Abstract
Unlike organs with defined stem cell compartments, such as the intestine, the pancreas has limited capacity to regenerate. The question of whether the adult pancreas harbors facultative stem/progenitor cells has been a prime subject of debate. Cumulative evidence from recent genetic lineage tracing studies, in which specific cell populations were marked and traced in adult mice, suggests that endocrine and acinar cells are no longer generated from progenitors in the adult pancreas. These studies further indicate that adult pancreatic ductal cells are not a source for endocrine cells following pancreatic injury, as previously suggested. Our own studies have shown that adult ductal cells reinitiate expression of some endocrine progenitor markers, including Ngn3, after injury by partial duct ligation (PDL), but that these cells do not undergo endocrine cell differentiation. Here, we present additional evidence that endocrine cells do not arise from ducts following β-cell ablation by streptozotocin or by a diphtheria toxin-expressing transgene or when β-cell ablation is combined with PDL. In this review, we discuss findings from recent lineage tracing studies of embryonic and adult pancreatic ductal cells. Based upon the combined evidence from these studies, we propose that multipotency is associated with a specific transcriptional signature.
Key words: Sox9, pancreas, regeneration, multipotent progenitor, neogenesis, partial duct ligation, β-cell ablation, streptozotocin, alloxan
Multipotent Cell Identity in the Embryonic Pancreas
The three major cell types of the adult pancreas, encompassing the exocrine acinar and ductal cells and the five different endocrine cell types (α-, β-, δ-, ϵ- and pancreatic polypeptide-cells), all arise from a common field of cells in the primitive gut tube of the embryo. The pancreatic anlage begins to form at around embryonic day (e) 8.5 in mice, and these earliest pancreatic epithelial cells are capable of forming cells of all pancreatic lineages.1–3 The question of whether multipotent pancreatic progenitors persist beyond these earliest stages and where such a cell population might reside within the organ has been the subject of intense investigation within the field of pancreas biology.
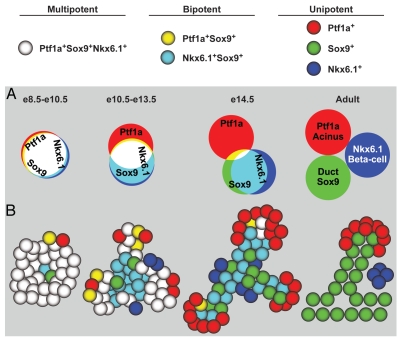
The early pancreatic progenitor epithelium proliferates and expands between e8.5 and e11.5, and the majority of cells at this stage express the transcription factors Pdx1, Sox9, Ptf1a, Hnf1b, Hes1 and Nkx6.1.4–9 At around e10.5, a subset of these progenitors also initiates expression of carboxypeptidase A1 (Cpa1) and Ngn3, two markers that are later associated with pre-acinar and pre-endocrine domains, respectively.2,10,11 Starting at around e12.5, the pancreatic epithelium is progressively compartmentalized into “tip” and “trunk” domains, a process that, upon completion, results in the lineage restriction of tip progenitors to an acinar fate and trunk progenitors to an endocrine/ductal fate.1,2,10,12 During tip-trunk separation, the expression of some transcription factors, as well as other cell markers, becomes increasingly restricted to only one of the two domains. Among the transcription factors that attain a trunk-specific pattern of expression are Hnf1b, Sox9 and Nkx6.1, while Ptf1a and its target gene Cpa1 become restricted to the tips.1,2,10,12 Mutual repression between Nkx6.1 and Ptf1a has been identified as a critical mechanism through which progenitors acquire distinct tip or trunk identities and are allocated to an endocrine/ductal or acinar cell fate, respectively.2 Lineage tracing experiments demonstrating contribution of Cpa1+ tip cells to all three pancreatic lineages at e12.5 have led to the prevailing view that the tips constitute the primary reservoir for multipotent progenitors in the developing pancreas.10,13,14 However, subsequent lineage analysis of cells expressing the trunk marker Hnf1b have challenged this idea, as Hnf1b+ progenitors were shown to be equally capable of giving rise to all pancreatic cell types at e12.5.12 Similar studies focusing upon the trunk markers Sox9, mucin-1 (Muc1) or Hes1 further demonstrated that these domains are also multipotent at this time.1,15,16 However, it is important to consider that lineage tracing experiments assess the developmental potential of an entire cell population and not of individual cells. It is therefore possible that only a subset of Hnf1b+ trunk or Cpa1+ tip cells retain multipotency at e12.5, and that some Hnf1b+ or Cpa1+ cells have already committed to an endocrine/ductal or acinar cell fate, respectively. This notion is consistent with the observation that Cpa1+ cells no longer generate endocrine or ductal cells after e13.5, whereas Hnf1b+ cells lose the ability to give rise to acinar cells.10,12 On the basis of their co-expression in the trunk and exclusion from the tips, the domains for Hnf1b and Sox9 were predicted to contain cells with similar lineage potential.12 Surprisingly, we found that the Sox9+ domain continued to contribute to acinar, ductal and endocrine cell compartments between e13.5 and birth,1 although acinar cells arose at a much lower frequency than prior to e13.5. Careful examination of Hnf1b and Sox9 expression domains at e14.5 revealed a small population of cells at the interface of tip and trunk co-expressing Pdx1, Sox9, Ptf1a and Nkx6.1, but not