Abstract
Drug-seeking behavior is maintained by encounters with drug-associated cues. Preventing retrieval of drug-associated memories that these cues provoke would therefore limit relapse susceptibility; however, little is known regarding the mechanisms of retrieval. Here, we show that β-adrenergic receptor activation is necessary for the retrieval of a cocaine-associated memory. Using a conditioned place preference (CPP) procedure, rats were conditioned to associate one chamber, but not another, with cocaine. When administered before a CPP trial, propranolol, but not saline, prevented retrieval of a cocaine-associated CPP. In subsequent drug-free trials, rats previously treated with propranolol continued to show a retrieval deficit, as no CPP was evident. This retrieval deficit was long lasting and robust, as the CPP did not re-emerge during a test for spontaneous recovery 14 days later or reinstate following a priming injection of cocaine. Moreover, the peripheral β-adrenergic receptor antagonist sotalol did not affect retrieval. Thus, retrieval of cocaine-associated memories is mediated by norepinephrine acting at central β-adrenergic receptors. Our findings support the use of propranolol, a commonly prescribed β-blocker, as an adjunct to exposure therapy for the treatment of addiction by preventing retrieval of drug-associated memories during and long after treatment, and by providing protection against relapse.
Keywords: noradrenergic β-receptor, norepinephrine, reinstatement, extinction learning, relapse, drug abuse
INTRODUCTION
Drug addiction is a chronically relapsing disorder characterized by compulsive drug-seeking behavior, and is maintained by encounters with drug-associated cues (Heather et al, 1991; Herman, 1974; O'Brien et al, 1990). Presentation of these cues can elicit craving in humans (Childress et al, 1986; Ehrman et al, 1992; Foltin and Haney, 2000; O'Brien et al, 1977) and stimulate active drug seeking in rodents (Banna et al, 2010; Chauvet et al, 2009; Fuchs et al, 2004; Mueller and Stewart, 2000). The associations between these cues and the drug reinforcer are acquired, consolidated, and readily retrieved in the presence of the cues. Despite the pivotal role of drug-associated memory retrieval in stimulating drug seeking and relapse, little is known about the neurobiological mechanisms of retrieval.
Emerging evidence implicates the noradrenergic system in memory retrieval in emotional learning paradigms (Sara, 2000, 2009; van Stegeren, 2008). Stimulation of noradrenergic neurons in the locus coeruleus facilitates retrieval of associative memories (Devauges and Sara, 1991; Sara and Devauges, 1988), and the presentation of cues associated with emotional stimuli activates these neurons (Sterpenich et al, 2006) and provokes norepinephrine release (Cassens et al, 1980; Feenstra et al, 2001; Mingote et al, 2004). Mice constitutively lacking dopamine β-hydroxylase (DBH), an essential enzyme in the synthesis of norepinephrine, are able to acquire contextual fear conditioning, but show a deficit in retrieval when tested on subsequent days (Murchison et al, 2004). Restoring noradrenergic function in these mice before testing reversed the deficit. Furthermore, systemic administration of propranolol, a β-adrenergic receptor antagonist, also reduced or prevented memory retrieval in mice and rats, indicating that retrieval of conditioned fear is dependent on these receptors (Murchison et al, 2004; Rodriguez-Romaguera et al, 2009). Whether retrieval of drug-associated memories is dependent on β-adrenergic receptors, however, remains unknown.
We assessed the necessity of β-adrenergic receptor activation for the retrieval of cocaine-associated memories using the conditioned place preference (CPP) procedure, an animal model of drug seeking in which environmental stimuli are associated with drug administration. First, we examined the effects of β-receptor blockade, using the β-receptor antagonist propranolol, on initial CPP trials. We then determined whether the retrieval deficit induced by propranolol is long lasting by examining subsequent CPP trials in the absence of propranolol. We also determined whether the propranolol-induced retrieval deficit provides protection against spontaneous recovery and cocaine-induced reinstatement. Our results provide clear evidence that propranolol, a commonly prescribed β-blocker, could be useful in treating addiction by preventing drug-associated memories from being retrieved during and long after treatment.
MATERIALS AND METHODS
Subjects
A total of 126 male long-Evans rats (Harlan Laboratories, Madison, WI) weighing 200–225 g were individually housed in clear plastic cages. Rats were maintained on a 14-h light/10 h dark cycle (lights on at 0700 hours) and had unlimited access to both water and standard laboratory rat chow (Harlan Laboratories). Rats were weighed and handled daily. All procedures were approved by the Institutional Animal Care and Use Committee at the University of Wisconsin-Milwaukee in compliance with National Institutes of Health guidelines.
Place Preference Apparatus
Behavioral testing and conditioning were conducted in a three-chamber apparatus in which two larger chambers (13′′ × 9′′ × 11.5′′) were separated by a smaller chamber (6′′ × 7′′ × 11.5′′). One of the larger chambers had wire mesh flooring with white walls, whereas the other had gold-grated flooring with a black wall. The center chamber had PVC plastic floors. All floors were raised to 1.5′′, with removable trays placed underneath. Removable partitions were used to isolate the rats within specific chambers during conditioning. During the CPP trials, the partitions were removed to allow free access to the entire apparatus. Each of the larger chambers contained two infrared photobeams separated by 3′′. If the beam furthest from the door was broken, the rat was determined to be in the larger chamber. If only the beam closest to the center chamber was broken, then the rat was determined to be in the center chamber. In addition, total number of photobeam breaks could be recorded to quantify locomotor activity. During all phases of the experiments, the room was kept in semidarkness.
Drugs
Cocaine HCl (National Institute on Drug Abuse) was dissolved in sterile 0.9% saline at a concentration of 10 mg/ml, and was administered intraperitoneally (i.p.) at a dose of 10 mg/kg for all experiments and 20 mg/kg in experiment 1 only. (+/−)-Propranolol HCl (Sigma-Aldrich, St Louis, MO) and sotalol HCl (Sigma-Aldrich) were dissolved in sterile 0.9% saline at a concentration of 10 mg/ml, and were administered subcutaneously (s.c.) at a dose of 10 mg/kg. (−)-Propranolol HCl (Sigma-Aldrich) was dissolved in sterile 0.9% saline at a concentration of 1 mg/ml, and was administered at a dose of 1 mg/kg, s.c.
