Abstract
Increased blood-brain barrier (BBB) Na-K-Cl cotransporter activity appears to contribute to cerebral edema formation during ischemic stroke. We have shown previously that inhibition of BBB Na-K-Cl cotransporter activity reduces edema and infarct in the rat middle cerebral artery occlusion (MCAO) model of ischemic stroke. We have also shown that the BBB cotransporter is stimulated by the ischemic factors hypoxia, aglycemia, and arginine vasopressin (AVP), although the mechanisms responsible are not well understood. AMP-activated protein kinase (AMPK), a key mediator of cell responses to stress, can be activated by a variety of stresses, including ischemia, hypoxia, and aglycemia. Previous studies have shown that the AMPK inhibitor Compound C significantly reduces infarct in mouse MCAO. The present study was conducted to evaluate the possibility that AMPK participates in ischemic factor-induced stimulation of the BBB Na-K-Cl cotransporter. Cerebral microvascular endothelial cells (CMEC) were assessed for Na-K-Cl cotransporter activity as bumetanide-sensitive 86Rb influx. AMPK activity was assessed by Western blot analysis and immunofluorescence methods using antibodies that detect total versus phosphorylated (activated) AMPK. We found that hypoxia (7% and 2% O2), aglycemia, AVP, and oxygen-glucose deprivation (5- to 120-min exposures) increase activation of AMPK. We also found that Compound C inhibition of AMPK reduces hypoxia-, aglycemia-, and AVP-induced stimulation of CMEC Na-K-Cl cotransporter activity. Confocal immunofluorescence of perfusion-fixed rat brain slices revealed the presence of AMPK, both total and phosphorylated kinase, in BBB in situ of both control and ischemic brain. These findings suggest that ischemic factor stimulation of the BBB Na-K-Cl cotransporter involves activation of AMPK.
Keywords: blood-brain barrier, stroke, cerebral edema, bumetanide
brain edema that occurs with ischemic stroke is a major cause of morbidity and mortality (5, 29). During the early hours of ischemia, brain edema forms by processes involving net influx of Na and water into the brain across an intact blood-brain barrier (BBB) (1, 37, 45). At the same time, perivascular astrocytes swell as they take up ions and water transported across the BBB into the brain (3, 24, 28). Breakdown of the barrier, with increased paracellular flux of solute and water into the brain occurs later, generally 4–6 h after the onset of ischemia (17, 21, 29, 36, 45). Previous studies have shown that luminal BBB Na transporters appear to be rate limiting in ischemia-induced brain edema formation (2, 36, 37, 45), presenting a possible therapeutic target for reduction of edema during stroke. We and others have hypothesized that ischemia-induced stimulation of one or more luminal BBB membrane Na transporters brings about increased secretion of Na from blood into the brain by coupling to the abluminal BBB membrane Na-K-ATPase (4, 10, 12, 26, 40, 43, 45). Previous studies in our laboratory have shown that Na-K-Cl cotransport and Na/H exchange reside predominantly in the luminal BBB membrane (31, 43) and that both cotransporter and exchanger are stimulated by ischemic factors, including hypoxia, aglycemia, and arginine vasopressin (AVP) (4, 12, 31, 40). We have also shown that inhibition of the BBB Na-K-Cl cotransporter and Na/H exchanger by intravenous bumetanide or HOE642, respectively, reduces edema, brain Na uptake, and infarct in the rat permanent middle cerebral artery occlusion (MCAO) model of stroke (7, 8, 43). Whereas administering inhibitors of the cotransporter and exchanger after the onset of ischemic stroke appears to be a promising therapeutic avenue, identifying the signaling pathways by which these Na transporters are stimulated during ischemia could lead to therapies aimed at preventing ischemia-induced activation of the transporters in patients at risk for stroke.
AMP-activated protein kinase (AMPK) has been shown to be one of the key mediators of cell responses to stress. The kinase is a heterotrimer containing an α catalytic subunit along with β and γ regulatory subunits (20, 22, 27). It is activated by phosphorylation of the α subunit by one or more upstream kinases (51). AMPK can be switched on by a variety of stresses, including hypoxia, glucose deprivation, and ischemia (6, 19, 20). The kinase is exquisitely sensitive to elevation of the AMP-to-ATP (AMP/ATP) ratio, becoming activated even when ATP levels have not detectably fallen (6, 20). AMPK can also be activated by hypoxia independently of changes in AMP/ATP ratio (14, 32). Activated AMPK is best known for regulating metabolic processes to maintain ATP levels during stress (6, 20, 27). However, it has also been shown to regulate activity of transport proteins, including GLUT1 and GLUT4 glucose transporters (6, 19, 20), the CFTR Cl channel (18), the KCa3.1 channel (30), and the renal-specific NKCC2 isoform of Na-K-Cl cotransport (13). With respect to ischemic stress, previous studies have shown that AMPK activity is increased following MCAO-induced transient ischemia in rat and mouse brain (33, 35). In addition, the highly selective AMPK inhibitor Compound C has been found to reduce cerebral infarct following transient MCAO (34, 35), and infarct is also reduced in AMPK knockout mice (34). These findings suggest the possibility that ischemia stimulation of BBB Na transporter activity occurs via a mechanism involving AMPK. Previous studies have shown that there are two isoforms of the α catalytic subunit (α1 and α2) and that expression of these isoforms varies with cell type. Studies of brain AMPK have revealed that both isoforms are present in neurons and astrocytes, with more robust expression in neurons than astrocytes and α2 the predominant isoform in neurons (33, 35, 47). Endothelial cells of the peripheral vasculature have been shown to predominantly express the α1 AMPK isoform (9, 11, 53). Little is known, however, about AMPK expression in BBB endothelial cells, including which AMPKα isoforms are found in the cells.
The present study was conducted as an initial investigation of signaling pathways that mediate ischemia-induced stimulation of BBB Na transporters, focusing in particular on examining the role of AMPK in ischemic factor activation of the BBB Na-K-Cl cotransporter. Our findings reveal that AMPK activity in cultured cerebral microvascular endothelial cells (CMEC) is rapidly increased following exposure to hypoxia, aglycemia, AVP, or oxygen glucose deprivation (OGD). The results of our studies also show that Compound C inhibits ischemic factor-induced stimulation of CMEC Na-K-Cl cotransporter activity as well as ischemic factor activation of AMPK. Finally, we demonstrate the presence of activated AMPK in BBB endothelial cells in situ.
MATERIALS AND METHODS
Cerebral microvascular endothelial cell culture.
Cultured bovine CMEC were maintained in DMEM containing 5 mM d-glucose and 1 mM Na-pyruvate, supplemented with 2 mM l-glutamine, 50 μg/ml gentamicin, 1 ng/ml basic fibroblast growth factor, 5% calf serum, and 5% horse serum in a 95% air-5% CO2, atmosphere at 37°C, as described previously (12). For Western blot analysis and Na-K-Cl cotransporter activity experiments, CMEC were grown to confluence on 6- and 96-well plates, respectively, coated with 50 mg/l rat tail collagen, 5 mg/l fibronectin, and 10 mg/l bovine serum albumin. CMEC growth medium was replaced with fresh medium every other day. At 48 h before each experiment, the medium was changed to a 50:50 (vol/vol) mixture of fresh DMEM containing 5% calf serum-5% horse serum and astrocyte-conditioned medium (ACM) containing 10% fetal bovine serum (FBS). ACM was prepared from primary cultures of rat neonatal astrocytes as described previously (41, 46).
Western blot analysis of AMPK activation.