Hnf1b.1 The transcriptional signature of this population is similar to the one found in multipotent progenitors of the early pancreatic anlage, suggesting that this domain may constitute a residual reservoir of still-multipotent pancreatic progenitors at later developmental stages (Fig. 1A and B). One possible model explaining the results of all lineage tracing experiments is that multipotency requires the concomitant expression of several transcription factors, and that this pool of cells becomes progressively depleted as development proceeds (Fig. 1A). Based on their co-expression in early multipotent pancreatic progenitors and their subsequent segregation into distinct cell compartments, Pdx1, Sox9, Ptf1a and Nkx6.1 are possible candidates (Fig. 1A). However, until more refined lineage tracing and/or cell isolation tools allow for direct fate mapping of a select cell population, it also remains possible that multipotent progenitors no longer exist beyond e13.5, and that the Sox9+ domain contains distinct pre-endocrine/ductal and pre-acinar progenitor cell populations. Future experiments should address whether cells at the border of tip and trunk are particularly plastic and able to easily activate differentiation programs specific to the different cell compartments. In this light, it will be important to determine whether cells with similar characteristics can also be detected in the adult pancreas. The location of Ptf1a, Nkx6.1 and Sox9 copositive cells at the boundary of the pre-acinar and pre-ductal compartments in the embryo raises the intriguing possibility that this domain harbors the precursors for centroacinar cells, a cell type that connects acini with the intercalated ducts and that is thought to possess facultative progenitor cell characteristics in the mature pancreas.17,18
Figure 1.
Multipotent cell identity in the embryonic pancreas. (A) The Venn diagram represents the expression domains of Ptf1a, Sox9 and Nkx6.1 from early embryonic stages until adulthood. While most early pancreatic epithelial cells coexpress all three factors (white color), their expression domains gradually diverge as pancreatic development progresses. In the adult pancreas, each transcription factor is restricted to a particular cell compartment: Ptf1a, acinar cells; Sox9, ductal cells; Nkx6.1, β cells. We propose that multipotency is associated with the concomitant expression of all three transcription factors and that the developmental potential of cells becomes restricted as expression domains diverge. Ptf1a, Sox9 and Nkx6.1 were chosen to illustrate the idea. However, multipotent pancreatic progenitors express numerous transcription factors, and it is currently unclear which combination of these factors is critical to maintain multipotency. (B) As the expression domains of transcription factors diverge, cells progressively lose their ability to contribute to all pancreatic lineages and become bi- or unipotent. Acinar-committed cells are confined to the tip domain, while duct- and endocrine-committed cells are localized in the trunk. It should be noted that aspects of this model need to be experimentally confirmed; as an example, the bipotent or multipotent state of cells at the border between tip and trunk at e14.5. e, embryonic day.
Loss of Multipotency in Adult Ductal Cells
Since endocrine cells originate from the bipotential primitive ductal epithelium (also called “embryonic cords”) in the trunk region of the developing pancreas, the ducts of the adult pancreas have long been considered a potential source of endocrine cells during adulthood. In the adult pancreas, Sox9 and Hnf1b are restricted to ductal and centroacinar cells, whereas Nkx6.1 and Ptf1a are expressed in β or acinar cells, respectively.4,9,12,19 Interestingly, terminal duct/centroacinar cells coexpress Sox9 and Hes1 together with Ptf1a and thus bear a distinct transcriptional signature among the cell population comprising the ductal tree.1,17 Concordant with previous suggestions that centroacinar cells are facultative progenitor cells in the adult pancreas,18,20,21 it has been shown that isolated terminal duct/centroacinar cells can generate multiple pancreatic cell types after culture in vitro or upon transplantation into embryonic pancreatic explants (further discussion below).17 The ability of centroacinar cells to function as endocrine progenitors appears, however, to be limited to ex vivo conditions, as lineage tracing of the adult Sox9+ ductal tree, which included the centroacinar cells, failed to demonstrate endocrine cell neogenesis from Sox9+ cells in vivo.1,22 Similar lineage tracing studies that employed mouse lines expressing CreER under control of Hes1, Muc1 or Hnf1b regulatory sequences further support the notion that endocrine cells do not arise from postnatal ducts in situ.12,15,16 Thus, unlike their embryonic counterparts, adult ducts do not appear to give rise to endocrine cells. In contrast to embryonic ducts, adult ducts do not express (at any appreciable level) the progenitor cell markers Nkx6.1 and Pdx1,1 which could account for their lineage restriction to the ductal cell compartment. Comprehensive examination of the transcriptional and epigenetic changes that occur in the Sox9+ population between embryogenesis and early adulthood might aid in identifying the molecular mechanisms that restrict Sox9+ cells solely to the ductal lineage.
