci A, Robinson T, Malenka RC. Cocaine-induced potentiation of synaptic strength in dopamine neurons: behavioral correlates in GluRA(−/−) mice. Proc Natl Acad Sci U S A. 2004;101:14282–14287. doi: 10.1073/pnas.0401553101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmer GI, Gorelick DA, Goldberg SR, Rothman RB. Acute sensitivity vs. context-specific sensitization to cocaine as a function of genotype. Pharmacol Biochem Behav. 1996;53:623–628. doi: 10.1016/0091-3057(95)02062-4. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Dickinson A, Robbins TW. The neuropsychological basis of addictive behaviour. Brain Res Rev. 2001;36:129–138. doi: 10.1016/s0165-0173(01)00088-1. [DOI] [PubMed] [Google Scholar]
- Fabre V, Beaufour C, Evrard A, Rioux A, Hanoun N, Lesch KP, Murphy DL, Lanfumey L, Hamon M, Martres MP. Altered expression and functions of serotonin 5-HT1A and 5-HT1B receptors in knock-out mice lacking the 5-HT transporter. Eur J Neurosci. 2000;12:2299–2310. doi: 10.1046/j.1460-9568.2000.00126.x. [DOI] [PubMed] [Google Scholar]
- Fadda F, Garau B, Marchei F, Colombo G, Gessa GL. MDL 72222, a selective 5-HT3 receptor antagonist, suppresses voluntary ethanol consumption in alcohol-preferring rats. Alcohol Alcohol. 1991;26:107–110. doi: 10.1093/oxfordjournals.alcalc.a045088. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Chintoh AF, Sinyard J, Higgins GA. Injection of the 5-HT2C receptor agonist Ro60-0175 into the ventral tegmental area reduces cocaine-induced locomotor activity and cocaine self-administration. Neuropsychopharmacology. 2004;29:308–318. doi: 10.1038/sj.npp.1300319. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Korth KM. Activation of 5-HT1B receptors in the nucleus accumbens reduces amphetamine-induced enhancement of responding for conditioned reward. Psychopharmacology (Berl) 1999;142:165–174. doi: 10.1007/s002130050876. [DOI] [PubMed] [Google Scholar]
- Fukushima S, Shen H, Hata H, Ohara A, Ohmi K, Ikeda K, Numachi Y, Kobayashi H, Hall FS, Uhl GR, Sora I. Methamphetamine-induced locomotor activity and sensitization in dopamine transporter and vesicular monoamine transporter 2 double mutant mice. Psychopharmacology (Berl) 2007;193:55–62. doi: 10.1007/s00213-007-0749-4. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Mohn AR, Bohn LM, Caron MG. Glutamatergic modulation of hyperactivity in mice lacking the dopamine transporter. Proc Natl Acad Sci U S A. 2001;98:11047–11054. doi: 10.1073/pnas.191353298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- Gold LH, Swerdlow NR, Koob GF. The role of mesolimbic dopamine in conditioned locomotion produced by amphetamine. Behav Neurosci. 1988;102:544–552. doi: 10.1037//0735-7044.102.4.544. [DOI] [PubMed] [Google Scholar]
- Gonon F, Burie JB, Jaber M, Benoit-Marand M, Dumartin B, Bloch B. Geometry and kinetics of dopaminergic transmission in the rat striatum and in mice lacking the dopamine transporter. Prog Brain Res. 2000;125:291–302. doi: 10.1016/S0079-6123(00)25018-8. [DOI] [PubMed] [Google Scholar]
- Hall FS, Li XF, Centeno M, Murphy DL, Lesch KP, Uhl GR. Effect of combined dopamine receptor D2/serotonin transporter gene knockout on the behavioral effects of cocaine. Genes Brain Behav. Submitted. [Google Scholar]
- Hall FS, Li XF, Sora I, Xu F, Caron M, Lesch KP, Murphy DL, Uhl GR. Cocaine mechanisms: Enhanced cocaine, fluoxetine and nisoxetine place preferences following monoamine transporter deletions. Neuroscience. 2002;115:153–161. doi: 10.1016/s0306-4522(02)00379-2. [DOI] [PubMed] [Google Scholar]
