Abstract
The cellular protein synthesis machinery is tightly regulated and capable of rapid reaction to a variety of physiological inputs critical in stress-response, cell cycle control, cancer biology and virus infection. One important strategy for stimulating protein synthesis involves the ser/thr kinase Akt, which subsequently triggers inactivation of the cap-dependent translational repressor 4E-BP1 by an mTOR-containing protein complex (mTORC1). A recent paper demonstrated that herpes simplex virus utilizes a remarkable tactic to activate mTOR in infected cells. Instead of using the cellular Akt, the virus produces a ser/thr kinase called Us3 that doesn't look like Akt, but masquerades as Akt. By making the Akt-like protein unrecognizable, this disguise allows it to bypass the strict limits normally imposed on the real cellular Akt. Importantly, preventing the virus Akt-imposter from triggering mTORC1 inhibited viral growth, suggesting a new way to block herpes simplex virus. This study also raises the possibility that other Akt-impersonators may lurk hidden in our own genomes, possibly contributing to diseases ranging from diabetes to cancer.
Key words: Akt signaling, translational control, mTORC1 activation, virus replication, viral kinase
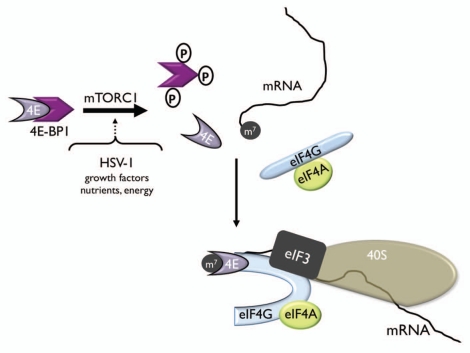
By manipulating activity of the translation repressor 4E-BP1, Akt signaling through mTORC1 controls a critical step regulating the initiation of protein synthesis in eukaryotes.1 4E-BP1 represses translation by binding to the cellular cap-binding protein, eukaryotic translation initiation factor 4E (eIF4E), preventing its incorporation into the eIF4F multi-subunit complex required to initiate translation.2 Hyperphosphorylation of 4E-BP1 by mTORC1 inactivates the repressor, allowing eIF4E to associate with the large molecular scaffold eIF4G and assemble eIF4F (Fig. 1).1 Subsequently, hyperphosphorylated 4E-BP1 can be degraded by the proteasome.3–5 Regulating assembly of eIF4F is a major point through which 40S ribosome subunits are recruited and loaded onto the mRNA 5′ end. Indeed, numerous biological regulatory processes where differential control of translation plays a fundamental role often involve 4E-BP1 hyperphosphorylation by mTORC1.6
Figure 1.
Repression of eIF4F assembly and cap-dependent mRNA translation by 4E-BP1 phosphorylation. The cellular cap-binding protein (4E) is depicted bound to the translational repressor eIF4E-binding protein 1 (4E-BP1) and is unable to assemble into an eIF4F complex with the other translation initiation factors, eIF4G and eIF4A. Activation of the kinase mTOR in response to a variety of cues such as HSV-1 infection, growth factor signaling, alterations to the nutrient pool or changes in cellular energy reserves results in phosphorylation of the translational repressor protein 4E-BP1 and the release of eIF4E from 4E-BP1. Binding of eIF4E to eIF4G and eIF4A results in assembly of the multisubunit initiation factor eIF4F complex, which in turn recognizes the 7-methyl guanine cap (m7) at the mRNA 5′ end. The 40S ribosome is recruited through its association with eIF3.