While a recent study by Furuyama et al. similarly concluded that endocrine cells do not arise from the ductal cell population,22 the study challenged previous conclusions that acinar cells do not originate from ducts postnatally.18,22,23 Furuyama and colleagues generated a knock-in Sox9IRES-CreERT2 mouse line, and showed that Sox9+ cells can produce acinar cells in adult mice.22 However, using a bacterial artificial chromosome transgenic Sox9CreERT2 mouse line, we failed to observe a contribution from the Sox9+ population to the acinar cell lineage in the pancreas after birth.1 While we do not fully understand the reason for this discrepancy, differences in experimental design may account for the divergent findings. For example, the tamoxifen dosages used by Furuyama and colleagues were extremely high,22 and in our model, we observed that the extent of acinar cell pre-labeling was contingent upon the administered dosage of tamoxifen.1 It is possible that acinar cells transcribe Sox9, and hence CreER in Sox9IRES-CreERT2 mice, at a level that is sufficient to induce recombination above a certain tamoxifen threshold. With the tamoxifen dosages used in our study, we observed some acinar cell pre-labeling but no increase in the percentage of labeled acinar cells during the chase period.1 As it is unclear how long CreER remains active after high dosages of tamoxifen, it is possible that, rather than arising from Sox9+ ductal cells, in Furuyama's study, acinar cells were continuously labeled for an extended period of time after the tamoxifen pulse. Alternatively, the disparate findings could be a result of altered Sox9 dosage through disruption of the 3′ untranslated region in Sox9IRES-CreERT2 mice.22,24 Furthermore, tamoxifen itself might alter Sox9 expression.25 Importantly, our finding that adult ducts do not generate acinar cells is consistent with other studies that directly traced lineage-labeled ducts in the adult pancreas.12,15 Moreover, direct labeling experiments of the acinar cell compartment have also failed to provide evidence for a non-acinar cell contribution to acinar cell formation in the adult pancreas.23
The Fate of Ductal Cells after Pancreatic Injury
While cumulative evidence from many studies argues against cell neogenesis from ducts during normal aging, substantial controversy exists as to whether ducts might serve as the source of endocrine cells after pancreatic injury.12,26–28 Since the expression of a few embryonic progenitor markers is induced in ducts following some forms of pancreatic injury, the ductal compartment has long been postulated to harbor a facultative progenitor cell population.18,20,28
No endocrine cell neogenesis from ducts after partial duct ligation (PDL).
After PDL, Pdx1 and Ngn3 become detectable in the ductal epithelium.28 In conjunction with the observation that endocrine cells reside in or close to the ductal epithelium after PDL,28,29 this has led to the hypothesis that ductal cells are able to give rise to new β cells following PDL.28 Five groups, including ours, have examined the PDL model for evidence of β-cell neogenesis from lineage-labeled ductal cells.1,12,15,22,27 While four of these studies observed no endocrine cell neogenesis from ducts after PDL, findings by Inada et al. suggested endocrine neogenesis did, in fact, occur from ducts.27 One caveat of the study concerned is that the extent of endocrine cell labeling was not examined immediately after tamoxifen administration, which makes it difficult to determine whether endocrine cells indeed arose from the ductal epithelium. In our study, we found that Pdx1 and Ngn3 are induced in lineage-labeled Sox9+ ductal cells following PDL.1 However, these cells did not appear to progress further along the endocrine differentiation pathway, as evidenced by their failure to express insulin or additional endocrine differentiation factors, including Nkx6.1.1 This suggests that the effects of PDL are not sufficient to induce expression of the complete complement of transcription factors required to induce endocrine cell neogenesis from ductal cells.
While lineage tracing experiments have been extensively performed in the PDL model, other injury models have not been rigorously analyzed for a possible contribution of non-β cells to the β-cell compartment. Current knowledge about different injury models is summarized in a recent review by Desgraz et al.30 To extend our analysis of whether Sox9+ cells are able to give rise to endocrine cells in vivo, we examined three additional injury models in which β-cell regeneration has been suggested to occur.
Ductal cells do not generate endocrine cells following streptozotocin (STZ)-induced β-cell ablation.