Total AMPKα (catalytic subunit) protein and phosphorylated (activated) AMPKα (p-AMPKα) were assessed by Western blot analysis, using antibodies that specifically detect AMPKα (both nonphosphorylated and phosphorylated) or only p-AMPKα. Western blot analysis was performed as described by us previously (12) with some modifications. Briefly, CMEC monolayers on 6-well plates were rinsed three to four times with ice-cold PBS plus protease inhibitors (Complete Protease Inhibitor Cocktail tablet, Roche Diagnostic) and phosphatase inhibitors (100 nM NaF and 100 mM Na pyrophosphate). Cells were then lysed with PBS containing 5 mM EDTA, 20 mM HEPES, 1% SDS plus protease inhibitors, and phosphatase inhibitors. The protein content of each lysate was determined using the bicinchoninic acid method to ensure equal loading of membrane protein onto gel lanes. Lysate samples and prestained molecular weight markers (Bio-Rad, Hercules, CA) were denatured in SDS reducing buffer containing DTT (Invitrogen NuPage, Carlsbad CA), heated to 70°C for 10 min, and then loaded onto precast 12% Tris·HCl gels (Lonza PAGEr Gold Precast; Rockland, ME). Protein was separated by electrophoresis (Bio-Rad Mini-Protean II), and resolved proteins were then transferred to nitrocellulose membranes using a Bio-Rad Trans-Blot apparatus. The blots were rinsed three times in PBS-0.1% Tween and then blocked with 7.5% bovine serum albumin (BSA) in PBS-0.1% Tween for 1 h at room temperature. Blots were then incubated overnight at 4°C in 7.5% BSA/PBS-0.1% Tween with either AMPKα (rabbit polyclonal), phospho-AMPKα1 (Ser485 rabbit monoclonal), or phospho-AMPKα1/α2 (p-AMPK pan-α, Thr 172 rabbit monoclonal) primary antibodies (Cell Signaling, Danvers, MA). Blots were again rinsed three times with PBS-0.1% Tween and then incubated in 7.5% nonfat dry milk/PBS-0.1% Tween containing secondary antibody (horseradish peroxidase-conjugated goat anti-rabbit IgG; Zymed, San Francisco, CA) for 1 h at room temperature. Blots were rinsed three times with PBS-0.1% Tween and then protein was visualized using enhanced chemiluminescence (ECL; GE Healthcare, Buckinghamshire, UK) on a Fuji Film LAS-4000 Imaging Machine (Medford, UK). MultiGauge software (Science Lab 2005, FujiFilm) was used to quantitate band density. All antibodies were used at a 1:1,000 dilution. Densitometry values for AMPK and p-AMPK kinase abundances observed in hypoxia-, aglycemia-, OGD-, and AVP-treated cells were all normalized to their respective normoxic controls, run as an internal standard on each gel. Lane positions for control and experimental lysates were randomized between experiments to avoid any possible lane bias.
Na-K-Cl cotransporter activity assay.
Na-K-Cl cotransport activity was assessed as ouabain-insensitive, bumetanide-sensitive K influx, using 86Rb as a tracer for K as described by us previously (40, 42). For these studies, CMEC monolayers on 96-well plates were placed in a hypoxia chamber (COY Laboratory Products; Grass Lake, MI) preset to 37°C with an atmosphere of 5% CO2 and 19% O2, 7% O2, or 2% O2 (normoxia, moderate hypoxia, and severe hypoxia, respectively). O2 levels in the chamber and in treatment media (after equilibration in the chamber atmosphere) were verified by the chamber O2 sensor/regulator and Corning dissolved oxygen sensor with Checkmate II meter (Corning, NY), respectively. Immediately upon entry into the chamber, growth medium of the cells was removed and the cells were rinsed once with the appropriate pretreatment medium preequilibrated to the desired O2 level and then incubated in that medium for 30 min. The pretreatment/assay media used for these assays was DMEM with 10 mM HEPES (DMEM HEPES) containing (in mM) 5.6 d-glucose, 1.0 Na-pyruvate, 156 Na+, 119 Cl−, 5.3 K+, 1.8 Ca2+, 44.1 HCO3−, 0.91 H2PO4, 0.81 Mg2+, and 0.81 SO42−. For experiments testing the effects of aglycemia, both glucose and Na pyruvate were omitted from this DMEM HEPES medium. In experiments testing OGD, cells were exposed to glucose- and pyruvate-free DMEM HEPES hypoxic (2% O2) medium. In some experiments, pretreatment media also contained the AMPK inhibitor Compound C (20 μM) or vehicle. During the last 5 min of the pretreatment period, bumetanide (10 μM) and/or ouabain (100 μM) or vehicle were added to the media. To initiate the assay for Na-K-Cl cotransporter activity, 86Rb (1 μC/ml) was then added to the pretreatment/assay medium, and the cells were incubated for an additional 5 min. To terminate the assay, wells were aspirated and rapidly washed three times with ice-cold 0.1 M MgCl2. Cells were then extracted using 1% sodium dodecyl sulfate (SDS) for 86Rb quantitation by liquid scintillation analysis (Tri-Carb 2500 TR liquid scintillation counter) and protein determination by bicinchoninic acid assay (Pierce, Rockford, IL). 86Rb uptake was calculated as the slope of an uptake versus time plot as described previously (39).
Immunofluorescence detection of BBB endothelial cell AMPK in situ.
This study was conducted in accordance with the animal use and care guidelines issued by the National Institutes of Health, and the protocol was approved by the Animal Use and Care Committee at the University of California, Davis. Normotensive male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) weighing 250–300 g were anesthetized by intraperitoneal injection of pentobarbital sodium (1 mg/kg), then either immediately perfusion fixed or first subjected to 30 min of MCAO, and then perfusion fixed. MCAO was performed as described previously (16, 43) with a few modifications. Briefly, for MCAO a nylon suture was advanced in the internal carotid artery to the origin of the middle cerebral artery. Cerebral blood flow (CBF) was continuously monitored by Laser Dopler (LD; Moor Instruments, Wilmington, DE) through a probe holder attached to the skull surface by adhesive. Successful occlusion at the start of MCAO and throughout the 30-min occlusion period was verified by a 75–80% reduction of CBF. Brains were immediately perfusion fixed after 30 min of MCAO. For this, the abdomen was opened, exposing the heart and the descending aorta and vena cava. A blunt 15-gauge × 1.5-inch needle was inserted through an incision in right atrium, advanced into the aorta, and clamped in place. Ice-cold saline (50 ml) was injected to flush out blood, followed by 4% paraformaldehyde (400 ml) by gravity feed at a rate of ∼3 ml/min. The brain was then removed and further fixed in 4% paraformaldehyde overnight at 4°C and then in 10% paraformaldehyde for 7 days. After paraffin embedding and sectioning, 5-μm brain slices were mounted on slides. For immunofluorescence studies, slides were washed with xylene three times for 5 min, then hydrated using an alcohol gradient of 100% EtOH, 95% EtOH, 70% EtOH, and 50% EtOH for 5 min each, and finally placed in PBS for 5 min. For antigen recovery the slides were heated to 70°C in 10 mM Na citrate (pH 6.0) for 25 min. After being cooled for 10 min, the slides were again placed in PBS for 5 min and then incubated for 2 h at room temperature with 10% goat serum followed by overnight incubation at 4°C with 100 μl 2% goat serum containing a 1:50 dilution of primary antibody. Antibodies used were those that recognize total or phosphorylated AMPK (rabbit AMPKα polyclonal or rabbit phospho-AMPKα1 monoclonal). Also used were primary antibodies that recognize glial fibrillary acidic protein (GFAP), an astrocyte marker (guinea pig GFAP polyclonal) and SMI71, a brain microvascular endothelial cell marker (mouse SMI71 monoclonal). GFAP and SMI71 antibodies were used at 1:1,000 dilution and were obtained from Advanced ImmunoChemical (Long Beach, CA) and Covance (Emeryville, CA), respectively. After being washed in PBS three times for 5 min each, the slides were incubated in 2% goat serum with a 1:400 dilution of secondary antibody (Alexa 488 anti-rabbit IgG or Alexa 546 anti-mouse IgG; Molecular Probes; Eugene, OR). Slides were again washed in PBS three times for 5 min each and then dehydrated through an alcohol gradient 50% EtOH, 70% EtOH, 95% EtOH, 100% EtOH for 5 min each. After being washed in xylene three times for 5 min each, a coverslip was mounted using Permount (Fisher; Pittsburgh, PA). Slides were allowed to dry for 24 h. Images were captured using an Axiovert 100M Zeiss confocal microscope and Zeiss LSM 510 software (Thornwood, NY).