Diverse inputs including nutrient, energy and growth factor availability are integrated by the tuberous sclerosis heterodimer complex (TSC1/2), which controls mTORC1 activation.1 TSC is a GTPase activating protein (GAP) for the small G-protein rheb (ras-homolog enriched in brain). Whereas rheb•GTP activates mTORC1, rheb•GDP cannot. Thus, TSC GAP activation results in rheb•GDP accumulation and inactive mTORC1, while TSC GAP inhibition results in rheb•GTP accumulation and stimulates mTORC1 (Fig. 2). TSC activity is controlled by phosphorylation of the TSC2 subunit by different cellular kinases. One of these is Akt. In response to PI3-kinase activation, recruitment of PDK1 and Akt to the plasma membrane results in Akt phosphorylation at T308. Fully active Akt results after a second mTOR-containing complex, mTORC2, phosphorylates S473. Phosphorylation of TSC2 by Akt on S939 and T1462 inhibits TSC Gap activity and thereby activates mTORC1. Although mTORC1 can be regulated by inputs from other signaling pathways including Erk/RSK, which also can phosphorylate TSC2 to inhibit its GAP function and Rag-GTPases, which are required for mTORC1 activation in response to amino acids, Akt signaling is required for growth factors and hormone-responsive mTORC1 activation and the resulting stimulation of protein synthesis.7 In addition to TSC2 phosphorylation, Akt can also phosphorylate and inactivate PRAS40, an mTORC1 inhibitory subunit.8 Phosphorylation of the mTORC1 substrates ATG1, ribosomal protein S6 kinase and 4E-BP1 regulate autophagy, cell size, cell proliferation and protein synthesis.9,10 In addition, inhibition of mTORC1 with rapamycin decelerates cellular senescence.11 These basic processes are important in a variety of pathophysiological settings, including response to stress, cell cycle control, age-related diseases, cancer biology and virus infection.
Figure 2.
Phosphorylation of TSC2 by Akt or Us3 activates mTORC1. Growth factor-mediated activation of mTORC1 is illustrated. IRS1 is recruited to the cytoplasmic face of activated growth factor receptors and stimulates PI3-kinase signaling. PDK1 and Akt are both localized to the plasma membrane via a lipid-binding plekstrin homology domain. Upon activation by PI3-kinase, PDK1 phosphorylates Akt on T308, thereby contributing to Akt activation. Full Akt activation also requires S473 phosphorylation by mTORC2. Akt inhibits TSC rheb-GAP activity by phosphorylating TSC2 on T1462/S939, promoting rheb•GTP-mediated mTORC1 activation and subsequent 4E-BP1 hyperphosphorylation and activation of p70 S6K (S6K). Inactivation of the translational repressor 4E-BP1 promotes binding of eIF4E to eIF4G, stimulating cap-dependent translation. An intrinsic feedback control circuit whereby inactivation of IR S1 and mTORC2 by activated p70 S6K limits Akt activation. Even though it is unrelated to Akt at the primary sequence level, the HSV1 ser/thr kinase Us3 phosphorylates TSC2 on the same residues targeted by Akt (T1462, S939). By targeting TSC2, this strategy allows Us3 to bypass the intrinsic feedback controls designed to limit Akt activation, allowing HSV1 to constitutively inactivate 4E-BP1 and maintain high levels of viral mRNA translation in infected cells.
Following lytic infection of a host cell or reactivation from latency, herpesviruses stimulate the cap-dependent translation machinery of their hosts by promoting eIF4F assembly.3,12–15 This is often a critical step in the virus replication cycle, as viruses are completely dependent on the translation machinery resident in their cellular hosts to produce viral proteins required for their productive growth. Similar to uninfected cells, herpesvirus-induced eIF4F assembly was sensitive to recently-developed mTOR active site inhibitors and impaired by expression of a dominant 4E-BP1 repressor allele with T→A substitutions at the key T37 and T46 sites.16–18 However, in a major departure from findings in uninfected cells, 4E-BP1 was constitutively hyperphosphorylated in HSV-1-infected cells in the presence of allosteric Akt inhibitors.17 Precisely how HSV-1 was able to stimulate mTORC1 in the absence of Akt signaling was not known.