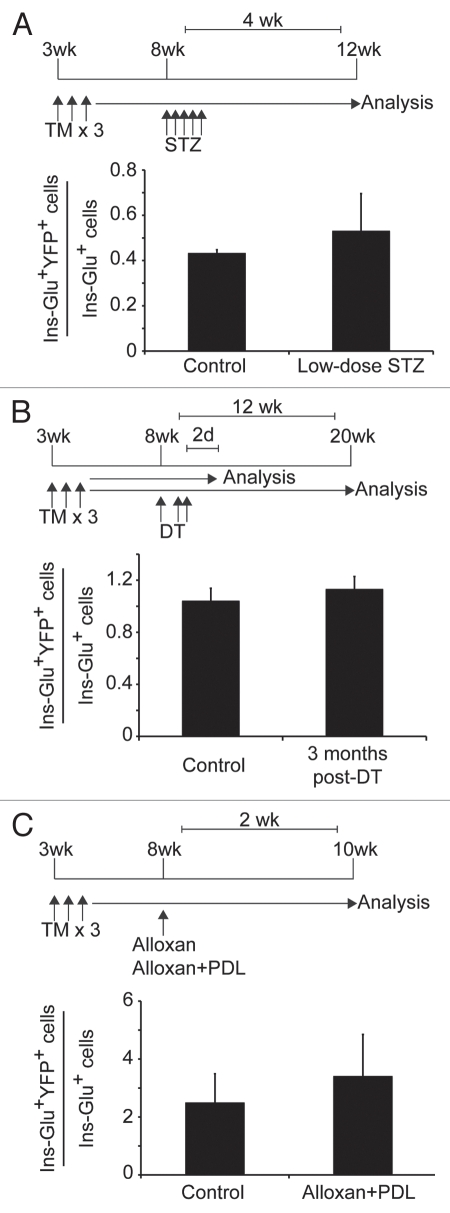
Following administration of five daily low doses of the β-cell-ablating agent STZ, the ductal marker CK-20 was found to be co-expressed with insulin,31 suggesting that β cells might arise from ducts in this model. To test this idea, we treated Sox9CreERT2; R26RYFP mice with 50 mg/ kg of STZ for five consecutive days and analyzed the percentage of lineage-labeled YFP+ endocrine cells one month later. The percentage of glucagon+ or insulin+ cells labeled by YFP was similar in STZ- and vehicle-treated control mice (Fig. 2A, n = 3), suggesting that β cells do not arise from ducts after STZ-induced β-cell ablation. Similarly, Solar and colleagues failed to observe β-cell regeneration from Hnf1b+ ductal cells following alloxan-mediated β-cell ablation and subsequent treatment with epidermal growth factor and gastrin.12
Figure 2.
Pancreatic injury does not induce β-cell neogenesis from Sox9+ ductal cells. Three-week-old Sox9CreERT2; R26RYFP mice were injected with tamoxifen (5 mg/40 g body weight) for three consecutive days. Individual groups of mice were then subjected to one of three different treatments. (A) At eight weeks of age, Sox9CreERT2; R26RYFP mice were injected for five consecutive days with 50 mg/kg of streptozotocin (STZ) or vehicle (control). One month later, the number of glucagon+/insulin+/YFP+ (Ins-Glu+YFP+) cells was quantified as a percentage of the total number of glucagon and insulin-positive cells. A minimum of 60 insulin+/glucagon+ cell clusters were scored per mouse (n = 3). (B) Sox9CreERT2; R26RYFP mice also carrying the RIP-DTR transgene were given three intraperitoneal injections of diphtheria toxin (DT) over five days (126 ng of DT per injection on days 0, 3 and 4) at eight weeks of age. Two days (control) and three months post-DT injection, the number of Ins-Glu+YFP+ cells was quantified as a percentage of the total number of glucagon and insulin-positive cells. A minimum of 1,300 insulin+/glucagon+ cells were scored per mouse (n = 3). (C) At eight weeks of age, Sox9CreERT2; R26RYFP mice were administered 90 mg/kg of alloxan intravenously. Partial duct ligation (PDL) was performed immediately thereafter. Only mice displaying blood glucose levels exceeding 500 mg/dL within two days post-alloxan administration were examined. Two weeks post-alloxan + PDL or post-alloxan (control), the number of Ins-Glu+YFP+ cells was quantified as a percentage of the total number of glucagon and insulin-positive cells. A minimum of 700 insulin+/glucagon+ cells were analyzed per mouse (n = 3), representing approximately 100 islets. None of the pancreatic injury models examined resulted in a significant increase in the percentage of insulin+/glucagon+ cells la beled by YFP. d, day; DT, diphtheria toxin; glu, glucagon; ins, insulin; STZ, streptozotocin; PDL, partial duct ligation; TM, tamoxifen; wk, week. Values are shown as mean ± standard error of the mean.
Endocrine cells do not arise from ducts following near-complete α-cell ablation.