Conditioning and Testing
Baseline preferences were determined by placing the rats in the center chamber and allowing free access to all chambers for 15 min. Time spent in each chamber was recorded. Overall, rats showed no preference for either of the larger conditioning chambers during this baseline trial, although less time was spent in the smaller center chamber. Analysis of variance (ANOVA) revealed a significant effect of chamber (F2,250=237.70, p<0.001), and post hoc tests showed that rats spent less time in the center chamber than either of the larger chambers (p<0.05) and an equivalent amount of time in the larger chambers (p>0.05). Because of the absence of an initial preference for either conditioning chamber, we used an unbiased procedure in which rats were randomly assigned to receive cocaine in one or the other larger chamber. Following baseline testing, rats were conditioned to associate one chamber, but not another, with cocaine in a counterbalanced fashion over 8 days. Injections of saline or cocaine were given on alternating days immediately before 20-min conditioning sessions during which the rats were confined to their respective chambers. Following four conditioning sessions in each chamber, the rats were subjected to a 2-day break, followed by daily CPP trials in which they were placed in the center chamber, with access to the entire apparatus for 15 min. A CPP was determined when rats spent significantly more time in the previously cocaine-paired chamber than the saline-paired chamber.
Experimental Manipulations
To examine whether β-adrenergic receptor activation is necessary for retrieval of cocaine-associated memories, we administered the non-selective β-adrenergic receptor antagonist (+/−)-propranolol or saline 20 min before cocaine-free CPP extinction trials. Rats were tested daily, receiving injections before the first trial only (experiment 1). In experiment 2, we investigated the possible nonspecific effects of propranolol on CPP expression. First, to ensure that the effects of propranolol were specific to β-receptor blockade, we examined the effect of a low dose of the more active enantiomer (−)-propranolol (1 mg/kg, s.c.), with greater specificity for β-receptors, on CPP retrieval. Rats were tested daily, receiving (−)-propranolol or saline 20 min before the first CPP trial only. Second, we tested the possible effects of propranolol on reconsolidation. Rats were tested daily, receiving (+/−)-propranolol immediately before, as opposed to 20 min before, the first CPP trial, thereby allowing CPP retrieval during the first trial. Third, we examined whether propranolol itself induces a CPP or aversion. Following baseline testing, rats were conditioned to associate one chamber, but not another, with propranolol in a counterbalanced fashion over 2 days. Injections of (+/−)-propranolol or saline were given on either day 20 min before normal conditioning sessions, during which the rats were confined to their respective chambers. Following conditioning, the rats were subjected to a drug-free CPP extinction trial. Finally, the effect of propranolol on locomotor activity was measured as photobeam breaks.
We next examined the effects of propranolol administered 20 min before the second trial only (experiment 3) or each of the second, third, and fourth trials (experiment 4). The multiple injection procedure was adopted to maximize the retrieval deficit and model the use of a pharmacological adjunct to exposure therapy. Rats were then tested repeatedly with daily drug-free CPP extinction trials. In experiment 4, rats were given an additional CPP trial 14 days later to test for spontaneous recovery of the CPP.
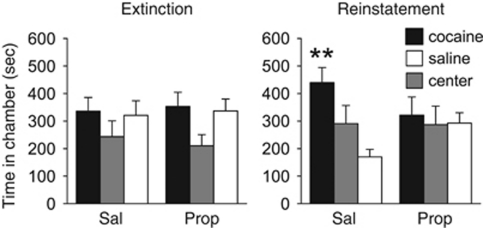
In experiment 5, we examined whether propranolol treatment during initial CPP trials would prevent later cocaine-induced reinstatement. Rats received propranolol or saline before each of the second, third, and fourth CPP trials, and were then tested again in a drug-free CPP trial. To match the lack of CPP expressed by the propranolol group after treatment, saline-treated rats were subjected to 30-min drug-free CPP extinction trials (instead of the normal 15-min CPP trials), with full access to the apparatus until no preference was observed (12 days). One day following the final CPP extinction trial for each treatment group, rats were given a priming injection of cocaine (10 mg/kg, i.p.) 5 min before an additional CPP trial to test for cocaine-induced reinstatement.
In experiment 6, we examined whether the effects of propranolol were due to β-receptor blockade within the central or peripheral nervous system. We injected either the non-selective β-adrenergic receptor antagonist sotalol or saline 20 min before the second, third, and fourth CPP trials. Sotalol does not cross the blood–brain barrier (Dahlof, 1981), and has been shown to effectively decrease heart rate in rats to a similar extent as propranolol (Rodriguez-Romaguera et al, 2009).
Data Analysis
Drug-seeking behavior during single or across multiple CPP trials was analyzed by comparing time spent in the previously cocaine-paired, saline-paired, and center chambers using a repeated measures ANOVA. Following a significant effect of chamber, Tukey's Honestly Significant Difference post hoc tests were used to compare the amount of time spent in the previously cocaine-paired vs saline-paired chambers for single or across multiple CPP trials. In no cases did animals spend significantly more time in the center chamber than in the cocaine-paired chamber (p>0.05). Locomotor activity was measured as the number of photobeam breaks during the CPP trials, and was analyzed using an independent sample t-test.
RESULTS
Propranolol Prevents Retrieval of a CPP when Administered Before an Initial Test
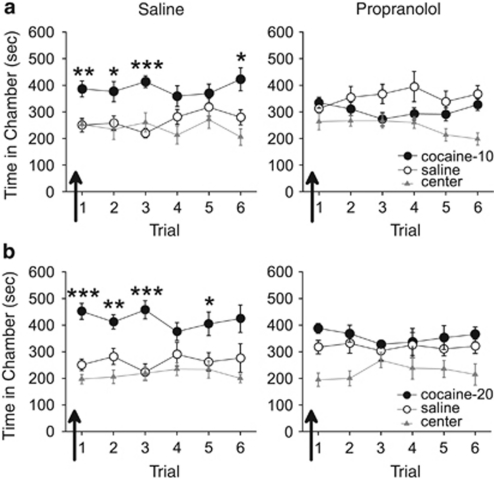
We first examined the necessity of β-adrenergic receptor activation for the retrieval of a cocaine-associated CPP. In experiment 1, rats were pretested, conditioned with either 10 or 20 mg/kg of cocaine, and subjected to daily CPP trials, with administration of the β-adrenergic receptor antagonist propranolol (10 mg/kg) or saline (n=6–8 per group) 20 min before the first CPP trial. Rats injected with saline demonstrated a CPP for the previously cocaine-paired chamber during this trial, but rats injected with propranolol did not (see Figure 1). ANOVA revealed a significant effect of chamber for saline-treated rats conditioned with either dose of cocaine on the first trial (10 mg/kg cocaine, F2,14=8.51, p<0.01; 20 mg/kg cocaine, F2,14=21.35, p<0.001), and post hoc analyses confirmed that significantly more time was spent in the previously cocaine-paired chamber than the saline-paired chamber (p<0.01). Propranolol-treated rats conditioned with either dose of cocaine, however, spent an equal amount of time in the previously cocaine-paired chamber and saline-paired chamber (effect of chamber: 10 mg/kg cocaine, F2,14=3.73, p>0.05, post hoc p>0.05; 20 mg/kg cocaine, F2,10=11.83, p<0.01, post hoc p>0.05), indicating a lack of a CPP. Across all subsequent CPP trials (ie, trials 2–6), saline-treated rats conditioned with either dose of cocaine continued to spend significantly more time in the cocaine-paired chamber than the saline-paired chamber overall (effect of chamber: 10 mg/kg cocaine, F2,14=10.82, p=0.001, post hoc p<0.001; 20 mg/kg cocaine, F2,14=9.04, p<0.01, post hoc p<0.01), whereas propranolol-treated rats spent an equivalent amount of time in all chambers (10 mg/kg cocaine, F2,14=2.78, p>0.05; 20 mg/kg cocaine, F2,10=1.71, p>0.05). Thus, a single treatment of propranolol disrupted initial and subsequent retrieval of a CPP.
