Abstract
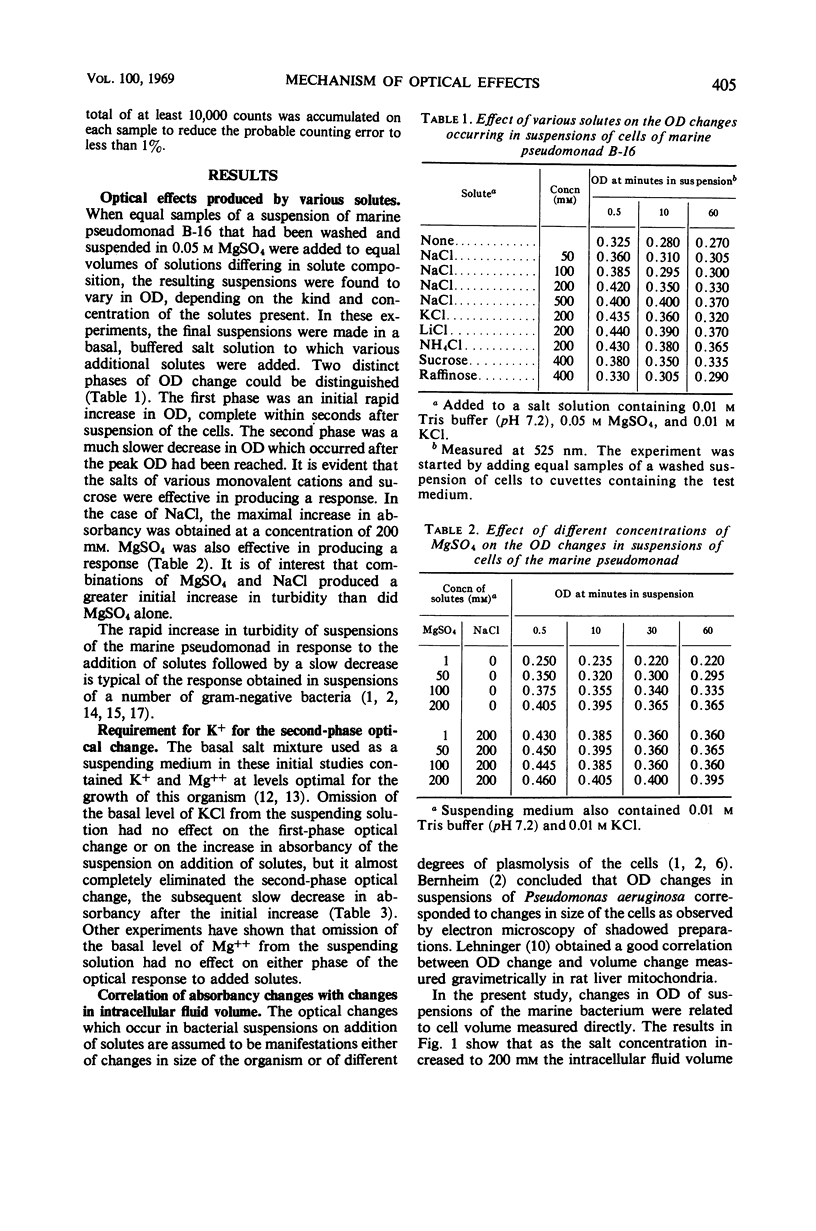
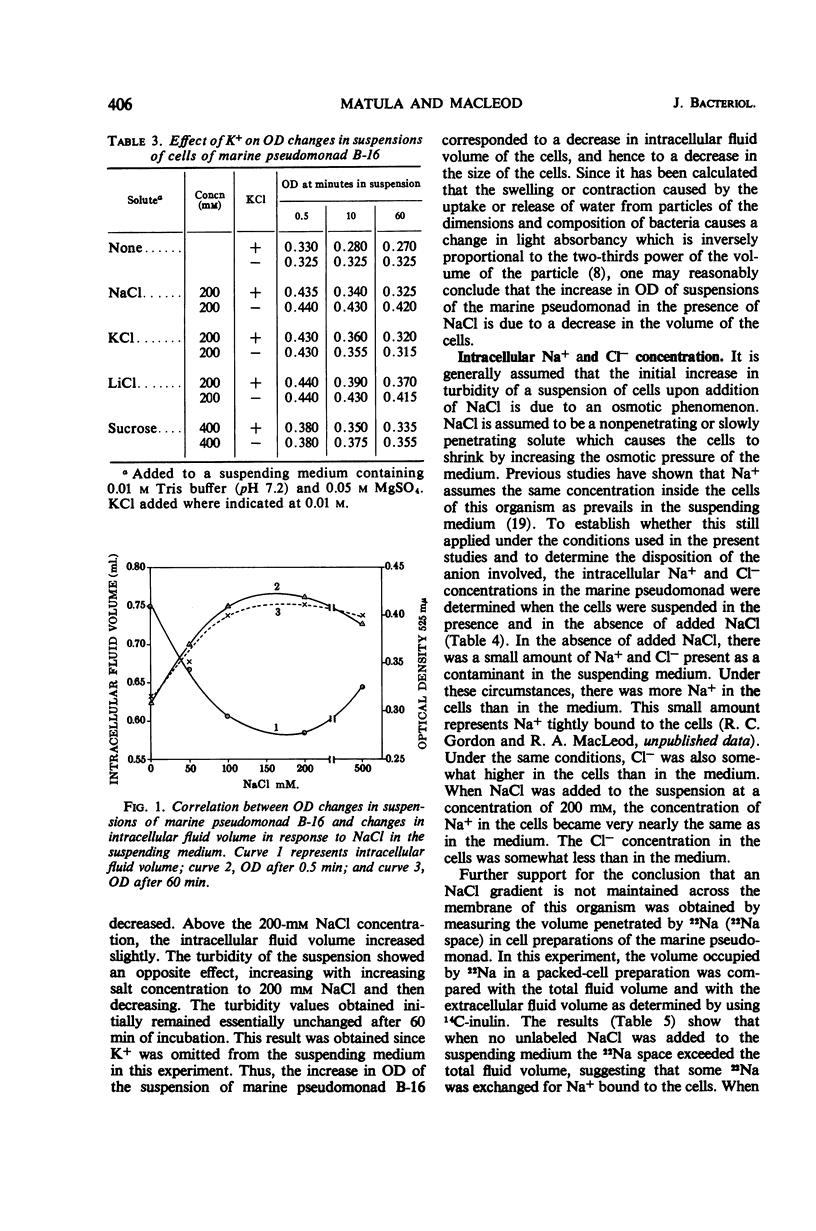
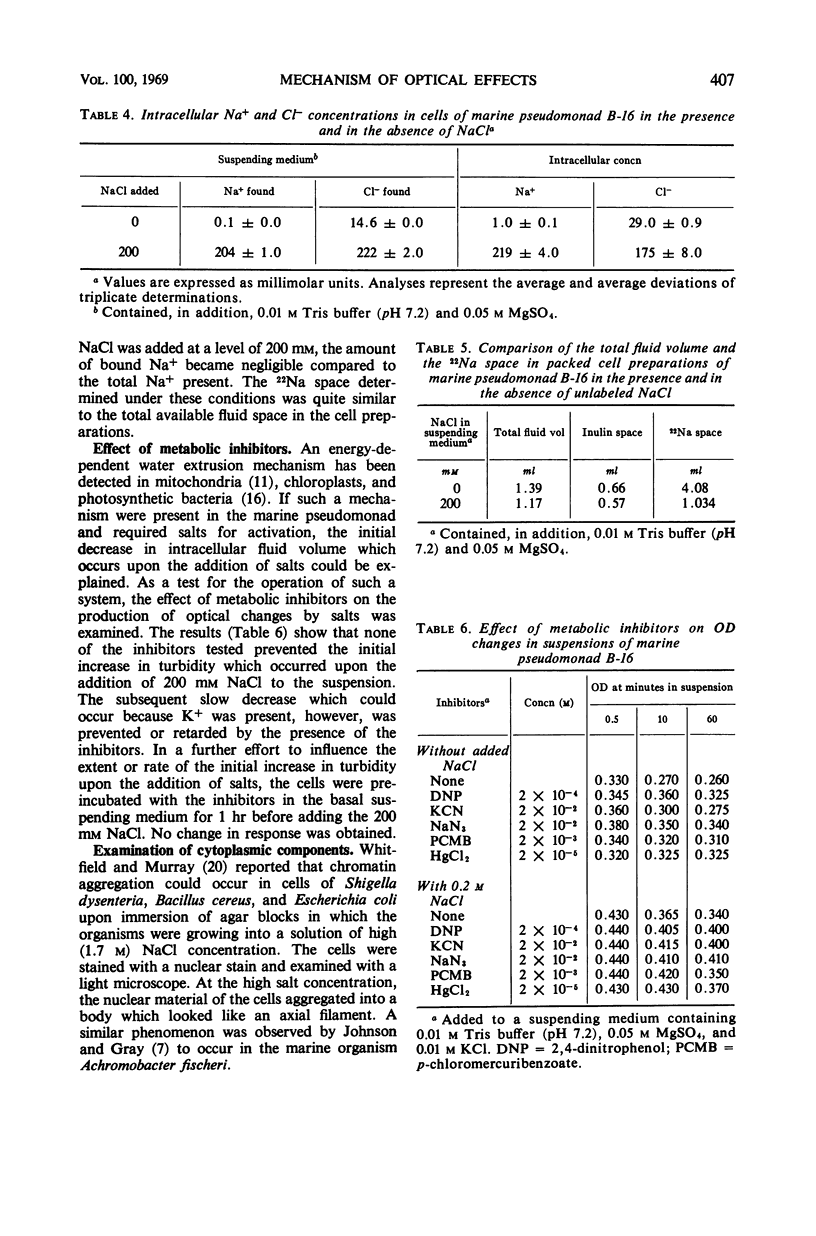
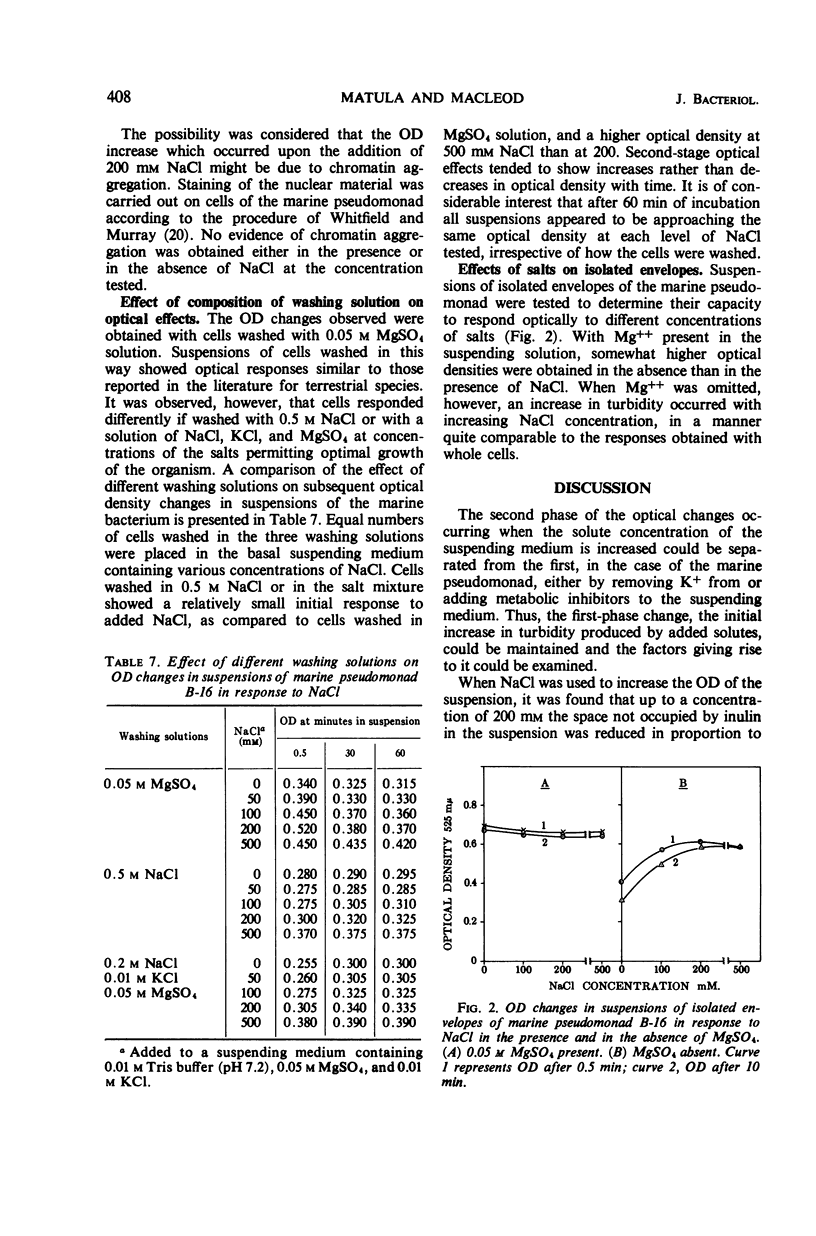
When cells of a marine pseudomonad washed free of medium components with 0.05 m MgSO4 were suspended in solutions containing 200-mm concentrations of various salts, there was an immediate increase in optical density (OD), followed by a slow decrease. The decrease following the initial increase, but not the increase itself, could be prevented by omitting K+ from or by adding metabolic inhibitors to the suspending solution. With NaCl, the initial increase in OD rose to a maximum as the salt concentration was increased to 200 mm and then declined at 500 mm. There was a corresponding decrease in intracellular fluid volume to a minimum at 200-mm NaCl and then a rise. When the increased OD produced by NaCl was maintained, the internal Na+ and Cl− could be shown to have reached essentially the same concentration in the cells as in the medium. Thus, the OD changes could not have been due to osmotic effects. No evidence was obtained of a salt-induced aggregation of nuclear material. The OD of suspensions of isolated cell envelopes increased