- Harrison AA, Parsons LH, Koob GF, Markou A. RU 24969, a 5-HT1A/1B agonist, elevates brain stimulation reward thresholds: an effect reversed by GR 127935, a 5-HT1B/1D antagonist. Psychopharmacology (Berl) 1999;141:242–250. doi: 10.1007/s002130050831. [DOI] [PubMed] [Google Scholar]
- Heusner CL, Palmiter RD. Expression of mutant NMDA receptors in dopamine D1 receptor-containing cells prevents cocaine sensitization and decreases cocaine preference. J Neurosci. 2005;25:6651–6657. doi: 10.1523/JNEUROSCI.1474-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins GA, Joharchi N, Nguyen P, Sellers EM. Effect of the 5-HT3 receptor antagonists, MDL72222 and ondansetron on morphine place conditioning. Psychopharmacology. 1992a;106:315–320. doi: 10.1007/BF02245411. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Tomkins DM, Fletcher PJ, Sellers EM. Effect of drugs influencing 5-HT function on ethanol drinking and feeding behaviour in rats: studies using a drinkometer system. Neurosci Biobehav Rev. 1992b;16:535–552. doi: 10.1016/s0149-7634(05)80195-2. [DOI] [PubMed] [Google Scholar]
- Hotsenpiller G, Giorgetti M, Wolf ME. Alterations in behaviour and glutamate transmission following presentation of stimuli previously associated with cocaine exposure. Eur J Neurosci. 2001;14:1843–1855. doi: 10.1046/j.0953-816x.2001.01804.x. [DOI] [PubMed] [Google Scholar]
- Hotsenpiller G, Wolf ME. Conditioned locomotion is not correlated with behavioral sensitization to cocaine: an intra-laboratory multi-sample analysis. Neuropsychopharmacology. 2002;27:924–929. doi: 10.1016/S0893-133X(02)00370-6. [DOI] [PubMed] [Google Scholar]
- Jones SR, Gainetdinov RR, Jaber M, Giros B, Wightman RM, Caron MG. Profound neuronal plasticity in response to inactivation of the dopamine transporter. Proc Natl Acad Sci U S A. 1998;95:4029–4034. doi: 10.1073/pnas.95.7.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Repeated cocaine administration alters extracellular glutamate in the ventral tegmental area. J Neurochem. 1998;70:1497–1502. doi: 10.1046/j.1471-4159.1998.70041497.x. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Fox MA, Gallagher PS, Murphy DL. Hypolocomotion, anxiety and serotonin syndrome-like behavior contribute to the complex phenotype of serotonin transporter knockout mice. Genes Brain Behav. 2007a;6:389–400. doi: 10.1111/j.1601-183X.2006.00270.x. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Jensen CL, Murphy DL. Locomotory patterns, spatiotemporal organization of exploration and spatial memory in serotonin transporter knockout mice. Brain Res. 2007b;1169:87–97. doi: 10.1016/j.brainres.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Kim HS, Park WK, Jang CG, Oh S. Inhibition by MK-801 of cocaine-induced sensitization, conditioned place preference, and dopamine-receptor supersensitivity in mice. Brain Res Bull. 1996;40:201–207. doi: 10.1016/0361-9230(96)00006-8. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Miserendino MJ. Dissociation of novelty- and cocaine-conditioned locomotor activity from cocaine place conditioning. Pharmacol Biochem Behav. 1998;60:785–791. doi: 10.1016/s0091-3057(97)00388-2. [DOI] [PubMed] [Google Scholar]
- Kostowski W, Dyr W, Krzascik P. The abilities of 5-HT3 receptor antagonist ICS 205-930 to inhibit alcohol preference and withdrawal seizures in rats. Alcohol. 1993;10:369–373. doi: 10.1016/0741-8329(93)90022-g. [DOI] [PubMed] [Google Scholar]