Figure 1.
Propranolol disrupts retrieval of a cocaine-associated conditioned place preference (CPP). Following conditioning with (a) 10 mg/kg or (b) 20 mg/kg doses of cocaine, propranolol but not saline injections (arrows) prevented rats from expressing a CPP for the previously cocaine-paired chamber over the previously saline-paired chamber during the first CPP trial. Propranolol- but not saline-treated rats continued to show no CPP during subsequent propranolol-free trials. ***p<0.001, **p<0.01, and *p<0.05.
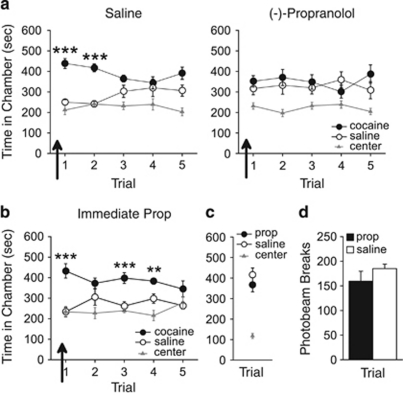
At high doses, propranolol has been shown to have effects in addition to β-receptor blockade, including serotonergic receptor blockade (Alexander and Wood, 1987) and protein kinase C inhibition (Sozzani et al, 1992). Thus, in experiment 2, we examined the effect of a low dose of (−)-propranolol, the more active enantiomer that has greater specificity for β-receptors (Dawson, 1986), on CPP expression. Rats were pretested, conditioned, and subjected to daily CPP trials, with administration of (−)-propranolol (1 mg/kg; n=8) or saline (n=8) 20 min before the first CPP trial. Rats injected with saline demonstrated a CPP for the previously cocaine-paired chamber during this trial, but rats injected with (−)-propranolol did not (see Figure 2a). ANOVA revealed that saline-treated rats spent significantly more time in the previously cocaine-paired chamber during this trial (effect of chamber: F2,14=23.18, p<0.001, post hoc p<0.001), whereas propranolol-treated rats spent an equivalent amount of time in the cocaine- and saline-paired chambers (effect of chamber F2,14=8.99, p<0.05, post hoc p>0.05). During subsequent propranolol-free trials (ie, trials 2–5), saline-treated rats continued to spend significantly more time in the cocaine-paired chamber overall (effect of chamber: F2,14=13.14, p<0.001, post hoc p<0.01), whereas propranolol-treated rats spent an equivalent amount of time in the cocaine-paired and saline-paired chambers (F2,14=39.46, p<0.001, post hoc p>0.05). Thus, (−)-propranolol disrupted the initial and subsequent retrieval of a CPP, indicating that the effects of propranolol on CPP retrieval are specific to β-receptor blockade.
Figure 2.
Propranolol-induced conditioned place preference (CPP) retrieval disruption is not due to nonspecific effects of propranolol or reconsolidation blockade. (a) Following conditioning, the more active enantiomer of propranolol, (−)-propranolol but not saline injections (arrows) prevented rats from expressing a CPP for the previously cocaine-paired chamber over the previously saline-paired chamber during the first and subsequent CPP trials. (b) Propranolol injection immediately before the first CPP trial did not prevent CPP expression during this trial or subsequent trials. (c) Following conditioning with propranolol or saline, rats spent an equivalent amount of time in the previously propranolol-paired and saline-paired chambers. (d) Propranolol had no effect on locomotor activity, as measured by photobeam breaks, when injected before an initial CPP trial. Prop, propranolol. ***p<0.001, **p<0.01, and *p<0.05.
Propranolol has been demonstrated to impair reconsolidation of a cocaine-associated memory when administered after a retrieval trial (Bernardi et al, 2006; Fricks-Gleason and Marshall, 2008), and therefore reconsolidation blockade could explain the persistent retrieval deficit observed in our study. To test this, we administered propranolol immediately before, as opposed to 20 min before, an initial CPP trial. Rats injected with propranolol (n=7) demonstrated a CPP for the previously cocaine-paired chamber during this trial and during subsequent drug-free trials (see Figure 2b). ANOVA revealed that propranolol-treated rats spent significantly more time in the previously cocaine-paired chamber during this trial (effect of chamber: F2,12=15.01, p<0.001, post hoc p<0.001). During subsequent drug-free trials, propranolol-treated rats continued to spend significantly more time in the previously cocaine-paired chamber (effect of chamber: F2,12=7.89, p=0.01, post hoc p<0.05). Thus, a single propranolol treatment immediately before an initial CPP trial did not disrupt subsequent CPP expression. Taken together, these results indicate that propranolol induces a persistent CPP retrieval deficit independently of reconsolidation blockade.
We next examined whether propranolol itself induced an affective state or influenced locomotor activity. We conditioned rats (n=11) to associate one chamber, but not another, with propranolol. Propranolol treatment had no effect on time spent in the conditioning chambers during a subsequent drug-free CPP trial (see Figure 2c). ANOVA revealed a significant effect of chamber during the CPP trial (F2,20=18.58, p<0.001), but post hoc analyses indicated that an equivalent amount of time was spent in the previously propranolol- and saline-paired chambers (p>0.05). Thus, propranolol did not generate an affective state sufficient to induce a CPP or aversion, consistent with previous findings (Milton et al, 2008). We also evaluated the effects of propranolol on locomotor activity. Rats injected with saline (n=8) or propranolol (n=8) before an initial CPP trial had similar levels of locomotor activity during the trial (t14=1.16, p=0.25; see Figure 2d). Furthermore, rats injected with saline (n=8) or (−)-propranolol (n=8) before an initial CPP trial had similar levels of locomotor activity during the trial (beam breaks: saline, 218.8±11.2; (−)-propranolol, 228.3±10.3; t14=0.62, p>0.05; data not shown). Thus, retrieval deficits induced by propranolol are not due to nonspecific motor impairments.