Using lineage tracing approaches, Thorel et al. recently demonstrated that α cells transdifferentiate into β cells following near-complete β-cell ablation.32 In this model, diphtheria toxin was administered to specifically ablate over 99% of β cells in mice expressing the diphtheria toxin receptor under the control of the rat insulin promoter (RIP-DTR mice). While 65% of new β cells were found to originate from α cells,32 the origin of the remaining 35% of regenerated β cells has remained elusive. To investigate whether ductal cells give rise to new β or α cells in the RIP-DTR regeneration model, we crossed RIP-DTR mice with our Sox9CreERT2; R26RYFP line. YFP labeling of Sox9+ cells was induced by tamoxifen injection at three weeks of age, and diphtheria toxin was subsequently administered to male mice at eight weeks of age as per the protocol devised by Thorel and colleagues.32 Control mice were analyzed 48 hours following the final dose of diphtheria toxin to determine the extent of β- and α-cell labeling in the remnant islet prior to β-cell regeneration. The experimental group was analyzed after a three-month regeneration period. Although we noted a significant increase in the numbers of insulin+ cells three months after diphtheria toxin-mediated β-cell ablation (data not shown), we found no appreciable difference in the percentage of glucagon+ or insulin+ cells labeled with YFP in mice analyzed before and after the three-month regenerative period (Fig. 2B, n = 3). This suggests that Sox9+ cells do not contribute to the β-cell regeneration induced by near-total β-cell ablation.
β-cell ablation combined with PDL does not result in endocrine cell neogenesis from ducts.
While little to no β-cell regeneration is observed following alloxan-induced β-cell ablation,33,34 Chung and colleagues recently reported a recovery of β-cell mass within two weeks after alloxan administration when combined with PDL.33 Based upon an expression analysis of cell markers, Chung et al. concluded that the newly formed β cells arise by transdifferentiation of α cells, paralleling the mechanism established by Thorel and colleagues.32 However, the origin of β cells in this so-called “alloxan + PDL” model was not tested through the use of genetic lineage tracing studies. To determine whether β-cell ablation combined with PDL leads to the formation of β cells either directly through β-cell neogenesis or indirectly through α-cell neogenesis from ducts, we induced expression of YFP in Sox9CreERT2; R26RYFP mice by tamoxifen injection at three weeks of age then, five weeks later, administered 90 mg/kg of alloxan followed by PDL, as described in reference 33. Only mice exhibiting blood glucose levels exceeding 500 mg/dL within two days post-alloxan administration were examined. Two weeks following alloxan-induced β-cell ablation, we compared the numbers of YFP-labeled insulin+ or glucagon+ cells in alloxan-treated and alloxan + PDL-treated mice. While the majority of β cells were effectively ablated by alloxan injection, α cells remained (data not shown), allowing for identification of the islets. Similar to the results observed after PDL treatment alone,1 there was no significant increase in the number of YFP-labeled insulin+ or glucagon+ cells present following alloxan + PDL treatment compared to alloxan treatment alone (Fig. 2C; n = 3). These findings suggest that Sox9+ ductal cells do not generate endocrine cells in the alloxan + PDL model.
Complementing our studies, Furuyama and colleagues traced the progeny of Sox9+ cells following high-dose STZ treatment, caerulein-induced pancreatitis or 70% partial pancreatectomy. Concordant with our findings, no contribution to the endocrine compartment was observed.22 Furthermore, work conducted in the Means laboratory has demonstrated that β cells residing in or near ductal structures in the MT-TGFα mouse model arise from pre-existing islets and not from a non-β-cell source.35 Combined, our lineage analyses, as well as studies by others, strongly suggest that ductal cells in the adult mouse pancreas do not significantly contribute to endocrine cell neogenesis in vivo. While this data does not conclusively exclude the possibility that ductal cells may be capable of endocrine differentiation, these recent studies are part of a growing body of evidence suggesting that this does not occur in vivo.