Recently, we established that the HSV-1 ser/thr kinase encoded by the Us3 gene is required to activate mTORC1, inactivate the 4E-BP1 translational repressor and stimulate eIF4F assembly. Surprisingly, Us3 displays no sequence homology with the cellular kinase Akt, yet directly phosphorylates tuberous sclerosis complex 2 (TSC2) on S939 and T1462, the same sites targeted by Akt to inhibit TSC activity and activate mTORC1 in uninfected cells.17 While it is not unusual for virus infection to stimulate Akt signaling, this typically involves PI3-kinase activation by a virus-encoded gene product, such as Influenza virus A NS1, HSV1 VP11/12, KSHV-encoded GPCR, HCMV IE1/2, EBV LMP2A and Adenovirus E4 orf1 or a less well-understood protein phosphatase 2A-dependent process involving HPV E7 or Adenovirus E4 orf4 that may prevent dephosphorylation of mTORC1 substrates.19–22 Akt activated in this manner is potentially limited by intrinsic feedback circuitry built into this important pathway, prohibiting sustained Akt activation (Fig. 2).23 Indeed, transient Akt activation early in the replication cycle is observed in primary human fibroblasts infected with wild-type HSV-1 or HCMV (C. McKinney, IM, in preparation).24 Failure to continuously stimulate Akt could limit mTORC1 activation, imposing substantial constraints on viruses that require the host cap-dependent translation machinery and seek to inactivate the 4E-BP1 translational repressor. Some viruses encode multiple functions capable of activating Akt or downstream targets, further illustrating the importance of this task in the virus lifecycle.22 Significantly, TSC-inactivation by Us3 allows HSV1 to activate mTORC1 even when Akt activity is low or undetectable, as may be the case in non-proliferating cells. Furthermore, by acting at the level of TSC, mTORC1 activation by Us3 is not responsive to p70 S6K-mediated cellular feedback controls in place to limit both receptor-mediated activation of the PI3K/Akt/mTORC1 signaling axis and mTORC2-mediated Akt activation (Fig. 2).23,25 Other viruses likewise activate mTORC1 via TSC, albeit via different mechanism, illustrating the potential advantages of targeting TSC2 to stimulate mTORC1 in infected cells. The related herpesvirus HCMV encodes a TSC2-binding protein (UL38) that inhibits TSC activity, while HPV E6 binds TSC2 and targets it for proteasome degradation.26,27 None of these strategies, however, involve direct phosphorylation of TSC2 by viral enzymes.
Disabling TSC allows viruses to overcome a natural antiviral checkpoint. Interestingly, Us3-deficient virus replication is impaired relative to wild-type in normal primary human fibroblasts and replication is significantly restored upon siRNA-mediated TSC2-depletion. WT virus replication, however, is not impacted by TSC2-depletion, consistent with the observation that TSC is already inactivated in cells infected with WT HSV-1.17 Thus, TSC comprises an antiviral checkpoint for viruses that require the host cap-dependent translation machinery to produce virus-encoded polypeptides. Replication of viruses that are not equipped to counteract TSC-mediated mTORC1 repression will be limited, unless they bypass this requirement by using an alternative, cap-independent mode of translation initiation that does not require eIF4E.
Not only is TSC regulated by Akt, it is also a critical juncture integrating signaling inputs from other pathways. In contrast to Akt, differential TSC2 phosphorylation by AMP-activated protein kinase (AMPK), which is responsive to elevated AMP levels resulting from energy deprivation, stimulates TSC Rheb-GAP activity and prevents mTORC1 activation.28 Induction of the p53-responsive sestrins 1 and 2 in response to genotoxic stress likewise activate AMPK and stimulate TSC Rheb-GAP.29 TSC2 phosphorylation by glycogen synthase kinase 3 cooperates with AMPK-mediated phosphorylation to inhibit mTORC1, linking bioenergetic state with Wnt-signaling responsive GSK3.30 Finally, hypoxia-induced REDD1 activates TSC by interfering with phosphorylation-dependent association of TSC with 14-3-3 proteins.31 Binding of phosphorylated TSC2 to 14-3-3 has been proposed to account for Akt-mediated inhibition of TSC. Thus, REDD1-mediated displacement of 14-3-3 from phospho-TSC2 prevents mTORC1 activation even though Akt is constitutively active. In HSV1 infected primary human fibroblasts, Erk activation, which can also phosphorylate TSC2 and inhibit TSC rheb-GAP, is suppressed, making it unlikely that Erk/Rsk signaling plays a role in mTORC1 activation.3 However, how other TSC regulators respond to HSV-1 infection and the potential for Us3 to repress TSC Rheb-GAP activity in response to different stress inputs remains largely unexplored.