- Li Y, Acerbo MJ, Robinson TE. The induction of behavioural sensitization is associated with cocaine-induced structural plasticity in the core (but not shell) of the nucleus accumbens. Eur J Neurosci. 2004;20:1647–1654. doi: 10.1111/j.1460-9568.2004.03612.x. [DOI] [PubMed] [Google Scholar]
- Li Y, Hu XT, Berney TG, Vartanian AJ, Stine CD, Wolf ME, White FJ. Both glutamate receptor antagonists and prefrontal cortex lesions prevent induction of cocaine sensitization and associated neuroadaptations. Synapse. 1999;34:169–180. doi: 10.1002/(SICI)1098-2396(19991201)34:3<169::AID-SYN1>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Li Y, Vartanian AJ, White FJ, Xue CJ, Wolf ME. Effects of the AMPA receptor antagonist NBQX on the development and expression of behavioral sensitization to cocaine and amphetamine. Psychopharmacology (Berl) 1997;134:266–276. doi: 10.1007/s002130050449. [DOI] [PubMed] [Google Scholar]
- Lu MR, Wagner GC, Fisher H. Ethanol consumption following acute treatment with methysergide, fluoxetine, fenfluramine, and their combination. Alcohol Clin Exp Res. 1994;18:60–63. doi: 10.1111/j.1530-0277.1994.tb00881.x. [DOI] [PubMed] [Google Scholar]
- Mateo Y, Budygin EA, John CE, Banks ML, Jones SR. Voltammetric assessment of dopamine clearance in the absence of the dopamine transporter: no contribution of other transporters in core or shell of nucleus accumbens. J Neurosci Methods. 2004a;140:183–187. doi: 10.1016/j.jneumeth.2004.05.018. [DOI] [PubMed] [Google Scholar]
- Mateo Y, Budygin EA, John CE, Jones SR. Role of serotonin in cocaine effects in mice with reduced dopamine transporter function. Proc Natl Acad Sci U S A. 2004b;101:372–377. doi: 10.1073/pnas.0207805101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews TA, Fedele DE, Coppelli FM, Avila AM, Murphy DL, Andrews AM. Gene dose-dependent alterations in extraneuronal serotonin but not dopamine in mice with reduced serotonin transporter expression. J Neurosci Methods. 2004;140:169–181. doi: 10.1016/j.jneumeth.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Mattson BJ, Koya E, Simmons DE, Mitchell TB, Berkow A, Crombag HS, Hope BT. Context-specific sensitization of cocaine-induced locomotor activity and associated neuronal ensembles in rat nucleus accumbens. Eur J Neurosci. 2008;27:202–212. doi: 10.1111/j.1460-9568.2007.05984.x. [DOI] [PubMed] [Google Scholar]
- Maurel S, De Vry J, De Beun R, Schreiber R. 5-HT2A and 5-HT2C/5-HT1B receptors are differentially involved in alcohol preference and consummatory behavior in cAA rats. Pharmacol Biochem Behav. 1999;62:89–96. doi: 10.1016/s0091-3057(98)00115-4. [DOI] [PubMed] [Google Scholar]
- McMillen BA, Walter S, Williams HL, Myers RD. Comparison of the action of the 5-HT2 antagonists amperozide and trazodone on preference for alcohol in rats. Alcohol. 1994;11:203–206. doi: 10.1016/0741-8329(94)90032-9. [DOI] [PubMed] [Google Scholar]
- Mead AN, Rocha BA, Donovan DM, Katz JL. Intravenous cocaine induced-activity and behavioural sensitization in norepinephrine-, but not dopamine-transporter knockout mice. Eur J Neurosci. 2002;16:514–520. doi: 10.1046/j.1460-9568.2002.02104.x. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Schindler CW. Conditioned locomotor-activating and reinforcing effects of discrete stimuli paired with intraperitoneal cocaine. Behav Pharmacol. 1997;8:691–698. doi: 10.1097/00008877-199712000-00003. [DOI] [PubMed] [Google Scholar]
- Parsons LH, Weiss F, Koob GF. Serotonin1B receptor stimulation enhances cocaine reinforcement. J Neurosci. 1998;18:10078–10089. doi: 10.1523/JNEUROSCI.18-23-10078.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pert A, Post R, Weiss SRB. Conditioning as a critical determinant of sensitization induced by psychomotor stimulants. Neurobiology of Drug Abuse: Learning and Memory. 1990;97:208–241. [PubMed] [Google Scholar]