Materials.
DMEM and l-glutamine were obtained from GIBCO-BRL (Grand Island, NY), and gentamicin was from AG Scientific (San Diego, CA). FBS and calf serum were purchased from Hyclone (Logan, UT), and horse serum and fibronectin were from Sigma (St. Louis, MO). Bumetanide and ouabain were obtained from INC Biomedicals (Costa Mesa, CA). 86Rb was purchased from Perkin-Elmer (Welles, MA). Compound C was purchased from Calbiochem (San Diego CA).
Statistics.
Data are presented as mean values ± SE. All experiments were performed with an n value of at least 6. Statistical analyses were done using Graph Pad Prism 4 software. Western blot densitometry data and K flux activity data for different treatment groups were analyzed by one-way repeated measure ANOVA unless stated otherwise. The criterion for statistical significance was P < 0.05.
RESULTS
Ischemic factors rapidly activate AMPK in cerebral microvascular endothelial cells.
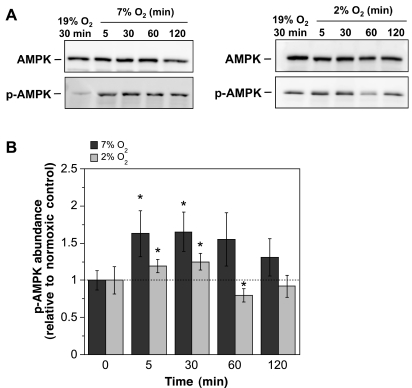
If AMPK participates in ischemic factor stimulation of BBB endothelial cell Na-K-Cl cotransporter activity during the early stages of stroke when edema is rapidly forming, we predict that one or more ischemic factors will activate AMPK with a time course similar to that of edema formation, i.e., within 30 min. To test this, we first evaluated AMPK activity in bovine CMEC following exposure to hypoxia, aglycemia, AVP, or OGD. Total AMPKα protein and p-AMPKα were assessed by Western blot analysis using antibodies that specifically detect AMPKα (both the nonphosphorylated and phosphorylated) or only p-AMPKα. In these experiments, we used antibodies that either detect both α1 and α2 isoforms of p-AMPKα (p-AMPK pan-α antibody) or specifically detect the α1 isoform, as described in materials and methods. Figure 1 shows the results of Western blot experiments generated using the p-AMPKα1 antibody. Evaluating lysates of CMEC exposed to either moderate or severe levels of hypoxia (7% O2 and 2% O2, respectively) for 5 to 120 min, we found prominent bands of ∼62 kDa for both AMPKα and p-AMPKα1. Densitometric analyses of multiple Western blots showed significant increases in p-AMPKα1 abundance following 5- and 30-min exposures to 7% O2 (1.63- and 1.65-fold increases, respectively), indicating rapid activation of CMEC AMPK by a moderate level of hypoxia (Fig. 1B). Sixty and 120-min exposures to 7% O2 showed a trend for increased p-AMPKα1 but did not reach statistical significance. Exposing CMEC to 2% O2 for 5 or 30 min also caused significant increases in CMEC p-AMPKα1 abundance, indicating that AMPK is activated by severe hypoxia as well as moderate hypoxia in the CMEC. For these cells exposed to severe hypoxia (2% O2) no increases in p-AMPKα1 abundance were observed following 60- and 120-min exposures. Total AMPKα abundance was not altered by exposure to either 7% or 2% O2 at any of the treatment times evaluated (1.00 ± 0.14, 1.20 ± 0.22, 1.19 ± 0.19, and 1.10 ± 0.19 for 5-, 30-, 60-, and 120-min exposures to 7% O2 and 0.97 ± 0.12, 1.14 ± 0.17, 0.93 ± 0.19, and 0.95 ± 0.12 for 5-, 30-, 60-, and 120-min exposures to 2% O2, means ± SE values relative to normoxic control, n = 8 and 7 experiments for 7% and 2% O2, respectively).
Fig. 1.
Hypoxia-induced activation of AMP-activated protein kinase (AMPK). Confluent cerebral microvascular endothelial cell (CMEC) monolayers were exposed to 19% O2 (normoxic control) or to 7% or 2% O2 in HEPES DMEM media for 5, 30, 60, or 120 min as described in materials and methods. Cell lysates were subjected to Western blot analysis using antibodies that recognize only phosphorylated (activated) AMPK (p-AMPK) or both p-AMPK and nonphosphorylated AMPK (i.e., total AMPK protein). A: representative Western blots. Bands shown for AMPK and p-AMPK are ∼62 kDa. B: p-AMPK abundance was quantitated as described in materials and methods. Values shown for the 5- to 120-min bars are kinase abundances for 7% or 2% O2 exposure relative to the internal normoxic control (0 min bars) for each experiment and are means ± SE of 8 and 7 separate experiments for 7% and 2% O2, respectively. The error bars shown for the 0-min bars are SE and represent between-experiment variance for the internal normoxic control p-AMPK densities. *Significantly different from normoxic control, P < 0.05 by one-tailed paired t-test.
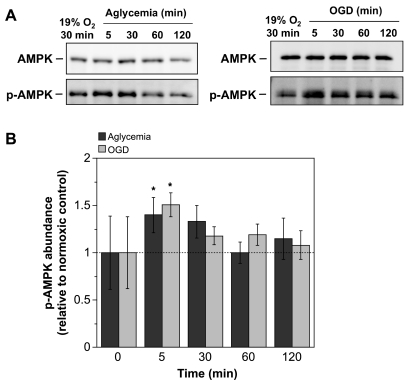
Figure 2 shows that exposing CMEC to aglycemia or OGD also caused rapid increases in p-AMPKα1 abundance while having no effect on AMPKα abundance. Here, statistically significant increases were observed following 5-min exposures to either aglycemia or OGD (1.40- and 1.51-fold increases, respectively). Trends for increased p-AMPKα1 abundance were seen with 30 min exposure to aglycemia and 30- and 60-min exposures to OGD, although these did not reach statistical significance. No increases were observed for either aglycemia or OGD following 120-min exposures. Densitometric analysis of AMPKα Western blots revealed no statistically significant changes in total AMPK (data not shown). In experiments using the p-AMPK pan-α antibody, we tested the effects of exposing CMEC to 7% O2 and found increases in p-AMPK abundance of 1.42 ± 0.05 and 1.43 ± 0.31 for 30- and 60-min exposures, respectively (means ± SE, values relative to normoxic control, n = 3 separate experiments). In two experiments we also found a 1.32-fold increase in p-AMPK abundance following 30 min exposure to aglycemia. Given that previous studies have reported pAMPKα1 to be the predominant isoform in endothelial cells from other vascular beds (9, 11, 53), and because the p-AMPK pan α antibody does not discriminate between α1 and α2 isoforms, we chose to pursue the remainder of our studies using the antibody to pAMPKα1.
Fig. 2.