Given the lack of primary sequence homology between Akt and Us3, it is amazing that Us3 stimulates phosphorylation of Akt substrates other than TSC2 in an Akt-independent manner. Both FOXO1 and GSK3 were among the Akt substrates phosphorylated by Us3 on the same residues targeted by Akt.17 Thus, Us3 appears to be an Akt surrogate with overlapping substrate specificity that activates mTORC1, stimulating translation and virus replication. As a unique viral kinase unrelated to any single cellular kinase, Us3 is a potential drug development target. Small molecule Us3 inhibitors could prevent HSV1-induced mTORC1 activation and effectively suppress replication without the immune suppressive side-effects associated with targeting mTOR itself.32 The benefits of such a strategy may not be confined to HSV1, given the prevalence of different virus-encoded TSC-inhibitory functions all focused on activating mTORC1 in virus-infected cells.
Viruses are masters at encoding multifunctional proteins like Us3, extracting maximum functionality from limited coding regions. Nevertheless, all known Us3 functions are impaired by mutations that eliminate its kinase activity. In addition to the cellular targets TSC2, GSK3 and FOXO1, Us3 has anti-apoptotic activity that likely involves phosphorylation of yet another Akt substrate BAD.33,34 Us3 also phosphorylates viral proteins some of which stimulate nuclear lamina disassembly and egress of newly assembled progeny virions.35,36 While not essential for replication, Us3-deficient viruses exhibit cell type-specific replication defects in culture and are severely impaired in mouse pathogenesis models.37–39 A recently developed cultured rat neuron model of HSV latency/reactivation dependent upon PI-3K/Akt signaling provides an exciting opportunity to probe the role of Us3 in reactivation.40 Indeed, by allowing Us3 access to a diverse palate of substrates, a multitude of host and virus-specific tasks can be subverted through the catalytic actions of a single virus enzyme. Given the sheer breadth of these diverse processes involving cellular and viral substrates, Us3 substrate specificity may not be restricted to those of a single cellular kinase like Akt. This raises some important questions regarding the limits of Us3 substrate targeting. For example, are all Akt substrates targeted by Us3, or are some Akt substrates effectively excluded, as their phosphorylation by Us3 may interfere somehow with viral replication? By mimicking Akt activity downstream of the cellular kinase, Us3 could effectively cherry pick which Akt substrates will be phosphorylated and which will be left untouched. Alternatively, the substrate specificity of Us3 could conceivably be so broad that some substrates make little or no detectable contribution to infected cell physiology, representing biological “noise” tolerated but neither advantageous nor detrimental to virus biology. Ultimately, understanding the role of each Us3 substrate in the virus lifecycle will require detailed genetic and biochemical analysis.
How Us3 recognizes its full spectrum of substrates, including those that overlap with Akt, remains unknown. Since Us3 bears little resemblance to any specific Ser/Thr kinase, target site prediction is problematic.17 Studies with synthetic peptide substrates in vitro have not yielded clear-cut results. Although capable of phosphorylating PKA and PKC peptide substrates, Us3 appeared to have a distinct specificity.41 Sequence recognition motifs, however, are not likely to provide the required in vivo specificity, since (1) many kinases share in vitro recognition motifs (i.e., Arg residues N-terminal to phospho-acceptor site for p90 RSK, PKA and Akt), (2) recognition motifs may not be physiologically phosphorylated, (3) peptides may not mimic intact protein phosphorylation kinetics and (4) not all kinases have a clear consensus motif in peptide substrates.42 Instead, interactions beyond the active site that tether the kinase to physiological substrates are often responsible for biological specificity.43,44 Despite the lack of significant primary sequence homology between Akt and Us3, their functional motifs governing substrate recognition may in fact be related structurally. Such structural similarity in the presence of only limited primary sequence homology has been observed previously among other proteins including globin, lysozyme and thioredoxin family members.45–47 Finally, the existence of an Akt-like kinase such as Us3 that shares Akt substrates, but not primary sequence homology has potential consequences for the biology of its human host. Is encoding an Akt mimic unrelated at the primary sequence level confined to virus biology, defining an effective strategy to escape from normal constraints that limit Akt activation? Or are there other ways to make kinases with Akt-like substrate specificity lurking in our own genomes? Their unrelatedness at the primary sequence level would render them invisible to present day functional genomic-based identification methods other than pinning them as kinases. Should their identity ever be unmasked, they are likely to play important roles given the critical contributions of Akt signaling to human health and disease.