- Pierce RC, Bell K, Duffy P, Kalivas PW. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J Neurosci. 1996;16:1550–1560. doi: 10.1523/JNEUROSCI.16-04-01550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post RM, Lockfeld A, Squillace KM, Contel NR. Drug-environment interaction: context dependency of cocaine-induced behavioral sensitization. Life Sci. 1981;28:755–760. doi: 10.1016/0024-3205(81)90157-0. [DOI] [PubMed] [Google Scholar]
- Post RM, Weiss SR, Pert A. The role of context and conditioning in behavioral sensitization to cocaine. Psychopharmacol Bull. 1987;23:425–429. [PubMed] [Google Scholar]
- Reid MS, Berger SP. Evidence for sensitization of cocaine-induced nucleus accumbens glutamate release. Neuroreport. 1996;7:1325–1329. doi: 10.1097/00001756-199605170-00022. [DOI] [PubMed] [Google Scholar]
- Rocha BA, Fumagalli F, Gainetdinov RR, Jones SR, Ator R, Giros B, Miller GW, Caron MG. Cocaine self-administration in dopamine-transporter knockout mice. Nat Neurosci. 1998;1:132–137. doi: 10.1038/381. [DOI] [PubMed] [Google Scholar]
- Rompre PP, Injoyan R, Hagan JJ. Effects of granisetron, a 5-HT3 receptor antagonist, on morphine- induced potentiation of brain stimulation reward. Eur J Pharmacol. 1995;287:263–269. doi: 10.1016/0014-2999(95)00497-1. [DOI] [PubMed] [Google Scholar]
- Shen HW, Hagino Y, Kobayashi H, Shinohara-Tanaka K, Ikeda K, Yamamoto H, Yamamoto T, Lesch KP, Murphy DL, Hall FS, Uhl GR, Sora I. Regional Differences in Extracellular Dopamine and Serotonin Assessed by In Vivo Microdialysis in Mice Lacking Dopamine and/or Serotonin Transporters. Neuropsychopharmacology. 2004 doi: 10.1038/sj.npp.1300476. [DOI] [PubMed] [Google Scholar]
- Smith JA, Mo Q, Guo H, Kunko PM, Robinson SE. Cocaine increases extraneuronal levels of aspartate and glutamate in the nucleus accumbens. Brain Res. 1995;683:264–269. doi: 10.1016/0006-8993(95)00383-2. [DOI] [PubMed] [Google Scholar]
- Sora I, Hall FS, Andrews AM, Itokawa M, Li XF, Wei HB, Wichems C, Lesch KP, Murphy DL, Uhl GR. Molecular mechanisms of cocaine reward: combined dopamine and serotonin transporter knockouts eliminate cocaine place preference. Proc Natl Acad Sci U S A. 2001;98:5300–5305. doi: 10.1073/pnas.091039298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sora I, Wichems C, Takahashi N, Li XF, Zeng Z, Revay R, Lesch KP, Murphy DL, Uhl GR. Cocaine reward models: conditioned place preference can be established in dopamine- and in serotonin-transporter knockout mice. Proc Natl Acad Sci U S A. 1998;95:7699–7704. doi: 10.1073/pnas.95.13.7699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J. Conditioned and unconditioned drug effects in relapse to opiate and stimulant drug self-adminstration. Prog Neuropsychopharmacol Biol Psychiatry. 1983;7:591–597. doi: 10.1016/0278-5846(83)90030-1. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Frys KA, Kalivas PW. Dissociable roles for the dorsal and median raphe in the facilitatory effect of 5-HT1A receptor stimulation upon cocaine-induced locomotion and sensitization. Neuropsychopharmacology. 2004;29:1675–1687. doi: 10.1038/sj.npp.1300473. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Hall FS, Uhl GR, Caine SB. Dramatically decreased cocaine self-administration in dopamine but not serotonin transporter knock-out mice. J Neurosci. 2009;29:1087–1092. doi: 10.1523/JNEUROSCI.4037-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirelli E, Michel A, Brabant C. Cocaine-conditioned activity persists for a longer time than cocaine-sensitized activity in mice: implications for the theories using Pavlovian excitatory conditioning to explain the context-specificity of sensitization. Behav Brain Res. 2005;165:18–25. doi: 10.1016/j.bbr.2005.06.029. [DOI] [PubMed] [Google Scholar]