Aglycemia- and oxygen-glucose deprivation (OGD)-induced activation of AMPK. CMEC monolayers were exposed to either aglycemia or to OGD (glucose- and pyruvate-free HEPES-buffered DMEM at 2% O2) or control normoxic media (glucose-containing HEPES DMEM) for 5, 30, 60, or 120 min. Cell lysates were subjected to Western blot analysis to assess AMPK and p-AMPK abundance as described in materials and methods. A: representative Western blots. B: p-AMPK abundance was quantitated as described in materials and methods. Values shown for the 5- to 120-min bars are kinase abundances for aglycemia or OGD exposure relative to the internal normoxic control with glucose and are means ± SE of 7 and 6 separate experiments for aglycemia and OGD, respectively. Error bars shown for the 0-min bars are SE and represent between-experiment variance for the internal normoxic control p-AMPK densities. *Significantly different from control, P < 0.05 by one-way repeated measure ANOVA.
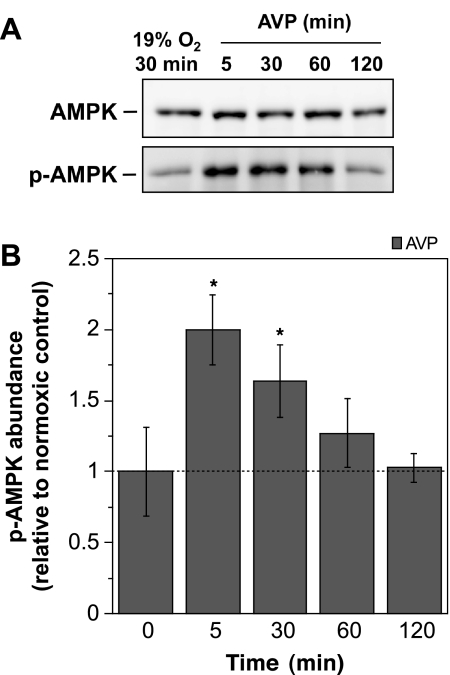
As shown in Fig. 3, we found that exposing CMEC to AVP (100 nM) for 5 or 30 min also caused statistically significant increases in p-AMPKα1 abundance (2.00- and 1.64-fold increases, respectively). As with the other ischemic factors, AVP exposures of 5 min and up to 120 min were without effect on total AMPKα, indicating that the increase in p-AMPKα1 was not simply due to an increase in total AMPKα protein in the cells.
Fig. 3.
Arginine vasporessin (AVP)-induced activation of AMPK. CMEC monolayers were exposed to either control normoxic media (glucose-containing HEPES DMEM) or to AVP (100 nM) in normoxic media for 5, 30, 60, or 120 min. AMPK and p-AMPK abundances of CMEC lysates were assessed by Western blot analysis as described in materials and methods. A: representative Western blots. B: p-AMPK abundance was quantitated as described in materials and methods. Values shown for the 5- to 120-min bars are kinase abundances for AVP exposure relative to the internal normoxic control and are means ± SE of 7 separate experiments. Error bars shown for the 0-min bars are SE and represent between-experiment variance for the internal normoxic control p-AMPK densities. *Significantly different from control, P < 0.05 by one-way repeated measure ANOVA.
Ischemic factor-induced stimulation of CMEC Na-K-Cl cotransporter activity is dependent on AMP kinase activity.
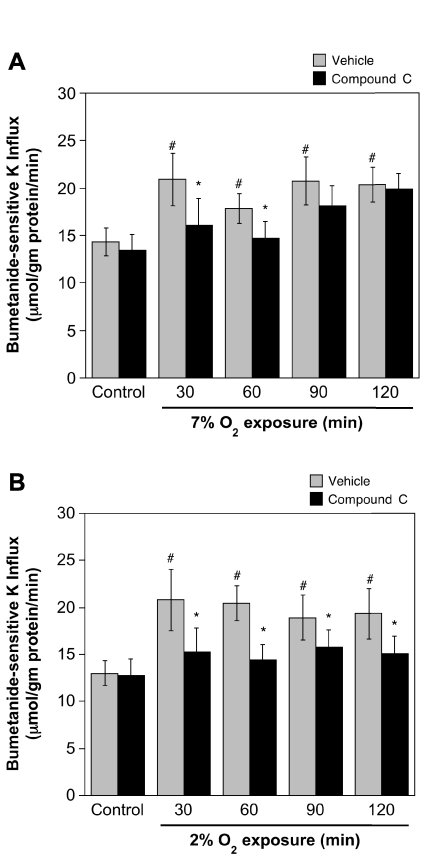
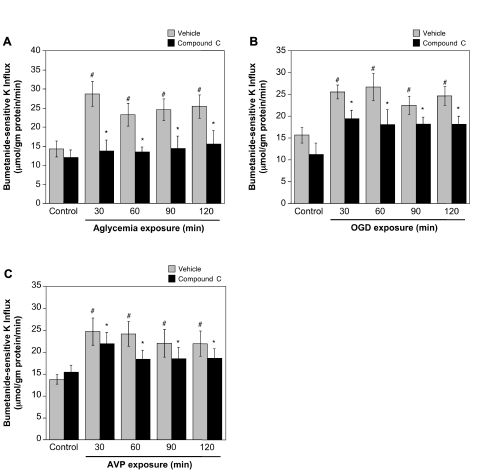
A role for AMPK in ischemia-induced stimulation of BBB Na-K-Cl cotransporter activity would predict that inhibition of AMPK should reduce or abolish ischemic factor stimulation of cotransporter activity. To test this, we evaluated the effects of the highly selective AMPK inhibitor Compound C on Na-K-Cl cotransporter activity in CMEC following exposure to hypoxia, aglycemia, OGD, or AVP. We found that Compound C (20 μM) significantly attenuated hypoxia-induced stimulation of CMEC Na-K-Cl cotransporter activity as shown in Fig. 4. For cells exposed to 2% O2 for 30 to 120 min, Compound C reduced cotransporter activity to levels not significantly different from normoxic control (Fig. 4A). This was also true for cells exposed to 7% O2 for 30 or 60 min (Fig. 4B). However, a significant reduction of cotransporter activity by Compound C was not observed for 90- or 120-min exposures to 7% O2. Figure 5 shows that Compound C also reduced or abolished CMEC Na-K-Cl cotransporter activity stimulated by aglycemia (Fig. 5A), OGD (Fig. 5B), or 100 nM AVP (Fig. 5C). For aglycemia, whether cells were exposed for 30 min or up to 120 min, the AMPK inhibitor abolished stimulation of cotransporter activity, reducing activity to the normoxic control level. Compound C significantly reduced, but did not abolish, stimulation of cotransporter activity following exposures of 30 to 120 min to either OGD or AVP. While Compound C reduced or abolished ischemic factor stimulation of CMEC Na-K-Cl cotransporter activity, the inhibitor did not alter cotransporter activity observed under control, normoxic conditions. During these experiments we evaluated the dose response for Compound C (0 to 60 μM) inhibition of aglycemia-stimulated Na-K-Cl cotransporter activity and found that maximal inhibition was obtained at 20 μM (data not shown). This is consistent with early dose response studies showing that maximal inhibition of AMPK activity by Compound C is achieved at 20 μM in cultured hepatocytes (52) and is in keeping with previous studies in a variety of cell types that have used Compound C in doses ranging from 10 to 20 μM (15, 25, 32, 38, 44).
Fig. 4.
Compound C inhibition of hypoxia-stimulated CMEC Na-K-Cl cotransporter activity. CMEC monolayers were pretreated for 30 min with Compound C (20 μM) or vehicle and then exposed for 30–120 min to normoxic control (19% O2) or hypoxia 7% O2 (A) or 2% O2 (B) in glucose-containing HEPES DMEM containing Compound C or vehicle. Na-K-Cl cotransporter activity of the cells was then assessed as ouabain-insensitive, bumetanide-sensitive K+ influx as described in materials and methods, with pretreatment conditions maintained throughout the assay. Values shown are means ± SE of 9 and 11 separate experiments for 7% and 2% O2, respectively. *Significantly different from vehicle, P < 0.05 by paired t-test. #Significantly different from normoxic control, P < 0.05 by ANOVA.