Propranolol Prevents Retrieval after a CPP has Already been Expressed
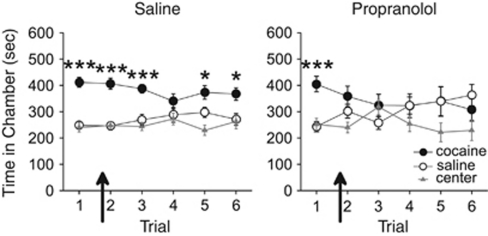
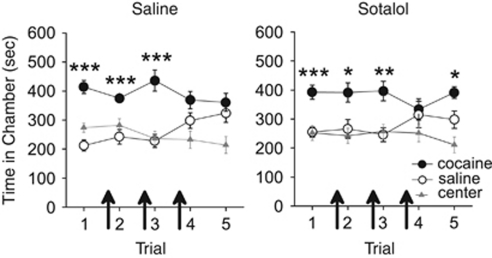
To test whether retrieval could be disrupted after a CPP had been previously expressed, in experiment 3, we examined the effects of propranolol on retrieval during the second CPP trial. Following conditioning, all rats expressed a CPP for the previously cocaine-paired chamber during the first trial. Injections of propranolol (n=11), but not saline (n=15), before the second trial impaired expression of the CPP on that trial and all subsequent trials (see Figure 3). ANOVA revealed an effect of chamber for both groups during the first trial (saline F2,28=24.91, p<0.001; propranolol, F2,20=10.10, p<0.001), and post hoc analyses confirmed that both groups spent significantly more time in the cocaine-paired chamber than the saline-paired chamber (p<0.001). Following injections of propranolol or saline before the second trial, however, only the saline-treated rats spent significantly more time in the previously cocaine-paired chamber (effect of chamber: F2,28=19.71, p<0.001, post hoc p<0.001), whereas propranolol-treated rats spent an equivalent amount of time in all chambers (F2,20=2.68, p>0.05). During subsequent propranolol-free trials (ie, trials 3–6), saline-treated rats continued to spend significantly more time in the cocaine-paired chamber overall (effect of chamber: F2,20=5.90, p<0.01, post hoc p<0.001), whereas propranolol-treated rats spent an equivalent amount of time in all chambers (F2,12=0.86, p>0.05). Thus, propranolol disrupted the retrieval of a previously expressed CPP.
Figure 3.
Propranolol disrupts retrieval of an established conditioned place preference (CPP). All rats expressed a CPP for the previously cocaine-paired chamber over the previously saline-paired chamber during the first CPP trial. Propranolol but not saline injections (arrows) prevented rats from expressing a CPP during the second CPP trial. Propranolol- but not saline-treated rats continued to show no CPP during subsequent propranolol-free trials. ***p<0.001 and *p<0.05.
Repeated Propranolol Treatment Results in a Long-Lasting Retrieval Deficit
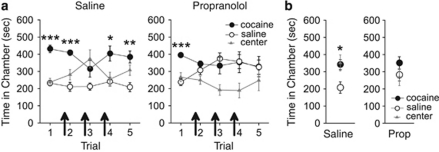
The passage of time is sufficient to induce spontaneous recovery of associative memories (Brooks and Bouton, 1993; Rescorla, 2004). Thus, we examined whether the propranolol-induced retrieval deficit would persist when rats were tested 14 days after treatment (experiment 4). Following conditioning, all rats expressed a CPP for the previously cocaine-paired chamber during the first trial. Propranolol treatment before each of the second, third, and fourth trials disrupted this CPP during these and a subsequent drug-free trial (see Figure 4a). ANOVA revealed that both treatment groups (saline, n=6; propranolol, n=7) spent significantly more time in the cocaine-paired chamber on the first trial (saline F2,12=20.67, p<0.001; propranolol, F2,10=10.86, p<0.01, post hoc p<0.05). During subsequent trials (ie, trials 2–5), saline-treated rats continued to spend significantly more time in the previously cocaine-paired chamber overall (effect of chamber: F2,12=8.48, p<0.01, post hoc p<0.001), whereas propranolol-treated rats spent an equivalent amount of time in all chambers (F2,10=3.34, p>0.05). Thus, repeated propranolol administration blocked the retrieval of a CPP during and following treatment. To determine whether this effect was resistant to spontaneous recovery, the rats were subjected to a CPP trial 14 days later. Propranolol-treated rats did not express a CPP during this trial as compared with saline-treated rats (see Figure 4b). ANOVA revealed that saline-treated rats spent significantly more time in the previously cocaine-paired chamber (effect of chamber: F2,12=5.59, p<0.05, post hoc p<0.05), whereas propranolol-treated rats spent an equivalent amount of time in all chambers (F2,10=0.81, p>0.05). Thus, repeated propranolol treatment results in a long-lasting retrieval deficit for a previously expressed CPP.
Figure 4.
Repeated propranolol treatment causes a long-lasting disruption in the retrieval of a conditioned place preference (CPP). (a) All rats expressed a CPP for the previously cocaine-paired chamber over the previously saline-paired chamber during the first CPP trial. Propranolol but not saline injections (arrows) before the second, third, and fourth CPP trials prevented the rats from expressing a CPP during those trials and a subsequent propranolol-free CPP trial. (b) Propranolol but not saline treatment resulted in a lack of expression of a CPP during a CPP trial 14 days later. Prop, propranolol. ***p<0.001, **p<0.01, and *p<0.05.
Propranolol Protects against Cocaine-Induced Reinstatement
A priming injection of cocaine has been shown to reliably induce reinstatement of a CPP after extinction (Kelley et al, 2007; Mantsch et al, 2010; Mueller and Stewart, 2000; Rodriguez-Arias et al, 2009). Behaviorally, both propranolol treatment and extinction training abolish the expression of a CPP; hence, in experiment 5, we determined whether the retrieval deficit induced by propranolol would prevent reinstatement of a CPP to a priming injection of cocaine. Rats were injected with saline (n=11) or propranolol (n=9) before the second, third, and fourth CPP trials, with saline-treated rats receiving an additional eight CPP trials of longer duration (30 min) to ensure extinction of the CPP. To compare the final extinction trial of saline-treated rats with that of propranolol-treated rats, we analyzed the first 15 min of the final CPP extinction trial. Rats in both groups showed no chamber preference by the final trial. One day later, rats were injected with cocaine (10 mg/kg, i.p.) 5 min before a CPP trial to test for reinstatement of the CPP. Previous propranolol treatment prevented cocaine-induced reinstatement of a CPP as compared with previous saline treatment (see Figure 5). ANOVA revealed that both groups spent an equivalent amount of time in all chambers during the final CPP trial (saline F2,20=0.57, p>0.05; propranolol, F2,16=0.68, p>0.05). During the propranolol-free cocaine-primed reinstatement trial, however, saline-treated rats spent significantly more time in the previously cocaine-paired chamber (effect of chamber: F2,20=4.54, p<0.05, post hoc p<0.01), whereas propranolol-treated rats spent an equivalent amount of time in all chambers (F2,16=0.07, p>0.05). Thus, only saline-treated rats expressed cocaine-induced reinstatement of a CPP, indicating that disrupting retrieval of a CPP with propranolol subsequently prevents cocaine-induced reinstatement in the absence of further propranolol treatment.
Figure 5.