Acknowledgments
We thank Derek Walsh for critical comments on the manuscript. This work was supported by grants from the NIH to I.M. (GM056927 and AI073898). U.C. was supported in part by an NIH training grant (T32 AI007647).
References
- 1.Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009;10:307–318. doi: 10.1038/nrm2672. [DOI] [PubMed] [Google Scholar]
- 2.Jackson RJ, Hellen CU, Pestova TV. The mechanism of eukaryotic translation initiation and principles of its regulation. Nat Rev Mol Cell Biol. 2010;11:113–127. doi: 10.1038/nrm2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walsh D, Mohr I. Phosphorylation of eIF4E by Mnk-1 enhances HSV-1 translation and replication in quiescent cells. Genes Dev. 2004;18:660–672. doi: 10.1101/gad.1185304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elia A, Constantinou C, Clemens MJ. Effects of protein phosphorylation on ubiquitination and stability of the translational inhibitor protein 4E-BP1. Oncogene. 2008;27:811–822. doi: 10.1038/sj.onc.1210678. [DOI] [PubMed] [Google Scholar]
- 5.Braunstein S, Badura ML, Xi Q, Formenti SC, Schneider RJ. Regulation of protein synthesis by ionizing radiation. Mol Cell Biol. 2009;29:5645–5656. doi: 10.1128/MCB.00711-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richter JD, Sonenberg N. Regulation of cap-dependent translation by eIF4E inhibitory proteins. Nature. 2005;433:477–480. doi: 10.1038/nature03205. [DOI] [PubMed] [Google Scholar]
- 7.Zoncu R, Efeyan A, Sabatini DM. mTOR: From growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nascimento EB, Ouwens DM. PRAS40: Target or modulator of mTORC1 signalling and insulin action? Arch Physiol Biochem. 2009;115:163–175. doi: 10.1080/13813450902988580. [DOI] [PubMed] [Google Scholar]
- 9.Sengupta S, Peterson TR, Sabatini DM. Regulation of the mTOR complex 1 pathway by nutrients, growth factors and stress. Mol Cell. 2010;40:310–322. doi: 10.1016/j.molcel.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruvinsky I, Meyuhas O. Ribosomal protein S6 phosphorylation: from protein synthesis to cell size. Trends Biochem Sci. 2006;31:342–348. doi: 10.1016/j.tibs.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Demidenko ZN, Zubova SG, Bukreeva EI, Pospelov VA, Pospelova TV, Blagosklonny MV. Rapamycin decelerates cellular senescence. Cell Cycle. 2009;8:1888–1895. doi: 10.4161/cc.8.12.8606. [DOI] [PubMed] [Google Scholar]
- 12.Walsh D, Mohr I. Assembly of an active translation initiation factor complex by a viral protein. Genes Dev. 2006;20:461–472. doi: 10.1101/gad.1375006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kudchodkar SB, Yu Y, Maguire TG, Alwine JC. Human cytomegalovirus infection induces rapamycin-insensitive phosphorylation of downstream effectors of mTOR kinase. J Virol. 2004;78:11030–11039. doi: 10.1128/JVI.78.20.11030-11039.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walsh D, Perez C, Notary J, Mohr I. Regulation of the translation initiation factor eIF4F by multiple mechanisms in human cytomegalovirus-infected cells. J Virol. 2005;79:8057–8064. doi: 10.1128/JVI.79.13.8057-8064.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arias C, Walsh D, Harbell J, Wilson AC, Mohr I. Activation of host translational control pathways by a viral developmental switch. PLoS Pathog. 2009;5:1000334. doi: 10.1371/journal.ppat.1000334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moorman NJ, Shenk T. Rapamycin-resistant mTORC1 kinase activity is required for herpesvirus replication. J Virol. 