Fig. 5.
Compound C inhibition of aglycemia-, OGD-, and AVP-stimulated CMEC Na-K-Cl cotransporter activity. CMEC monolayers were pretreated for 30 min with Compound C (20 μM) or vehicle and then exposed to normoxic control (19%O2, in glucose-containing HEPES DMEM), aglycemia (A, glucose- and pyruvate-free normoxic HEPES DMEM), OGD (B, glucose- and pyruvate-free HEPES DMEM at 2%O2), or to AVP (C, 100 nM, in control normoxic glucose-containing HEPES DMEM) and Compound C or vehicle for 30–120 min. Na-K-Cl cotransporter activity was assessed as ouabain-insensitive, bumetanide-sensitive K+ influx, with pretreatment retreatment conditions were maintained throughout the assay. Values shown are means ± SE of 6, 7, and 7 separate experiments for aglycemia, OGD, and AVP, respectively. *Significantly different from vehicle, P < 0.05 by paired t-test. #Significantly different from normoxic control, P < 0.05 by ANOVA.
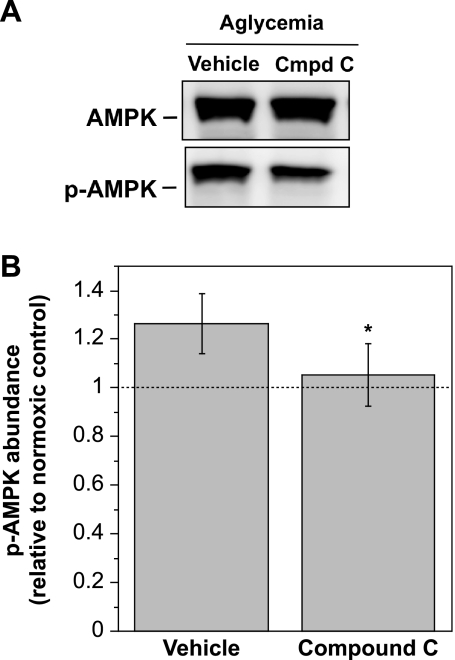
To verify that Compound C inhibits activation of AMPK in our CMEC, lysates of cells exposed to aglycemia and either vehicle or Compound C were evaluated for AMPKα and p-AMPKα1 abundance by Western blot analysis. As shown in Fig. 6, the presence of Compound C (20 μM) during a 30-min exposure to aglycemia abolished the aglycemia-induced increase in CMEC p-AMPKα1 abundance while having no effect on total AMPKα.
Fig. 6.
Compound C inhibition of aglycemia-induced AMPK activation. CMEC monolayers were pretreated for 30 min with Compound C (20 μM) or vehicle then exposed to normoxic control (19%O2, in glucose-containing HEPES DMEM) or aglycemia (glucose- and pyruvate-free normoxic HEPES DMEM) and Compound C or vehicle for 30 min. Cell lysates were subjected to Western blot analysis of AMPK and p-AMPK abundance as described in materials and methods. A: representative Western blots. B: p-AMPK abundance was quantitated as described in materials and methods. Kinase abundance values shown are relative to normoxic control and are means ± SE of 9 separate experiments. *Significantly different from vehicle P < 0.05 by paired t-test.
Detection of AMPK in BBB endothelial cells in situ.
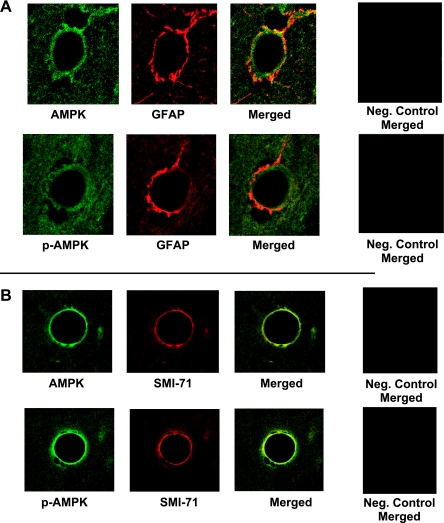
To determine whether our observations regarding AMPK involvement in ischemia-induced stimulation of CMEC Na-K-Cl cotransporter activity are relevant to the BBB in vivo, we conducted an initial investigation of AMPK protein and activity in perfusion-fixed rat brain, using confocal immunofluorescence methods and antibodies to detect total AMPK and p-AMPK, as well as antibodies to positively identify BBB endothelial cells and to distinguish them from perivascular astrocyte endfeet that lie in close apposition to the BBB. Figure 7 shows representative confocal images generated using perfusion-fixed normoxic rat brain, together with antibodies specific for AMPKα and p-AMPKα1. Here, we also used astrocyte-specific GFAP antibody and the BBB endothelial cell specific SMI-71 antibody. Figure 7A shows images obtained with antibodies for AMPKα or p-AMPKα1 (green) and GFAP (red). Both total and activated forms of AMPKα are present in BBB endothelial cells (green). Merged AMPK and GFAP images (yellow) show that AMPK is also observed in the perivascular astrocytes. p-AMPKα1 is also present in both BBB endothelial cells (green) and perivascular astrocytes (yellow). Figure 7B shows images obtained with antibodies to AMPKα or p-AMPKα1 (green) and the SMI-71 antibody (red). Here, the presence of both AMPKα and p-AMPKα1 can be seen in the endothelial cells (yellow).
Fig. 7.
Immunofluorescence detection of AMPK and p-AMPK in normoxic control blood-brain barrier (BBB) endothelial cells in situ. Perfusion-fixed control rat brains were embedded in paraffin, sectioned (5 μm), and mounted on glass slides. Paraffin was removed, sections were lightly rehydrated, and immunohistochemistry was performed with antibodies for AMPK, p-AMPK, and the astrocyte marker glial fibrillary acidic protein (GFAP) (A) or BBB endothelial cell marker SMI-71 (B). The bars shown are 10 μm. AMPK and p-AMPK (green) are detected in both BBB endothelial cells and perivascular astrocytes. GFAP (red) appears in the perivascular astrocytic endfeet surrounding the BBB endothelial cells, and SMI-71 (red) appears in the BBB endothelial cells. Merged images show AMPK and p-AMPK in endothelial cells (green) and astrocytes (yellow) (A), as well as just in endothelial cells (yellow) (B). For both A and B, right, negative controls in which secondary antibodies only were used are shown.
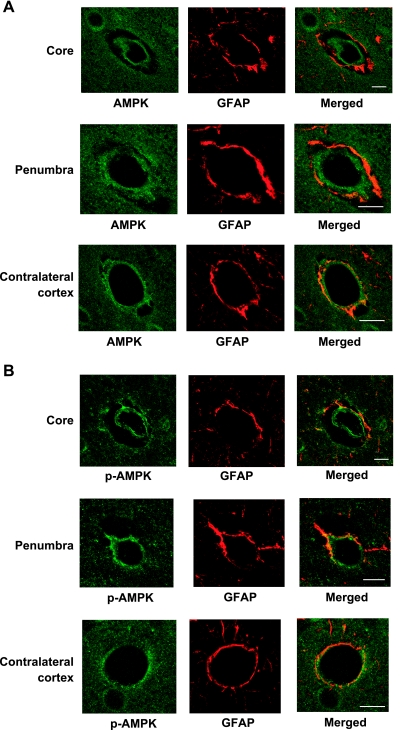
If AMPK activation participates in stimulation of BBB Na-K-Cl cotransporter activity during cerebral ischemia, we should also observe AMPK and p-AMPK in BBB of the ischemic brain. As an initial investigation to address this question, we subjected rats to 30 min of permanent MCAO, then used cardiac perfusion methods to immediately fix the brains and process them for confocal immunofluorescence evaluation as described in materials and methods. Figure 8 shows representative images of AMPKα, p-AMPKα1, and GFAP staining in the ipsilateral (ischemic) cortex of brains subjected to MCAO, both the core and penumbra, as well as contralateral (control normoxic) cortex. Both AMPKα and p-AMPKα1 (green) can be seen in BBB endothelial cells as well as perivascular astrocytes in ischemic core and penumbra of the ipsilateral cortex and in the control contralateral cortex. Here again GFAP staining (red) identifies perivascular astrocyte endfeet at the abluminal surface of BBB endothelial cells. Merged images show the presence of AMPKα and p-AMPKα1 in the astrocytes (orange yellow) and also the BBB endothelial cells (green) of both ischemic ipsilateral cortex and control contralateral cortex. These findings indicate that AMPK is indeed present in BBB endothelial cells in situ and that phosphorylated (activated) AMPKα1 is detected in the cells in both normoxic control and ischemic brain. The significance and limitations of these findings will be considered further in the discussion.