in response to increases in NaCl concentration in the absence but not in the presence of 0.05 m MgSO4. The data was interpreted to indicate that the salt-induced increases in OD occurring in suspensions of the cells resulted from an interaction of salts with components of the cell envelope, causing contraction of the envelopes and shrinkage of the cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVI-DOR Y., KUCZYNSKI M., SCHATZBERG G., MAGER J. Turbidity changes in bacterial suspensions: kinetics and relation to metabolic state. J Gen Microbiol. 1956 Feb;14(1):76–83. doi: 10.1099/00221287-14-1-76. [DOI] [PubMed] [Google Scholar]
- BERNHEIM F. Factors which affect the size of the organisms and the optical density of suspensions of Pseudomonas aeruginosa and Escherichia coli. J Gen Microbiol. 1963 Jan;30:53–58. doi: 10.1099/00221287-30-1-53. [DOI] [PubMed] [Google Scholar]
- BOVELL C. R., PACKER L., HELGERSON R. PERMEABILITY OF ESCHERICHIA COLI TO ORGANIC COMPOUNDS AND INORGANIC SALTS MEASURED BY LIGHT-SCATTERING. Biochim Biophys Acta. 1963 Sep 24;75:257–266. doi: 10.1016/0006-3002(63)90604-8. [DOI] [PubMed] [Google Scholar]