2010;84:5260–5269. doi: 10.1128/JVI.02733-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chuluunbaatar U, Roller R, Feldman ME, Brown S, Shokat KM, Mohr I. Constitutive mTORC1 activation by a herpesvirus Akt surrogate stimulates mRNA translation and viral replication. Genes Dev. 2010;24:2627–2639. doi: 10.1101/gad.1978310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perez C, McKinney C, Chulunbaatar U, Mohr I. Translational control of the abundance of cytoplasmic poly(A) binding protein in human cytomegalo-virus-infected cells. J Virol. 2011;85:156–164. doi: 10.1128/JVI.01778-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hale BG, Jackson D, Chen YH, Lamb RA, Randall RE. Influenza A virus NS1 protein binds p85beta and activates phosphatidylinositol-3-kinase signaling. Proc Natl Acad Sci USA. 2006;103:14194–14199. doi: 10.1073/pnas.0606109103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ehrhardt C, Wolff T, Pleschka S, Planz O, Beermann W, Bode JG, et al. Influenza A virus NS1 protein activates the PI3K/Akt pathway to mediate antiapoptotic signaling responses. J Virol. 2007;81:3058–3067. doi: 10.1128/JVI.02082-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wagner MJ, Smiley JR. Herpes simplex virus requires VP11/12 to activate Src family kinase-phosphoinositide-3-Kinase-Akt signaling. J Virol. 2011;85:2803–2812. doi: 10.1128/JVI.01877-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buchkovich NJ, Yu Y, Zampieri CA, Alwine JC. The TORrid affairs of viruses: Effects of mammalian DNA viruses on the PI3K-Akt-mTOR signaling pathway. Nat Rev Microbiol. 2008;6:266–275. doi: 10.1038/nrmicro1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harrington LS, Findlay GM, Lamb RF. Restraining PI3K: mTOR signaling goes back to the membrane. Trends Biochem Sci. 2005;30:35–42. doi: 10.1016/j.tibs.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 24.Benetti L, Roizman B. Protein kinase B/Akt is present in activated form throughout the entire replicative cycle of deltaU(S)3 mutant virus but only at early times after infection with wild-type herpes simplex virus 1. J Virol. 2006;80:3341–3348. doi: 10.1128/JVI.80.7.3341-3348.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dibble CC, Asara JM, Manning BD. Characterization of Rictor phosphorylation sites reveals direct regulation of mTOR complex 2 by S6K1. Mol Cell Biol. 2009;29:5657–5670. doi: 10.1128/MCB.00735-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moorman NJ, Cristea IM, Terhune SS, Rout MP, Chait BT, Shenk T. Human cytomegalovirus protein UL38 inhibits host cell stress responses by antagonizing the tuberous sclerosis protein complex. Cell Host Microbe. 2008;3:253–262. doi: 10.1016/j.chom.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu Z, Hu X, Li Y, Zheng L, Zhou Y, Jiang H, et al. Human papillomavirus 16 E6 oncoprotein interferences with insulin signaling pathway by binding to tuberin. J Biol Chem. 2004;279:35664–35670. doi: 10.1074/jbc.M403385200. [DOI] [PubMed] [Google Scholar]
- 28.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 29.Budanov AV, Karin M. p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell. 2008;134:451–460. doi: 10.1016/j.cell.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inoki K, Ouyang H, Zhu T, Lindvall C, Wang Y, Zhang X, et al. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell. 2006;126:955–968. doi: 10.1016/j.cell.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 31.DeYoung MP, Horak P, Sofer A, Sgroi D, Ellisen LW. Hypoxia regulates TSC1/2-mTOR signaling and tumor suppression through REDD1-mediated 14-3-3 shuttling. Genes Dev. 2008;22:239–251. doi: 10.1101/gad.1617608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sehgal SN. Sirolimus: Its discovery, biological properties and mechanism of action. Transplant Proc. 2003;35:7–14. doi: 10.1016/s0041-1345(03)00211-2. [DOI] [PubMed] [Google Scholar]