The Developmental Potential of Ductal Cells Ex Vivo
The strong evidence that adult ductal cells are unipotent in vivo does not exclude the possibility that ducts have the innate capacity to generate other pancreatic cell types. Pancreatic duct-like cells isolated by cell fractionation have been shown to expand in vitro and, given the correct environment, to generate endocrine marker-expressing cells in Matrigel cultures.36 Formation of endocrine cells ex vivo has also been observed from populations isolated by fluorescence-activated cell sorting (FACS) using cell surface proteins expressed on ductal cells.37–39 Yet another study has recently utilized FACS to isolate terminal duct/centroacinar cells based on their stem cell-associated aldehyde dehydrogenase-1 (ALDH-1) activity and E-cadherin expression.17 These cells were able to form spheres in vitro, a phenomenon that has been associated with progenitor cell activity in the nervous system and other organs.40–42 ALDH-1-enriched spheres initiated expression of endocrine and acinar markers in vitro while retaining expression of the centroacinar/duct marker Sox9.17 Additionally, ALDH-1+/E-cadherin+ cells differentiated into endocrine and acinar cells upon transplantation into embryonic pancreatic explants.17 Overall, these experiments suggest that ductal cells and specifically, terminal duct/centroacinar cells, can function as multipotent pancreatic progenitors when removed from their in vivo environment. However, it is important to consider the potential caveats in the interpretation of such ex vivo studies of sorted cells. First, while FACS can enrich for specific cell populations, it is not a strategy by which ductal cells can be completely separated from other cell types in the pancreas, thus leaving the possibility that the colonies are derived from non-ductal or even non-epithelial cells of the organ. The cellular origin of the in vitro-generated colonies could be more definitively determined by sorting these cell populations from mice in which pancreatic ducts are stably labeled by a reporter gene prior to cell sorting. However, as none of the mouse lines expressing CreER under the control of duct-specific genes induce recombination exclusively in the ductal cell compartment, there are also potential limitations to these experiments. If further evidence supports the notion that ductal cells are capable of differentiating into endocrine or acinar cells in vitro, the next step will be to identify the signals that promote expansion of the colonies and their differentiation into endocrine cells. By stimulating the same signaling pathways in vivo, it may then become possible to differentiate endocrine cells from native pancreatic ducts as a regenerative approach for the treatment of diabetes.
Conclusions
Cumulative evidence from numerous lineage tracing studies suggests that the ability of the ductal epithelium to generate endocrine or acinar cells ceases around birth. Since loss of the neogenic potential of pancreatic epithelial cells is accompanied by the segregation of early pancreatic transcription factors into distinct cell compartments, we propose that the multipotent state requires concomitant expression of several of these early transcriptional regulators (Fig. 1). While the specific combination of transcription factors associated with multipotency remains to be defined, the concomitant expression of Ptf1a, Nkx6.1, Pdx1 and Sox9 in early pancreatic progenitors makes them good candidates. Similar to the approach taken in reprogramming strategies, reexpression of a combination of these factors may revert unipotent adult ductal cells to the multipotent state.
Acknowledgments
We appreciate the technical contribution of Ashleigh Schaffer and thank Philip Seymour for editing the manuscript. This work was supported by NIH/NIDDK R01-DK078803, the ARRA, JDRF-5-2007-280, JDRF-43-2009-791 to M.S. J.K. was supported by NIH/F32 CA136124 and C.D. by CIRM T1-00008. P.L.H. is supported by the NIH/NIDDK, the JDRF, the EU and the Swiss National Science Foundation.
Abbreviations
- ALDH-1
aldehyde dehydrogenase-1
- Cpa1
carboxypeptidase A1
- CreER
cre-recombinase-estrogen receptor hormone binding domain fusion protein
- DT
diphtheria toxin
- e
embryonic day
- FACS
fluorescence-activated cell sorting
- PDL
partial duct ligation
- RIP-DTR
rat insulin promoter-driven diphtheria toxin receptor
- STZ
streptozotocin
- YFP
yellow fluorescent protein
References
- 1.Kopp JL, Dubois CL, Schaffer AE, Hao E, Shih HP, Seymour PA, et al. Sox9+ ductal cells are multipotent progenitors throughout development but do not produce new endocrine cells in the normal or injured adult pancreas. Development. 2011;138:653–665. doi: 10.1242/dev.056499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schaffer AE, Freude KK, Nelson SB, Sander M. Nkx6 transcription factors and Ptf1a function as antagonistic lineage determinants in multipotent pancreatic progenitors. Dev Cell. 2010;18:1022–1029. doi: 10.1016/j.devcel.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. 2002;129:2447–2457. doi: 10.1242/dev.129.10.2447. [DOI] [PubMed] [Google Scholar]