Propranolol treatment prevents subsequent cocaine-induced reinstatement of a conditioned place preference (CPP). Saline- and propranolol-treated rats showed no CPP for the previously cocaine-paired chamber over the previously saline-paired chamber on the final extinction trials. The following day, saline- but not propranolol-treated rats expressed a cocaine-induced reinstatement of the CPP. Sal, saline; prop, propranolol. **p<0.01.
Propranolol-Induced Retrieval Deficits are Mediated Centrally
Propranolol acts both centrally and peripherally (Street et al, 1979), and therefore retrieval deficits could be mediated by feedback from the peripheral nervous system. To address this, in experiment 6, we administered sotalol, a β-adrenergic receptor antagonist that does not cross the blood–brain barrier (Dahlof, 1981). Following conditioning, all rats expressed a CPP for the previously cocaine-paired chamber during the first trial. Rats were then injected with saline (n=6) or sotalol (10 mg/kg, s.c.; n=9) before the second, third, and fourth CPP trials (see Figure 6). ANOVA revealed that both groups spent significantly more time in the previously cocaine-paired chamber during the first trial (saline, F2,10=18.67, p<0.001; sotalol F2,16=38.64, p<0.001) and during all trials following treatment (saline, F2,10=8.59, p<0.01; sotalol F2,16=6.31, p<0.01). Post hoc analyses confirmed that saline- and sotalol-treated rats spent significantly more time in the cocaine-paired chamber than the saline-paired chamber across all trials (p<0.01). Thus, β-adrenergic receptor blockade in the periphery alone does not affect retrieval of a CPP, indicating that the effects of propranolol must be mediated centrally.
Figure 6.
Peripheral β-adrenergic receptor antagonist sotalol does not impair retrieval of a conditioned place preference (CPP). All rats expressed a CPP for the previously cocaine-paired chamber over the previously saline-paired chamber during the first CPP trial. Peripheral β-adrenergic receptor antagonist sotalol and saline injections (arrows) before the second, third, and fourth CPP trials did not alter expression of a CPP across trials. ***p<0.001, **p<0.01, and *p<0.05.
DISCUSSION
We investigated the effects of the β-adrenergic receptor antagonist propranolol on the retrieval of a cocaine-associated CPP. Propranolol disrupted initial retrieval of the CPP and prevented subsequent retrieval in the absence of further propranolol treatment. This retrieval deficit was long lasting, perhaps permanent, as the CPP never re-emerged in subsequent trials and provided protection against cocaine-induced reinstatement. The effects of propranolol on memory retrieval were mediated centrally, as the peripheral β-receptor antagonist sotalol had no effect. Taken together, these data demonstrate for the first time that β-adrenergic receptor activation is necessary for the retrieval of a CPP, and preventing this activation results in a long-lasting, state-independent deficit in retrieval of a cocaine-associated memory.
Our finding that propranolol disrupts the retrieval of a cocaine-associated memory agrees with previous investigations. β-Adrenergic signaling has a pivotal role in retrieval of context-conditioned fear (Murchison et al, 2004; Ouyang et al, 2008) and the expression of cue-conditioned fear (Rodriguez-Romaguera et al, 2009), both of which are disrupted by propranolol. Propranolol prevents stress-induced reinstatement of drug seeking (Mantsch et al, 2010) and context-induced reinstatement of cue-conditioned fear (Morris et al, 2005), which could be attributed to memory retrieval blockade. In humans, declarative memory enhancement of emotional words is persistently disrupted by a single propranolol treatment (Kroes et al, 2010). Similarly, in abstinent heroin addicts, propranolol disrupts stress-enhanced drug-associated memory retrieval (Zhao et al, 2010). In light of our results, these findings may be due to memory retrieval blockade rather than simply a reduction in stress. In our hands, propranolol treatment resulted in a permanent retrieval deficit, as the CPP did not re-emerge after 14 days in a test for spontaneous recovery or after a priming injection of cocaine. This latter effect is remarkable, as cocaine has been shown to reliably induce reinstatement of a CPP after extinction (Kelley et al, 2007; Mantsch et al, 2010; Mueller and Stewart, 2000; Rodriguez-Arias et al, 2009). Because the passage of time and cocaine itself did not reinstate the CPP, we conclude that retrieval of the original association between cocaine and the conditioning context was disrupted.
Our finding that β-adrenergic signaling is necessary for CPP retrieval is supported by studies using mice lacking DBH, an essential enzyme for the synthesis of norepinephrine from dopamine. Although DBH-knockout mice were able to express a CPP to one of three conditioning doses of cocaine in one study (Schank et al, 2006), this was not true in another (Jasmin et al, 2006). In addition, DBH-knockout mice were unable to express a CPP after conditioning with five different doses of morphine (Olson et al, 2006). Importantly, both cocaine- and morphine-induced CPP expression was rescued in DBH-knockout mice following norepinephrine restoration by administration of DOPS during conditioning (Jasmin et al, 2006; Olson et al, 2006). The authors concluded that norepinephrine mediates the rewarding properties of addictive drugs. However, because norepinephrine was restored in both studies 24 h before the CPP trial, detectable levels of norepinephrine would have remained in the brain (Thomas et al, 1998). Taken together with the results of the current study, these results demonstrate a critical role of noradrenergic signaling in retrieval of a drug-induced CPP. The necessity of norepinephrine for retrieval may be specific to drug-associated memories, as DBH-knockout mice are able to express a food-induced CPP (Jasmin et al, 2006; Olson et al, 2006). Whether norepinephrine is necessary for retrieval of drug-associated memories in a self-administration paradigm remains to be determined.
Recent investigations have demonstrated a role for β-adrenergic signaling in the reconsolidation of drug-associated memories. Post-retrieval propranolol treatment disrupts subsequent expression of both cocaine- and morphine-associated CPP memories (Bernardi et al, 2006; Fricks-Gleason and Marshall, 2008; Robinson and Franklin, 2007), an effect attributable to reconsolidation blockade rather than facilitated extinction learning (Fricks-Gleason and Marshall, 2008). In the present study, we found that pre-retrieval propranolol treatment blocked the initial retrieval of a CPP memory. This cannot be explained by impaired reconsolidation, as memory retrieval is not disrupted until several hours following reconsolidation blockade (Lewis, 1979; Lubin and Sweatt, 2007; Nader and Hardt, 2009; Nader et al, 2000). Moreover, initial memory retrieval is required for reconsolidation to occur (Nader and Hardt, 2009; Nader et al, 2000). Our results show that the initial retrieval was blocked by propranolol independently of CPP expression deficits, as propranolol had no effect on locomotor activity and did not induce a CPP or aversion on its own. We also showed that allowing memory retrieval by administering propranolol immediately rather than 20 min before CPP testing spared CPP expression during subsequent trials. Thus, our results are congruent with a permanent impairment in memory retrieval rather than disrupted memory reconsolidation. This is in agreement with studies in other learning and memory paradigms, demonstrating that manipulations can induce persistent memory retrieval deficits independently of blocking memory reconsolidation (Kim and Fanselow, 1992; Kroes et al, 2010; Miller and Springer, 1972; Sara, 1973, 2000).