Fig. 8.
Immunofluorescence detection of AMPK and p-AMPK in ischemic BBB endothelial cells in situ. Rats were subjected to 30 min of permanent middle cerebral artery occlusion (MCAO), and then brains were perfusion-fixed, embedded in paraffin, sectioned (5 μm), and mounted on glass slides as described in materials and methods. Paraffin was removed, sections were lightly rehydrated, and immunohistochemistry was performed with antibodies for AMPKα (A), p-AMPKα (B), and the astrocyte marker GFAP (A and B). The bars shown are 10 μm. AMPK and p-AMPK (green) are detected in both BBB endothelial cells and perivascular astrocytes. GFAP (red) appears in the perivascular astrocytic endfeet surrounding the BBB endothelial cells. Merged images show AMPK and p-AMPK in endothelial cells (green) and astrocytes (yellow).
DISCUSSION
Previous studies from this group have provided evidence that BBB Na-K-Cl cotransporter activity is stimulated by the ischemic factors hypoxia, aglycemia, and AVP and, further, that inhibition of BBB cotransporter activity reduces edema and infarct in the rat MCAO model of ischemic stroke (4, 12, 40, 43). However, the mechanisms underlying ischemic factor stimulation of the BBB cotransporter are not well understood. AMPK has been shown to be activated by ischemic stress (6, 19), and AMPK inhibitors reduce damage in mouse and rat models of transient cerebral ischemia (33–35). However, it has not been known whether AMPK plays a role in events occurring at the BBB during stroke, including edema formation. In the present study we demonstrate that AMPK is present in BBB endothelial cells and that it is activated by ischemic factors that also stimulate CMEC Na-K-Cl cotransporter activity. In addition, we demonstrate that inhibition of AMPK activity with Compound C reduces or abolishes ischemic factor stimulation of CMEC cotransporter activity. Finally, we present evidence that AMPK is present and active in BBB endothelial cells in situ. Together, our findings indicate that the signaling pathway whereby ischemia stimulates BBB Na-K-Cl cotransport activity involves activation of AMPK.
The results of our studies reveal that AMPK is present in primary cultures of cerebral microvascular endothelial cells and that a portion of the AMPK is phosphorylated (activated) even under control, normoxic conditions. In addition, these studies demonstrate for the first time that the ischemic factors hypoxia, aglycemia, and AVP, all of which we have shown previously to stimulate CMEC Na-K-Cl cotransporter activity (4, 12, 40), also increase abundance of activated AMPK (p-AMPK) in the cells. While we found that each of these factors induces a significant increase in p-AMPK after just 5 min exposure, the increase is transient and decreases back toward control normoxic levels by 60 min of moderate or severe hypoxia and by 30 min of aglycemia or OGD. It is noteworthy that stimulation of CMEC Na-K-Cl cotransporter activity by these factors is sustained through at least 120 min in these and our previous studies (4, 12) and for AVP, even through 36 h (40). This suggests that a sustained elevation of Na-K-Cl cotransporter activity requires only transient activation of AMPK. In this regard, ongoing studies in our laboratory suggest that other kinases known to be activated by ischemic conditions, including p38 and JNK MAP kinases, also participate in ischemia stimulation of the BBB Na-K-Cl cotransporter (48, 49).
The present study also provides evidence that the α1 isoform of the AMPK catalytic subunit is present in BBB endothelial cells. This is in agreement with previous studies indicating that AMPKα1 is the predominant isoform in peripheral endothelial cells (9, 11, 53). While we conducted limited studies using the AMPK pan-α antibody that recognizes both AMPKα1 and α2 isoforms and found similar increases in AMPK activity in response to hypoxia and aglycemia, additional studies are needed to determine whether AMPKα2 is also activated by ischemic factors in BBB endothelial cells. It is noteworthy that in studies of mice subjected to cerebral ischemia-reperfusion, Western blot analysis of whole hemispheres showed elevated p-AMPK at 2 to 24 h after the start of reperfusion (33, 35). This is in contrast to our finding that ischemic factor elevation of p-AMPK abundance in CMEC was not sustained through 2 h exposure. However, the AMPK pan-α antibody was employed in the mouse whole hemisphere studies and because AMPKα2 has been reported to be the predominant isoform in neurons it is possible, if not likely, that the prolonged elevation of p-AMPK observed is attributable to activation of AMPKα2.
Our study further demonstrates that ischemic factor stimulation of CMEC Na-K-Cl cotransporter activity is reduced or abolished by the AMPK inhibitor Compound C. This occurs whether the cells are exposed to hypoxia, aglycemia, or AVP, suggesting that AMPK is involved in cotransporter stimulation by each of these factors present during ischemia. The observation that Compound C abolishes cotransporter stimulation by hypoxia and aglycemia while only reducing that stimulated by AVP or OGD further suggests the possibility that other ischemia-activated kinases also contribute to elevation of BBB Na-K-Cl cotransporter activity during ischemic stroke. In previous studies we have established that the AVP-induced stimulation of CMEC Na-K-Cl cotransporter activity is also Ca dependent (40). Ca/calmodulin-dependent protein kinase has been found to activate AMPK in some cells (23, 33, 50). Whether Ca and AMPK effects on the CMEC cotransporter are linked remains to be determined.
In the present study we have demonstrated that AMPK is present in BBB endothelial cells in situ using perfusion-fixed rat brain, both control, normoxic brain as well as ischemic brain. Using the astrocyte marker GFAP we show here prominent AMPKα and p-AMPKα1 staining in BBB endothelial cells as well as the surrounding perivascular astrocytes. In confirmation of BBB AMPK, our study also shows prominent colocalization of AMPK and p-AMPK with the BBB endothelial cell-specific marker. Phosphorylated, active kinase appears to be present in BBB of the normoxic brain as well as ischemic brain. It is important to note that these studies were intended as an initial investigation and examined AMPK in ischemic brain only after 30 min of permanent MCAO. Thus additional studies are needed to investigate BBB p-AMPK abundance at different times following induction of ischemia to determine whether and over what time course AMPK is activated in the BBB endothelial cells in situ. Nevertheless, our findings indicate that AMPK and p-AMPK are observed in BBB in situ and, together with our CMEC studies, support the hypothesis that AMPK is involved in the BBB response to ischemia. An immunohistochemical study of mouse cortex and hippocampus that used the p-AMPK pan-α antibody revealed the presence of both AMPK and p-AMPK in normoxic and ischemic mouse brain, with p-AMPK levels increased following ischemia-reperfusion (35). In that study, p-AMPK was prominent in neurons, identified using the neuronal marker NeuN, while minimal colocalization of p-AMPK with the astrocyte marker GFAP was observed, and no colabeling of AMPK or p-AMPK with the endothelial marker von Willebrand factor was seen. It should be noted that we examined perfusion-fixed rat brain using high magnification confocal immunofluorescence with specific BBB and astrocyte markers to positively identify AMPK staining in cerebral microvessels. We did not address AMPK staining in neurons, as the focus of our investigation was primarily the BBB. Clarifying whether the lack of observed AMPK staining in BBB endothelial cells of mouse brain is due to differences in species, fixation methods, or immunohistochemical markers employed will require further investigation.