- 4.Seymour PA, Freude KK, Tran MN, Mayes EE, Jensen J, Kist R, et al. SOX9 is required for maintenance of the pancreatic progenitor cell pool. Proc Natl Acad Sci USA. 2007;104:1865–1870. doi: 10.1073/pnas.0609217104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hald J, Sprinkel AE, Ray M, Serup P, Wright C, Madsen OD. Generation and characterization of Ptf1a antiserum and localization of Ptf1a in relation to Nkx6.1 and Pdx1 during the earliest stages of mouse pancreas development. J Histochem Cytochem. 2008;56:587–595. doi: 10.1369/jhc.2008.950675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jonsson J, Carlsson L, Edlund T, Edlund H. Insulin-promoter-factor 1 is required for pancreas development in mice. Nature. 1994;371:606–609. doi: 10.1038/371606a0. [DOI] [PubMed] [Google Scholar]
- 7.Haumaitre C, Barbacci E, Jenny M, Ott MO, Gradwohl G, Cereghini S. Lack of TCF2/vHNF1 in mice leads to pancreas agenesis. Proc Natl Acad Sci USA. 2005;102:1490–1495. doi: 10.1073/pnas.0405776102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Apelqvist A, Li H, Sommer L, Beatus P, Anderson DJ, Honjo T, et al. Notch signalling controls pancreatic cell differentiation. Nature. 1999;400:877–881. doi: 10.1038/23716. [DOI] [PubMed] [Google Scholar]
- 9.Sander M, Sussel L, Conners J, Scheel D, Kalamaras J, Dela Cruz F, et al. Homeobox gene Nkx6.1 lies downstream of Nkx2.2 in the major pathway of beta-cell formation in the pancreas. Development. 2000;127:5533–5540. doi: 10.1242/dev.127.24.5533. [DOI] [PubMed] [Google Scholar]
- 10.Zhou Q, Law AC, Rajagopal J, Anderson WJ, Gray PA, Melton DA. A multipotent progenitor domain guides pancreatic organogenesis. Dev Cell. 2007;13:103–114. doi: 10.1016/j.devcel.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Jørgensen MC, Ahnfelt-Rønne J, Hald J, Madsen OD, Serup P, Hecksher-Sørensen J. An illustrated review of early pancreas development in the mouse. Endocr Rev. 2007;28:685–705. doi: 10.1210/er.2007-0016. [DOI] [PubMed] [Google Scholar]
- 12.Solar M, Cardalda C, Houbracken I, Martín M, Maestro MA, De Medts N, et al. Pancreatic exocrine duct cells give rise to insulin-producing beta cells during embryogenesis but not after birth. Dev Cell. 2009;17:849–860. doi: 10.1016/j.devcel.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Collombat P, Xu X, Heimberg H, Mansouri A. Pancreatic beta-cells: From generation to regeneration. Semin Cell Dev Biol. 2010:1–7. doi: 10.1016/j.semcdb.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pan FC, Wright C. Pancreas organogenesis: From bud to plexus to gland. Dev Dyn. 2011;240:530–565. doi: 10.1002/dvdy.22584. [DOI] [PubMed] [Google Scholar]
- 15.Kopinke D, Brailsford M, Shea JE, Leavitt R, Scaife CL, Murtaugh LC. Lineage tracing reveals the dynamic contribution of Hes1+ cells to the developing and adult pancreas. Development. 2011;138:431–441. doi: 10.1242/dev.053843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kopinke D, Murtaugh LC. Exocrine-to-endocrine differentiation is detectable only prior to birth in the uninjured mouse pancreas. BMC Dev Biol. 2010;10:38. doi: 10.1186/1471-213X-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rovira M, Scott SG, Liss AS, Jensen J, Thayer SP, Leach SD. Isolation and characterization of centroacinar/terminal ductal progenitor cells in adult mouse pancreas. Proc Natl Acad Sci USA. 2010;107:75–80. doi: 10.1073/pnas.0912589107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jensen JN, Cameron E, Garay MVR, Starkey TW, Gianani R, Jensen J. Recapitulation of elements of embryonic development in adult mouse pancreatic regeneration. Gastroenterology. 2005;128:728–741. doi: 10.1053/j.gastro.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 19.Krapp A, Knofler M, Frutiger S, Hughes GJ, Hagenbuchle O, Wellauer PK. The p48 DNA-binding subunit of transcription factor PTF1 is a new exocrine pancreas-specific basic helix-loop-helix protein. EMBO J. 1996;15:4317–4329. [PMC free article] [PubMed] [Google Scholar]
- 20.Sharma A, Zangen DH, Reitz P, Taneja M, Lissauer ME, Miller CP, et al. The homeodomain protein IDX-1 increases after an early burst of proliferation during pancreatic regeneration. Diabetes. 1999;48:507–513. doi: 10.2337/diabetes.48.3.507. [DOI] [PubMed] [Google Scholar]
- 21.Nagasao J, Yoshioka K, Amasaki H, Mutoh K. Centroacinar and intercalated duct cells as potential precursors of pancreatic endocrine cells in rats treated with streptozotocin. Ann Anat. 2003;185:211–216. doi: 10.1016/s0940-9602(03)80025-0. [DOI] [PubMed] [Google Scholar]