- Tirelli E, Tambour S, Michel A. Sensitised locomotion does not predict conditioned locomotion in cocaine-treated mice: further evidence against the excitatory conditioning model of context-dependent sensitisation. Eur Neuropsychopharmacol. 2003;13:289–296. doi: 10.1016/s0924-977x(03)00037-3. [DOI] [PubMed] [Google Scholar]
- Tomkins DM, Higgins GA, Sellers EM. Low doses of the 5-HT1A agonist 8-hydroxy-2-(di-n-propylamino)-tetralin (8-OH DPAT) increase ethanol intake. Psychopharmacology (Berl) 1994a;115:173–179. doi: 10.1007/BF02244769. [DOI] [PubMed] [Google Scholar]
- Tomkins DM, Le AD, Sellers EM. Effect of the 5-HT3 antagonist ondansetron on voluntary ethanol intake in rats and mice maintained on a limited access procedure. Psychopharmacology (Berl) 1995;117:479–485. doi: 10.1007/BF02246222. [DOI] [PubMed] [Google Scholar]
- Tomkins DM, O'Neill MF. Effect of 5-HT(1B) receptor ligands on self-administration of ethanol in an operant procedure in rats. Pharmacol Biochem Behav. 2000;66:129–136. doi: 10.1016/s0091-3057(00)00232-x. [DOI] [PubMed] [Google Scholar]
- Tomkins DM, Sellers EM, Fletcher PJ. Median and dorsal raphe injections of the 5-HT1A agonist, 8-OH-DPAT, and the GABAA agonist, muscimol, increase voluntary ethanol intake in Wistar rats. Neuropharmacology. 1994b;33:349–358. doi: 10.1016/0028-3908(94)90065-5. [DOI] [PubMed] [Google Scholar]
- Valjent E, Pascoli V, Svenningsson P, Paul S, Enslen H, Corvol JC, Stipanovich A, Caboche J, Lombroso PJ, Nairn AC, Greengard P, Herve D, Girault JA. Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum. Proc Natl Acad Sci U S A. 2005;102:491–496. doi: 10.1073/pnas.0408305102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YM, Xu F, Gainetdinov RR, Caron MG. Genetic approaches to studying norepinephrine function: knockout of the mouse norepinephrine transporter gene. Biol Psychiatry. 1999;46:1124–1130. doi: 10.1016/s0006-3223(99)00245-0. [DOI] [PubMed] [Google Scholar]
- White FJ, Hu XT, Zhang XF, Wolf ME. Repeated administration of cocaine or amphetamine alters neuronal responses to glutamate in the mesoaccumbens dopamine system. J Pharmacol Exp Ther. 1995;273:445–454. [PubMed] [Google Scholar]
- Wilson AW, Neill JC, Costall B. An investigation into the effects of 5-HT agonists and receptor antagonists on ethanol self-administration in the rat. Alcohol. 1998;16:249–270. doi: 10.1016/s0741-8329(98)00013-5. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–492. [PubMed] [Google Scholar]
- Wise RA, Leeb K. Psychomotor-stimulant sensitization: a unitary phenomenon? Behav Pharmacol. 1993;4:339–349. [PubMed] [Google Scholar]
- Xu F, Gainetdinov RR, Wetsel WC, Jones SR, Bohn LM, Miller GW, Wang YM, Caron MG. Mice lacking the norepinephrine transporter are supersensitive to psychostimulants. Nat Neurosci. 2000;3:465–471. doi: 10.1038/74839. [DOI] [PubMed] [Google Scholar]
- Zhang XF, Hu XT, White FJ, Wolf ME. Increased responsiveness of ventral tegmental area dopamine neurons to glutamate after repeated administration of cocaine or amphetamine is transient and selectively involves AMPA receptors. J Pharmacol Exp Ther. 1997;281:699–706. [PubMed] [Google Scholar]