In summary, the present study provides evidence that AMPK activation participates in ischemia-induced stimulation of BBB Na-K-Cl cotransporter activity. This, together with our previous finding that inhibition of BBB Na-K-Cl cotransporter activity via IV bumetanide reduces edema and infarct in the rat permanent MCAO model of stroke, suggests that activation of BBB endothelial AMPK promotes edema formation. Compound C has been shown to reduce infarct following ischemia-reperfusion in mice and rats (33–35). Additional studies are needed to determine whether the AMPK inhibitor is also effective in reducing early events of ischemia that begin before reperfusion, including cerebral edema formation.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grant NS-039953 (to M. E. O'Donnell), American Heart Association Western States Affiliate Predoctoral Fellowship 10PRE330016 (to B. K. Wallace), and by Howard Hughes Integrating Medicine into Basic Science Fellowship and an ARCS Foundation Award (to B. K. Wallace). The investigation was conducted in part in a facility constructed with support from Research Facilities Improvement Program Grant Number C06 RR17348-01 from the National Center for Research Resources, National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Betz AL. Alterations in cerebral endothelial cell function in ischemia. Adv Neurol 71:301–313,1996 [PubMed] [Google Scholar]
- 2. Betz AL, Keep RF, Beer ME, Ren XD. Blood-brain barrier permeability and brain concentration of sodium, potassium, and chloride during focal ischemia. J Cereb Blood Flow Metab 14:29–37,1994 [DOI] [PubMed] [Google Scholar]
- 3. Bourke RS, Kimelberg HK, Nelson LR, Barron KD, Auen EL, Popp AJ, Waldman JB. Biology of glial swelling in experimental brain edema. Adv Neurol Brain Edema 28:99–109,1980 [PubMed] [Google Scholar]
- 4. Brillault J, Lam TI, Rutkowsky JM, Foroutan S, O'Donnell ME. Hypoxia effects on cell volume and ion uptake of cerebral microvascular endothelial cells. Am J Physiol Cell Physiol 294:C88–C96,2008 [DOI] [PubMed] [Google Scholar]
- 5. Bronner LL, Kanter DS, Manson JE. Primary prevention of stroke. N Engl J Med 333:1392–1400,1995 [DOI] [PubMed] [Google Scholar]
- 6. Carling D. The AMP-activated protein kinase cascade–a unifying system for energy control. Trends Biochem Sci 29:18–24,2004 [DOI] [PubMed] [Google Scholar]
- 7. Chen YJ, Anderson SE, O'Donnell ME. Bumetanide and HOE642 administered after initiation of middle cerebral artery occlusion effectively reduce rat brain Na uptake and infarct. FASEB J 23:A614.A613,2009 [Google Scholar]
- 8. Chen YJ, Lam TI, Anderson SE, Walton JH, O'Donnell ME. Blood-brain barrier Na-K-Cl cotransporter and Na/H exchanger: therapeutic targets for ischemia-induced brain Na uptake and edema formation. J Cereb Blood Flow Metab 29:S489,2009 [Google Scholar]
- 9. Davis BJ, Xie Z, Viollet B, Zou MH. Activation of the AMP-activated kinase by antidiabetes drug metformin stimulates nitric oxide synthesis in vivo by promoting the association of heat shock protein 90 and endothelial nitric oxide synthase. Diabetes 55:496–505,2006 [DOI] [PubMed] [Google Scholar]
- 10. Ennis SR, Ren XD, Betz AL. Mechanisms of sodium transport at the blood-brain barrier studied with in situ perfusion of rat brain. J Neurochem 66:756–763,1996 [DOI] [PubMed] [Google Scholar]
- 11. Fisslthaler B, Fleming I. Activation and signaling by the AMP-activated protein kinase in endothelial cells. Circ Res 105:114–127,2009 [DOI] [PubMed] [Google Scholar]
- 12. Foroutan S, Brillault J, Forbush B, O'Donnell ME. Moderate-to-severe ischemic conditions increase activity and phosphorylation of the cerebral microvascular endothelial cell Na+-K+-Cl− cotransporter. Am J Physiol Cell Physiol 289:C1492–C1501,2005 [DOI] [PubMed] [Google Scholar]
- 13. Fraser SA, Gimenez I, Cook N, Jennings I, Katerelos M, Katsis F, Levidiotis V, Kemp BE, Power DA. Regulation of the renal-specific Na+-K+-2Cl- co-transporter NKCC2 by AMP-activated protein kinase (AMPK). Biochem J 405:85–93,2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Frederich M, Zhang L, Balschi JA. Hypoxia and AMP independently regulate AMP-activated protein kinase activity in heart. Am J Physiol Heart Circ Physiol 288:H2412–H2421,2005 [DOI] [PubMed] [Google Scholar]
- 15. Fryer LGD, Parbu-Patel A, Carling D. The anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J Biol Chem 277:25226–25232,2002 [DOI] [PubMed] [Google Scholar]
- 16. Golshani P, Liu XB, Jones EG. Differences in quantal amplitude reflect GluR4- subunit number at corticothalamic synapses on two populations of thalamic neurons. Proc Natl Acad Sci USA 98:4172–4177,2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gotoh O, Asano T, Koide T, Takakura K. Ischemic brain edema following occlusion of the middle cerebral artery in the rat. I. The time courses of the brain water, sodium and potassium contents and blood-brain barrier permeability to 125I-albumin. Stroke 16:101–109,1985 [DOI] [PubMed] [Google Scholar]
- 18. Hallows KR, Kobinger GP, Wilson JM, Witters LA, Foskett JK. Physiological modulation of CFTR activity by AMP-activated protein kinase in polarized T84 cells. Am J Physiol Cell Physiol 284:C1297–C11308,2003 [DOI] [PubMed] [Google Scholar]
- 19. Hardie DG. The AMP-activated protein kinase pathway–new players upstream and downstream. J Cell Sci 117:5479–5487,2004 [DOI] [PubMed] [Google Scholar]
- 20. Hardie DG. Minireview: the AMP-activated protein kinase cascade: the key sensor of cellular energy status. Endocrinology 144:5179–5183,2003 [DOI] [PubMed] [Google Scholar]
- 21. Hatashita S, Hoff JT. Brain edema and cerebrovascular permeability during cerebral ischemia in rats. Stroke 21:582–588,1990 [DOI] [PubMed] [Google Scholar]
- 22. Hawley SA, Davison M, Woods A, Davies SP, Beri RK, Carling D, Hardie DG. Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J Biol Chem 271:27879–27887,1996 [DOI] [PubMed] [Google Scholar]
- 23. Hurley RL, Anderson KA, Franzone JM, Kemp BE, Means AR, Witters LA. The Ca2+/calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J Biol Chem 280:29060–29066,2005 [DOI] [PubMed] [Google Scholar]
- 24. Iadecola C. Mechanisms of cerebral ischemic damage. In: Cerebral Ischemia: Molecular and Cellular Pathophysiology, edited by Walz W. Totowa, NJ: Humana, 1999, p. 3–34 [Google Scholar]
- 25. Jing M, Ismail-Beigi F. Critical role of 5′-AMP-activated protein kinase in the stimulation of glucose transport in response to inhibition of oxidative phosphorylation. Am J Physiol Cell Physiol 292:C477–C487,2007 [DOI] [PubMed] [Google Scholar]