- Buckmire F. L., MacLeod R. A. Nutrition and metabolism of marine bacteria. XIV. On the mechanism of lysis of a marine bacterium. Can J Microbiol. 1965 Aug;11(4):677–691. doi: 10.1139/m65-091. [DOI] [PubMed] [Google Scholar]
- Drapeau G. R., Matula T. I., MacLeod R. A. Nutrition and metabolism of marine bacteria. XV. Relation of Na+-activated transport to the Na+ requirement of a marine pseudomonad for growth. J Bacteriol. 1966 Jul;92(1):63–71. doi: 10.1128/jb.92.1.63-71.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENNEMAN D. H., UMBREIT W. W. FACTORS WHICH MODIFY THE EFFECT OF SODIUM AND POTASSIUM ON BACTERIAL CELL MEMBRANES. J Bacteriol. 1964 Jun;87:1266–1273. doi: 10.1128/jb.87.6.1266-1273.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON F. H., GRAY D. H. Nuclei and large bodies of luminous bacteria in relation to salt concentration, osmotic pressure, temperature, and urethane. J Bacteriol. 1949 Nov;58(5):675-88, illust. doi: 10.1128/jb.58.5.675-688.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOCH A. L. Some calculations on the turbidity of mitochondria and bacteria. Biochim Biophys Acta. 1961 Aug 19;51:429–441. doi: 10.1016/0006-3002(61)90599-6. [DOI] [PubMed] [Google Scholar]