Another interpretation of our results is that propranolol facilitated the extinction of a CPP. This is unlikely, however, because of the complete absence of a CPP following propranolol administration before a CPP trial. Furthermore, it is well accepted that norepinephrine facilitates, rather than impedes, memory formation through β-receptor activation (McGaugh, 2000). Finally, research has demonstrated that propranolol impairs extinction learning (for review see Mueller and Cahill, 2010) in both aversive (Do-Monte et al, 2010; Merlo and Izquierdo, 1967; Mueller et al, 2008; Ouyang and Thomas, 2005) and appetitive paradigms (LaLumiere et al, 2010).
Our findings indicate that the long lasting, perhaps permanent, disruption of retrieval of cocaine-associated memories that results from β-adrenergic receptor blockade is mediated centrally, implicating central norepinephrine release in memory retrieval. The locus or loci at which these receptors mediate retrieval, however, remain to be determined. Evidence indicates that β-receptors in the dorsal hippocampus mediate retrieval of other contextual memories, although the β-receptor dependence is limited to 3 days following conditioning (Murchison et al, 2004). In experiments 2, 3, and 4, propranolol was administered 4 days following the last of eight conditioning trials, and induced a long-term deficit in CPP retrieval. Thus, the effect of propranolol on CPP retrieval may be independent of the hippocampus and is unlikely to have the same time limitations as the retrieval of other hippocampus-dependent contextual memories. Whether remote CPP memories (weeks or months after conditioning) are amenable to disruption by propranolol, however, remains to be determined.
Although the noradrenergic mechanisms underlying retrieval are poorly understood, activation of locus coeruleus noradrenergic neurons likely mediates retrieval. These neurons show a graded response to stimuli, responding greatest to particularly salient and relevant stimuli (Sara, 2009). Thus, the attribution of salience to stimuli following learning suggests that conditioned stimuli are particularly capable of evoking noradrenergic responses. In fact, locus coeruleus neurons respond vigorously to changes in stimulus-reinforcement contingencies, and this neural response precedes changes in behavioral responses (Aston-Jones et al, 1997; Bouret and Sara, 2004; Sara and Segal, 1991). Furthermore, recording studies in freely moving rats have shown that locus coeruleus neurons fire in response to salient stimuli on the first few presentations even in the absence of the unconditioned stimulus, but the neural response declines when the stimulus no longer evokes a conditioned response (Bouret and Sara, 2004; Sara and Segal, 1991). Direct stimulation of locus coeruleus neurons facilitates memory retrieval (Devauges and Sara, 1991; Sara and Devauges, 1988), supporting the notion that stimulus-evoked norepinephrine release initiates memory retrieval. As a result, disruption of noradrenergic signaling by propranolol prevents retrieval (Murchison et al, 2004; Ouyang et al, 2008; present findings) and may render drug-associated stimuli less salient. This effect could be due to a deficit in attention, such that rats are unaware of the salience previously attributed to the drug-associated chamber. Thus, subsequent presentations of those stimuli no longer evoke norepinephrine release, resulting in a permanent deficit in memory retrieval. Accordingly, noradrenergic stimulation (via noradrenergic agonists or direct locus coeruleus stimulation) may induce memory retrieval, as demonstrated in other paradigms (Devauges and Sara, 1991; Sara and Devauges, 1988, 1989). These data suggest that the original memory remains intact, but the retrieval mechanism has been disrupted. Thus, reconditioning previously propranolol-treated rats might be expected to strengthen the original CPP memory and re-engage retrieval mechanisms that were previously disrupted.
The presentation of drug-associated stimuli promotes drug seeking and relapse in addicts (Ehrman et al, 1992; Foltin and Haney, 2000; Heather et al, 1991; O'Brien et al, 1990; O'Brien et al, 1977). Thus, pharmacological manipulations that disrupt retrieval of long-term memories offer a particularly efficacious avenue for therapies targeting the elimination of these drug-associated memories. Rehabilitation programs utilizing exposure therapy, in which addicts are presented with drug-associated stimuli in the absence of the drug, have had limited success without the use of pharmacological adjuncts (Conklin and Tiffany, 2002; O'Brien et al, 1990). There are, however, no FDA-approved pharmacological adjuncts to exposure therapy for the treatment of addiction. Our findings suggest that propranolol would be a useful adjuvant during exposure therapy to permanently disrupt stimulus-induced drug seeking and prevent drug-induced relapse long after treatment cessation.
Acknowledgments
This research was supported by DA027870 and a grant from the University of Wisconsin-Milwaukee Research Growth Initiative to DM. We thank Kidane Dashew and Daniel Yang for technical assistance, Dr Robert Twining for insight into experimental design, and Patrick Reilly for design and construction of the CPP boxes.
The authors declare no conflict of interest.