- 26. Keep RF. Potassium transport at the blood-brain and blood-CSF barriers. In: Frontiers in Cerebral Vascular Biology: Transport and Its Regulation, edited by Drewes LR, Betz AL. New York: Plenum, 1993, p. 43–54 [DOI] [PubMed] [Google Scholar]
- 27. Kemp BE, Stapleton D, Campbell DJ, Chen ZP, Murthy S, Walter M, Gupta A, Adams JJ, Katsis F, van Denderen B, Jennings IG, Iseli T, Michell BJ, Witters LA. AMP-activated protein kinase, super metabolic regulator. Biochem Soc Trans 31:162–168,2003 [DOI] [PubMed] [Google Scholar]
- 28. Kimelberg HK. Cell Swelling in Cerebral Ischemia. In: Cerebral Ischemia: Molecular and Cellular Pathophysiology, edited by Walz W. Totowa, NJ: Humana, 1999, p. 45–68 [Google Scholar]
- 29. Kimelberg HK. Current concepts of brain edema. Review of laboratory investigations. J Neurosurg 83:1051–1059,1995 [DOI] [PubMed] [Google Scholar]
- 30. Klein H, Garneau L, Trinh NT, Prive A, Dionne F, Goupil E, Thuringer D, Parent L, Brochiero E, Sauve R. Inhibition of the KCa3.1 channels by AMP-activated protein kinase in human airway epithelial cells. Am J Physiol Cell Physiol 296:C285–C295,2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lam TI, Wise PM, O'Donnell ME. Cerebral microvascular endothelial cell Na/H exchange: evidence for the presence of NHE1 and NHE2 isoforms and regulation by arginine vasopressin. Am J Physiol Cell Physiol 297:C278–C289,2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lee M, Hwang JT, Lee HJ, Jung SN, Kang I, Chi SG, Kim SS, Ha J. AMP-activated protein kinase activity is critical for hypoxia-inducible factor-1 transcriptional activity and its target gene expression under hypoxic conditions in DU145 cells. J Biol Chem 278:39653–39661,2003 [DOI] [PubMed] [Google Scholar]
- 33. Li J, McCullough LD. Effects of AMP-activated protein kinase in cerebral ischemia. J Cereb Blood Flow Metab 30:480–492,2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li J, Zeng Z, Viollet B, Ronnett GV, McCullough LD. Neuroprotective effects of adenosine monophosphate-activated protein kinase inhibition and gene deletion in stroke. Stroke 38:2992–2999,2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McCullough LD, Zeng Z, Li H, Landree LE, McFadden J, Ronnett GV. Pharmacological inhibition of AMP-activated protein kinase provides neuroprotection in stroke. J Biol Chem 280:20493–204502,2005 [DOI] [PubMed] [Google Scholar]
- 36. Menzies SA, Betz AL, Hoff JT. Contributions of ions and albumin to the formation and resolution of ischemic brain edema. J Neurosurg 78:257–266,1993 [DOI] [PubMed] [Google Scholar]
- 37. Menzies SA, Hoff JT, Betz AL. Extravasation of albumin in ischaemic brain oedema. Acta Neurochir (Wien) 51:220–222,1990 [DOI] [PubMed] [Google Scholar]
- 38. Nakatsu Y, Kotake Y, Hino A, Ohta S. Activation of AMP-activated protein kinase by tributyltin induces neuronal cell death. Toxicol Appl Pharmacol 230:358–363,2008 [DOI] [PubMed] [Google Scholar]
- 39. O'Donnell ME. Regulation of Na-K-Cl cotransport in endothelial cells by atrial natriuretic factor. Am J Physiol Cell Physiol 257:C36–C44,1989 [DOI] [PubMed] [Google Scholar]
- 40. O'Donnell ME, Duong V, Suvatne J, Foroutan S, Johnson DM. Arginine vasopressin stimulation of cerebral microvascular endothelial cell Na-K-Cl cotransporter activity is V1 receptor and [Ca] dependent. Am J Physiol Cell Physiol 289:C283–C292,2005 [DOI] [PubMed] [Google Scholar]
- 41. O'Donnell ME, Martinez A, Sun D. Cerebral microvascular endothelial cell Na-K-Cl cotransport: regulation by astrocyte-conditioned medium. Am J Physiol Cell Physiol 268:C747–C754,1995 [DOI] [PubMed] [Google Scholar]
- 42. O'Donnell ME, Martinez A, Sun D. Endothelial Na-K-Cl cotransport regulation by tonicity and hormones: phosphorylation of cotransport protein. Am J Physiol Cell Physiol 269:C1513–C1523,1995 [DOI] [PubMed] [Google Scholar]
- 43. O'Donnell ME, Tran L, Lam TI, Liu XB, Anderson SE. Bumetanide inhibition of the blood-brain barrier Na-K-Cl cotransporter reduces edema formation in the rat middle cerebral artery occlusion model of stroke. J Cereb Blood Flow Metab 24:1046–1056,2004 [DOI] [PubMed] [Google Scholar]
- 44. Rotte A, Pasham V, Eichenmüller M, Bhandaru M, Föller M, Lang F. Upregulation of Na+/H+ exchanger by the AMP-activated protein kinase. Biochem Biophys Res Commun 398:677–682,2010 [DOI] [PubMed] [Google Scholar]
- 45. Schielke GP, Moises HC, Betz AL. Blood to brain sodium transport and interstitial fluid potassium concentration during focal ischemia in the rat. J Cereb Blood Flow Metab 11:466–471,1991 [DOI] [PubMed] [Google Scholar]
- 46. Sun D, Lytle C, O'Donnell ME. Astroglial cell-induced expression of Na-K-Cl cotransporter in brain microvascular endothelial cells. Am J Physiol Cell Physiol 269:C1506–C1512,1995 [DOI] [PubMed] [Google Scholar]
- 47. Turnley AM, Stapleton D, Mann RJ, Witters LA, Kemp BE, Bartlett PF. Cellular distribution and developmental expression of AMP-activated protein kinase isoforms in mouse central nervous system. J Neurochem 72:1707–1716,1999 [DOI] [PubMed] [Google Scholar]
- 48. Wallace BK, Jelks KA, Foroutan S, O'Donnell ME. Ischemia-induced stimulation of the BBB Na-K-Cl cotransporter: evidence for a role of p38 MAP kinase. FASEB J 22:A734.734, 2008 [Google Scholar]
- 49. Wallace BK, O'Donnell ME. Ischemic factor activation of p38, JNK and ERK1/2 MAP kinases in cerebral microvascular endothelial cells.2009 Society for Neuroscience Meeting Planner, Chicago IL: Society for Neuroscience; 2009:Program No. 148.110 2009 [Google Scholar]
- 50. Woods A, Dickerson K, Heath R, Hong SP, Momcilovic M, Johnstone SR, Carlson M, Carling D. Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab 2:21–33,2005 [DOI] [PubMed] [Google Scholar]
- 51. Woods A, Vertommen D, Neumann D, Turk R, Bayliss J, Schlattner U, Wallimann T, Carling D, Rider MH. Identification of phosphorylation sites in AMP-activated protein kinase (AMPK) for upstream AMPK kinases and study of their roles by site-directed mutagenesis. J Biol Chem 278:28434–28442,2003 [DOI] [PubMed] [Google Scholar]
- 52. Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirschman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 108:1167–1174,2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zou MH, Hou XY, Shi CM, Kirkpatick S, Liu F, Goldman MH, Cohen RA. Activation of 5′-AMP-activated kinase is mediated through c-Src and phosphoinositide 3-kinase activity during hypoxia-reoxygenation of bovine aortic endothelial cells. Role of peroxynitrite. J Biol Chem 278:34003–34010,2003 [DOI] [PubMed] [Google Scholar]