- KUCZYNSKI-HALMANN M., AVI-DOR Y., MAGER J. Turbidity changes in suspensions of gram-positive bacteria in relation to osmotic pressure. J Gen Microbiol. 1958 Apr;18(2):364–368. doi: 10.1099/00221287-18-2-364. [DOI] [PubMed] [Google Scholar]
- LEHNINGER A. L. Reversal of thyroxine-induced swelling of rat liver mitochondria by adenosine triphosphate. J Biol Chem. 1959 Aug;234(8):2187–2195. [PubMed] [Google Scholar]
- MACLEOD R. A., ONOFREY E. Nutrition and metabolism of marine bacteria. III. The relation of sodium and potassium to growth. J Cell Physiol. 1957 Dec;50(3):389–401. doi: 10.1002/jcp.1030500305. [DOI] [PubMed] [Google Scholar]
- MACLEOD R. A., ONOFREY E. Nutrition and metabolism of marine bacteria. VI. Quantitative requirements for halides, magnesium, calcium, and iron. Can J Microbiol. 1957 Aug;3(5):753–759. doi: 10.1139/m57-085. [DOI] [PubMed] [Google Scholar]
- MITCHELL P., MOYLE J. Permeability of the envelopes of Staphylococcus aureus to some salts, amino acids, and non-electrolytes. J Gen Microbiol. 1959 Apr;20(2):434–441. doi: 10.1099/00221287-20-2-434. [DOI] [PubMed] [Google Scholar]
- PACKER L., MARCHANT R. H., MUKOHATA Y. STRUCTURAL CHANGES RELATED TO PHOTOSYNTHETIC ACTIVITY IN CELLS AND CHLOROPLASTS. Biochim Biophys Acta. 1963 Jul 23;75:23–30. doi: 10.1016/0006-3002(63)90575-4. [DOI] [PubMed] [Google Scholar]