References
- Alexander BS, Wood MD. Stereoselective blockade of central [3H]5-hydroxytryptamine binding to multiple sites (5-HT1A, 5-HT1B and 5-HT1C) by mianserin and propranolol. J Pharm Pharmacol. 1987;39:664–666. doi: 10.1111/j.2042-7158.1987.tb03452.x. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Kubiak P. Conditioned responses of monkey locus coeruleus neurons anticipate acquisition of discriminative behavior in a vigilance task. Neuroscience. 1997;80:697–715. doi: 10.1016/s0306-4522(97)00060-2. [DOI] [PubMed] [Google Scholar]
- Banna KM, Back SE, Do P, See RE. Yohimbine stress potentiates conditioned cue-induced reinstatement of heroin-seeking in rats. Behav Brain Res. 2010;208:144–148. doi: 10.1016/j.bbr.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi RE, Lattal KM, Berger SP. Postretrieval propranolol disrupts a cocaine conditioned place preference. Neuroreport. 2006;17:1443–1447. doi: 10.1097/01.wnr.0000233098.20655.26. [DOI] [PubMed] [Google Scholar]
- Bouret S, Sara SJ. Reward expectation, orientation of attention and locus coeruleus-medial frontal cortex interplay during learning. Eur J Neurosci. 2004;20:791–802. doi: 10.1111/j.1460-9568.2004.03526.x. [DOI] [PubMed] [Google Scholar]
- Brooks DC, Bouton ME. A retrieval cue for extinction attenuates spontaneous recovery. J Exp Psychol Anim Behav Process. 1993;19:77–89. doi: 10.1037//0097-7403.19.1.77. [DOI] [PubMed] [Google Scholar]
- Cassens G, Roffman M, Kuruc A, Orsulak PJ, Schildkraut JJ. Alterations in brain norepinephrine metabolism induced by environmental stimuli previously paired with inescapable shock. Science. 1980;209:1138–1140. doi: 10.1126/science.7403874. [DOI] [PubMed] [Google Scholar]
- Chauvet C, Lardeux V, Goldberg SR, Jaber M, Solinas M. Environmental enrichment reduces cocaine seeking and reinstatement induced by cues and stress but not by cocaine. Neuropsychopharmacology. 2009;34:2767–2778. doi: 10.1038/npp.2009.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR, McLellan AT, O'Brien CP. Role of conditioning factors in the development of drug dependence. Psychiatr Clin North Am. 1986;9:413–425. [PubMed] [Google Scholar]
- Conklin CA, Tiffany ST. Applying extinction research and theory to cue-exposure addiction treatments. Addiction. 2002;97:155–167. doi: 10.1046/j.1360-0443.2002.00014.x. [DOI] [PubMed] [Google Scholar]
- Dahlof C. Studies on beta-adrenoceptor mediated facilitation of sympathetic neurotransmission. Acta Physiol Scand Suppl. 1981;500:1–147. [PubMed] [Google Scholar]
- Dawson RMC. Data for Biochemical Research. Oxford: New York; 1986. p. 347. [Google Scholar]
- Devauges V, Sara SJ. Memory retrieval enhancement by locus coeruleus stimulation: evidence for mediation by beta-receptors. Behav Brain Res. 1991;43:93–97. doi: 10.1016/s0166-4328(05)80056-7. [DOI] [PubMed] [Google Scholar]
- Do-Monte FH, Kincheski GC, Pavesi E, Sordi R, Assreuy J, Carobrez AP. Role of beta-adrenergic receptors in the ventromedial prefrontal cortex during contextual fear extinction in rats. Neurobiol Learn Mem. 2010;94:318–328. doi: 10.1016/j.nlm.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Ehrman RN, Robbins SJ, Childress AR, O'Brien CP. Conditioned responses to cocaine-related stimuli in cocaine abuse patients. Psychopharmacology (Berl) 1992;107:523–529. doi: 10.1007/BF02245266. [DOI] [PubMed] [Google Scholar]
- Feenstra MG, Vogel M, Botterblom MH, Joosten RN, de Bruin JP. Dopamine and noradrenaline efflux in the rat prefrontal cortex after classical aversive conditioning to an auditory cue. Eur J Neurosci. 2001;13:1051–1054. doi: 10.1046/j.0953-816x.2001.01471.x. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Haney M. Conditioned effects of environmental stimuli paired with smoked cocaine in humans. Psychopharmacology (Berl) 2000;149:24–33. doi: 10.1007/s002139900340. [DOI] [PubMed] [Google Scholar]
- Fricks-Gleason AN, Marshall JF. Post-retrieval beta-adrenergic receptor blockade: effects on extinction and reconsolidation of cocaine-cue memories. Learn Mem. 2008;15:643–648. doi: 10.1101/lm.1054608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MC, See RE. Differential involvement of the core and shell subregions of the nucleus accumbens in conditioned cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2004;176:459–465. doi: 10.1007/s00213-004-1895-6. [DOI] [PubMed] [Google Scholar]
- Heather N, Stallard A, Tebbutt J. Importance of substance cues in relapse among heroin users: comparison of two methods of investigation. Addict Behav. 1991;16:41–49. doi: 10.1016/0306-4603(91)90038-j. [DOI] [PubMed] [Google Scholar]
- Herman CP. External and internal cues as determinants of the smoking behavior of light and heavy smokers. J Pers Soc Psychol. 1974;30:664–672. doi: 10.1037/h0037440. [DOI] [PubMed] [Google Scholar]
- Jasmin L, Narasaiah M, Tien D. Noradrenaline is necessary for the hedonic properties of addictive drugs. Vascul Pharmacol. 2006;45:243–250. doi: 10.1016/j.vph.2005.08.030. [DOI] [PubMed] [Google Scholar]
- Kelley JB, Anderson KL, Itzhak Y. Long-term memory of cocaine-associated context: disruption and reinstatement. Neuroreport. 2007;18:777–780. doi: 10.1097/WNR.0b013e3280c1e2e7. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality specific retrograde amnesia of fear following hippocampal lesions. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Kroes MCW, Strange BA, Dolan RJ. β-adrenergic blockade during memory retrieval in humans evokes a sustained reduction of declarative emotional memory enhancement. J Neurosci. 2010;30:3959–3963. doi: 10.1523/JNEUROSCI.5469-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLumiere RT, Niehoff KE, Kalivas PW. The infralimbic cortex regulates the consolidation of extinction after cocaine self-administration. Learn Mem. 2010;17:168–175. doi: 10.1101/lm.1576810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DJ. Psychobiology of active and inactive memory. Psychol Bull. 1979;86:1054–1083. [PubMed] [Google Scholar]
- Lubin FD, Sweatt JD. The IkappaB kinase regulates chromatin structure during reconsolidation of conditioned fear memories. Neuron. 2007;55:942–957. doi: 10.1016/j.neuron.2007.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch JR, Weyer A, Vranjkovic O, Beyer CE, Baker DA, Caretta H. Involvement of noradrenergic neurotransmission in the stress- but not cocaine-induced reinstatement of extinguished cocaine-induced conditioned place preference in mice: role for beta-2 adrenergic receptors. Neuropsychopharmacology. 2010;35:2165–2178. doi: 10.1038/npp.2010.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL. Memory--a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- Merlo AB, Izquierdo I. The effect of catecholamines on learning in rats. Med Pharmacol Exp Int J Exp Med. 1967;16:343–349. doi: 10.1159/000137009. [DOI] [PubMed] [Google Scholar]