- 22.Furuyama K, Kawaguchi Y, Akiyama H, Horiguchi M, Kodama S, Kuhara T, et al. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat Genet. 2010;43:34–41. doi: 10.1038/ng.722. [DOI] [PubMed] [Google Scholar]
- 23.Desai BM, Oliver-Krasinski J, De Leon DD, Farzad C, Hong N, Leach SD, et al. Preexisting pancreatic acinar cells contribute to acinar cell, but not islet beta cell, regeneration. J Clin Invest. 2007;117:971–977. doi: 10.1172/JCI29988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trumpp A, Refaeli Y, Oskarsson T, Gasser S, Murphy M, Martin GR, et al. c-Myc regulates mammalian body size by controlling cell number but not cell size. Nature. 2001;414:768–773. doi: 10.1038/414768a. [DOI] [PubMed] [Google Scholar]
- 25.Kasahara T, Hashiba M, Harada T, Degawa M. Change in the gene expression of hepatic tamoxifen-metabolizing enzymes during the process of tamoxifen-induced hepatocarcinogenesis in female rats. Carcinogenesis. 2002;23:491–498. doi: 10.1093/carcin/23.3.491. [DOI] [PubMed] [Google Scholar]
- 26.Kushner JA, Weir GC, Bonner-Weir S. Ductal Origin Hypothesis of Pancreatic Regeneration under Attack. Cell Metab. 2010;11:2–3. doi: 10.1016/j.cmet.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inada A, Nienaber C, Katsuta H, Fujitani Y, Levine J, Morita R, et al. Carbonic anhydrase II-positive pancreatic cells are progenitors for both endocrine and exocrine pancreas after birth. Proc Natl Acad Sci USA. 2008;105:19915–19919. doi: 10.1073/pnas.0805803105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu X, D'Hoker J, Stangé G, Bonné S, De Leu N, Xiao X, et al. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008;132:197–207. doi: 10.1016/j.cell.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 29.Wang RN, Kloppel G, Bouwens L. Duct- to islet-cell differentiation and islet growth in the pancreas of duct-ligated adult rats. Diabetologia. 1995;38:1405–1411. doi: 10.1007/BF00400600. [DOI] [PubMed] [Google Scholar]
- 30.Desgraz R, Bonal C, Herrera PL. β-Cell regeneration: the pancreatic intrinsic faculty. Trends Endocrinol Metab. 2011;22:34–43. doi: 10.1016/j.tem.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 31.Anastasi E, Ponte E, Gradini R, Bulotta A, Sale P, Tiberti C, et al. Expression of Reg and cytokeratin 20 during ductal cell differentiation and proliferation in a mouse model of autoimmune diabetes. Eur J Endocrinol. 1999;141:644–652. doi: 10.1530/eje.0.1410644. [DOI] [PubMed] [Google Scholar]
- 32.Thorel F, Népote V, Avril I, Kohno K, Desgraz R, Chera S, et al. Conversion of adult pancreatic α-cells to β-cells after extreme β-cell loss. Nature. 2010:1–6. doi: 10.1038/nature08894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chung CH, Hao E, Piran R, Keinan E, Levine F. Pancreatic beta-Cell Neogenesis by direct conversion from mature alpha-cells. Stem Cells. 2010;28:1630–1638. doi: 10.1002/stem.482. [DOI] [PubMed] [Google Scholar]
- 34.Rooman I, Bouwens L. Combined gastrin and epidermal growth factor treatment induces islet regeneration and restores normoglycaemia in C57Bl6/J mice treated with alloxan. Diabetologia. 2004;47:259–265. doi: 10.1007/s00125-003-1287-1. [DOI] [PubMed] [Google Scholar]
- 35.Blaine SA, Ray KC, Anunobi R, Gannon MA, Washington MK, Means AL. Adult pancreatic acinar cells give rise to ducts but not endocrine cells in response to growth factor signaling. Development. 2010;137:2289–2296. doi: 10.1242/dev.048421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bonner-Weir S, Taneja M, Weir GC, Tatarkiewicz K, Song KH, Sharma A, et al. In vitro cultivation of human islets from expanded ductal tissue. Proc Natl Acad Sci USA. 2000;97:7999–8004. doi: 10.1073/pnas.97.14.7999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang CY, Gou SM, Liu T, Wu HS, Xiong JX, Zhou F, et al. Differentiation of CD24-pancreatic ductal cell-derived cells into insulin-secreting cells. Dev Growth Differ. 2008;50:633–643. doi: 10.1111/j.1440-169X.2008.01061.x. [DOI] [PubMed] [Google Scholar]
- 38.Oshima Y, Suzuki A, Kawashimo K, Ishikawa M, Ohkohchi N, Taniguchi H. Isolation of mouse pancreatic ductal progenitor cells expressing CD133 and c-Met by flow cytometric cell sorting. Gastroenterology. 2007;132:720–732. doi: 10.1053/j.gastro.2006.11.027. [DOI] [PubMed] [Google Scholar]
- 39.Yatoh S, Dodge R, Akashi T, Omer A, Sharma A, Weir GC, et al. Differentiation of affinity-purified human pancreatic duct cells to beta-cells. Diabetes. 2007;56:1802–1809. doi: 10.2337/db06-1670. [DOI] [PubMed] [Google Scholar]
- 40.Lawson DA, Xin L, Lukacs RU, Cheng D, Witte ON. Isolation and functional characterization of murine prostate stem cells. Proc Natl Acad Sci USA. 2007;104:181–186. doi: 10.1073/pnas.0609684104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 42.Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, et al. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17:1253–1270. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]