- PACKER L., PERRY M. Energy-linked light-scattering changes in Escherichia coli. Arch Biochem Biophys. 1961 Nov;95:379–388. doi: 10.1016/0003-9861(61)90163-1. [DOI] [PubMed] [Google Scholar]
- SANUI H., PACE N. Sodium and potassium binding by rat liver cell microsomes. J Gen Physiol. 1959 Jul 20;42(6):1325–1345. doi: 10.1085/jgp.42.6.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKACS F. P., MATULA T. I., MACLEOD R. A. NUTRITION AND METABOLISM OF MARINE BACTERIA. XIII. INTRACELLULAR CONCENTRATIONS OF SODIUM AND POTASSIUM IONS IN A MARINE PSEUDOMONAD. J Bacteriol. 1964 Mar;87:510–518. doi: 10.1128/jb.87.3.510-518.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITFIELD J. F., MURRAY R. G. The effects of the ionic environment on the chromatin structures of bacteria. Can J Microbiol. 1956 May;2(3):245–260. doi: 10.1139/m56-029. [DOI] [PubMed] [Google Scholar]
- Wong P. T., Thompson J., MacLeod R. A. Nutrition and metabolism of marine bacteria. XVII. Ion-dependent retention of alpha-aminoisobutyric acid and its relation to Na+ dependent transport in a marine pseudomonad. J Biol Chem. 1969 Feb 10;244(3):1016–1025. [PubMed] [Google Scholar]