- Miller RR, Springer AD. Induced recovery of memory in rats following electroconvulsive shock. Physiol Behav. 1972;8:645–651. doi: 10.1016/0031-9384(72)90089-3. [DOI] [PubMed] [Google Scholar]
- Milton AL, Lee JL, Everitt BJ. Reconsolidation of appetitive memories for both natural and drug reinforcement is dependent on β-adrenergic receptors. Learn Mem. 2008;15:88–92. doi: 10.1101/lm.825008. [DOI] [PubMed] [Google Scholar]
- Mingote S, de Bruin JP, Feenstra MG. Noradrenaline and dopamine efflux in the prefrontal cortex in relation to appetitive classical conditioning. J Neurosci. 2004;24:2475–2480. doi: 10.1523/JNEUROSCI.4547-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RW, Westbrook RF, Killcross AS. Reinstatement of extinguished fear by beta-adrenergic arousal elicited by a conditioned context. Behav Neurosci. 2005;119:1662–1671. doi: 10.1037/0735-7044.119.6.1662. [DOI] [PubMed] [Google Scholar]
- Mueller D, Cahill SP. Noradrenergic modulation of extinction learning and exposure therapy. Behav Brain Res. 2010;208:1–11. doi: 10.1016/j.bbr.2009.11.025. [DOI] [PubMed] [Google Scholar]
- Mueller D, Porter JT, Quirk GJ. Noradrenergic signaling in infralimbic cortex increases cell excitability and strengthens memory for fear extinction. J Neurosci. 2008;28:369–375. doi: 10.1523/JNEUROSCI.3248-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller D, Stewart J. Cocaine-induced conditioned place preference: reinstatement by priming injections of cocaine after extinction. Behav Brain Res. 2000;115:39–47. doi: 10.1016/s0166-4328(00)00239-4. [DOI] [PubMed] [Google Scholar]
- Murchison CF, Zhang XY, Zhang WP, Ouyang M, Lee A, Thomas SA. A distinct role for norepinephrine in memory retrieval. Cell. 2004;117:131–143. doi: 10.1016/s0092-8674(04)00259-4. [DOI] [PubMed] [Google Scholar]
- Nader K, Hardt O. A single standard for memory: the case for reconsolidation. Nat Rev Neurosci. 2009;10:224–234. doi: 10.1038/nrn2590. [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- O'Brien CP, Childress AR, McLellan T, Ehrman R. Integrating systemic cue exposure with standard treatment in recovering drug dependent patients. Addict Behav. 1990;15:355–365. doi: 10.1016/0306-4603(90)90045-y. [DOI] [PubMed] [Google Scholar]
- O'Brien CP, Testa T, O'Brien TJ, Brady JP, Wells B. Conditioned narcotic withdrawal in humans. Science. 1977;195:1000–1002. doi: 10.1126/science.841320. [DOI] [PubMed] [Google Scholar]
- Olson VG, Heusner CL, Bland RJ, During MJ, Weinshenker D, Palmiter RD. Role of noradrenergic signaling by the nucleus tractus solitarius in mediating opiate reward. Science. 2006;311:1017–1020. doi: 10.1126/science.1119311. [DOI] [PubMed] [Google Scholar]
- Ouyang M, Thomas SA. A requirement for memory retrieval during and after long-term extinction learning. Proc Natl Acad Sci USA. 2005;102:9347–9352. doi: 10.1073/pnas.0502315102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang M, Zhang L, Zhu JJ, Schwede F, Thomas SA. Epac signaling is required for hippocampus-dependent memory retrieval. Proc Natl Acad Sci USA. 2008;105:11993–11997. doi: 10.1073/pnas.0804172105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA. Spontaneous recovery varies inversely with the training-extinction interval. Learn Behav. 2004;32:401–408. doi: 10.3758/bf03196037. [DOI] [PubMed] [Google Scholar]
- Robinson MJ, Franklin KB. Central but not peripheral beta-adrenergic antagonism blocks reconsolidation for a morphine place preference. Behav Brain Res. 2007;182:129–134. doi: 10.1016/j.bbr.2007.05.023. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Arias M, Castillo A, Daza-Losada M, Aguilar MA, Minarro J. Effects of extended cocaine conditioning in the reinstatement of place preference. Physiol Behav. 2009;96:620–630. doi: 10.1016/j.physbeh.2008.12.011. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Romaguera J, Sotres-Bayon F, Mueller D, Quirk GJ. Systemic propranolol acts centrally to reduce conditioned fear in rats without impairing extinction. Biol Psychiatry. 2009;65:887–892. doi: 10.1016/j.biopsych.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara SJ. Recovery from hypoxia and ECS induced amnesia after a single exposure to the training environment. Physiol Behav. 1973;9:85–89. doi: 10.1016/0031-9384(73)90091-7. [DOI] [PubMed] [Google Scholar]
- Sara SJ. Retrieval and reconsolidation: toward a neurobiology of remembering. Learn Mem. 2000;7:73–84. doi: 10.1101/lm.7.2.73. [DOI] [PubMed] [Google Scholar]
- Sara SJ. The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci. 2009;10:211–223. doi: 10.1038/nrn2573. [DOI] [PubMed] [Google Scholar]
- Sara SJ, Devauges V. Priming stimulation of locus coeruleus facilitates memory retrieval in the rat. Brain Res. 1988;438:299–303. doi: 10.1016/0006-8993(88)91351-0. [DOI] [PubMed] [Google Scholar]
- Sara SJ, Devauges V. Idazoxan, an alpha-2 antagonist, facilitates memory retrieval in the rat. Behav Neural Biol. 1989;51:401–411. doi: 10.1016/s0163-1047(89)91039-x. [DOI] [PubMed] [Google Scholar]
- Sara SJ, Segal M. Plasticity of sensory responses of locus coeruleus neurons in the behaving rat: implications for cognition. Prog Brain Res. 1991;88:571–585. doi: 10.1016/s0079-6123(08)63835-2. [DOI] [PubMed] [Google Scholar]
- Schank JR, Ventura R, Puglisi-Allegra S, Alcaro A, Cole CD, Liles LC, et al. Dopamine β-hydroxylase knockout mice have alterations in dopamine signaling and are hypersensitive to cocaine. Neuropsychopharmacology. 2006;31:2221–2230. doi: 10.1038/sj.npp.1301000. [DOI] [PubMed] [Google Scholar]
- Sozzani S, Agwu DE, McCall CE, O'Flaherty JT, Schmitt JD, Kent JD, et al. Propranolol, a phosphatidate phosphohydrolase inhibitor, also inhibits protein kinase C. J Biol Chem. 1992;267:20481–20488. [PubMed] [Google Scholar]
- Sterpenich V, D'Argembeau A, Desseilles M, Balteau E, Albouy G, Vandewalle G, et al. The locus ceruleus is involved in the successful retrieval of emotional memories in humans. J Neurosci. 2006;26:7416–7423. doi: 10.1523/JNEUROSCI.1001-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street JA, Hemsworth BA, Roach AG, Day MD. Tissue levels of several radiolabelled beta-adrenoceptor antagonists after intravenous administration in rats. Arch Int Pharmacodyn Ther. 1979;237:180–190. [PubMed] [Google Scholar]
- Thomas SA, Marck BT, Palmiter RD, Matsumoto AM. Restoration of norepinephrine and reversal of phenotypes in mice lacking dopamine β-hydroxylase. J Neurochem. 1998;70:2468–2476. doi: 10.1046/j.1471-4159.1998.70062468.x. [DOI] [PubMed] [Google Scholar]
- van Stegeren AH. The role of the noradrenergic system in emotional memory. Acta Psychol (Amst) 2008;127:532–541. doi: 10.1016/j.actpsy.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Zhao LY, Shi J, Zhang XL, Epstein DH, Zhang XY, Liu Y, et al. Stress enhances retrieval of drug-related memories in abstinent heroin addicts. Neuropsychopharmacology. 2010;35:720–726. doi: 10.1038/npp.2009.179. [DOI] [PMC free article] [PubMed] [Google Scholar]