- 33.Munger J, Roizman B. The US3 protein kinase of herpes simplex virus 1 mediates the posttranslational modification of BAD and prevents BAD-induced programmed cell death in the absence of other viral proteins. Proc Natl Acad Sci USA. 2001;98:10410–10415. doi: 10.1073/pnas.181344498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kato A, Yamamoto M, Ohno T, Kodaira H, Nishiyama Y, Kawaguchi Y. Identification of proteins phosphorylated directly by the Us3 protein kinase encoded by herpes simplex virus 1. J Virol. 2005;79:9325–9331. doi: 10.1128/JVI.79.14.9325-9331.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mou F, Forest T, Baines JD. US3 of herpes simplex virus type 1 encodes a promiscuous protein kinase that phosphorylates and alters localization of lamin A/C in infected cells. J Virol. 2007;81:6459–6470. doi: 10.1128/JVI.00380-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leach N, Bjerke SL, Christensen DK, Bouchard JM, Mou F, Park R, et al. Emerin is hyperphosphorylated and redistributed in herpes simplex virus type 1-infected cells in a manner dependent on both UL34 and US3. J Virol. 2007;81:10792–10803. doi: 10.1128/JVI.00196-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meignier B, Longnecker R, Mavromara-Nazos P, Sears AE, Roizman B. Virulence of and establishment of latency by genetically engineered deletion mutants of herpes simplex virus 1. Virology. 1988;162:251–254. doi: 10.1016/0042-6822(88)90417-5. [DOI] [PubMed] [Google Scholar]
- 38.Nishiyama Y, Yamada Y, Kurachi R, Daikoku T. Construction of a US3 lacZ insertion mutant of herpes simplex virus type 2 and characterization of its phenotype in vitro and in vivo. Virology. 1992;190:256–268. doi: 10.1016/0042-6822(92)91212-d. [DOI] [PubMed] [Google Scholar]
- 39.Ryckman BJ, Roller RJ. Herpes simplex virus type 1 primary envelopment: UL34 protein modification and the US3-UL34 catalytic relationship. J Virol. 2004;78:399–412. doi: 10.1128/JVI.78.1.399-412.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Camarena V, Kobayashi M, Kim JY, Roehm P, Perez R, Gardner J, et al. Nature and duration of growth factor signaling through receptor tyrosine kinases regulates HSV-1 latency in neurons. Cell Host Microbe. 2010;8:320–330. doi: 10.1016/j.chom.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leader DP, Deana AD, Marchiori F, Purves FC, Pinna LA. Further definition of the substrate specificity of the alpha-herpesvirus protein kinase and comparison with protein kinases A and C. Biochim Biophys Acta. 1991;1091:426–431. doi: 10.1016/0167-4889(91)90210-o. [DOI] [PubMed] [Google Scholar]
- 42.Biondi RM, Nebreda AR. Signaling specificity of Ser/Thr protein kinases through docking-site-mediated interactions. Biochem J. 2003;372:1–13. doi: 10.1042/BJ20021641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pawson T, Nash P. Protein-protein interactions define specificity in signal transduction. Genes Dev. 2000;14:1027–1047. [PubMed] [Google Scholar]
- 44.Reményi A, Good MC, Lim WA. Docking interactions in protein kinase and phosphatase networks. Curr Opin Struct Biol. 2006;16:676–685. doi: 10.1016/j.sbi.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 45.Söderberg BO, Sjöberg BM, Sonnerstam U, Brändén CI. Three-dimensional structure of thioredoxin induced by bacteriophage T4. Proc Natl Acad Sci USA. 1978;75:5827–5830. doi: 10.1073/pnas.75.12.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matthews BW, Grutter MG, Anderson WF, Remington SJ. Common precursor of lysozymes of hen egg-white and bacteriophage T4. Nature. 1981;290:334–335. doi: 10.1038/290334a0. [DOI] [PubMed] [Google Scholar]
- 47.Lesk A, Chothia C. How different amino acid sequences determine similar protein structures: The structure and evolutionary dynamics of the globins. J Mol Biol. 1980;136:225–270. doi: 10.1016/0022-2836(80)90373-3. [DOI] [PubMed] [Google Scholar]