Abstract
Introduction
The purpose of this study was to characterize brain volumetric differences in HIV seropositive and seronegative men and to determine effects of age, cardiovascular risk, and HIV infection on structural integrity.
Methods
Magnetic resonance imaging was used to acquire high-resolution neuroanatomic data in 160 men aged 50 years and over, including 84 HIV seropositive and 76 seronegative controls. Voxel-based morphometry was used to derive volumetric measurements at the level of the individual voxel. Data from a detailed neuropsychological test battery were recombined into four summary scores representing psychomotor speed, visual memory, verbal memory, and verbal fluency.
Results
Both age and HIV status had a significant effect on both gray matter (GM) and white matter (WM) volume. The age-related GM atrophy was primarily in the superior temporal and inferior frontal regions; the HIV-related GM loss included the posterior and inferior temporal lobes, the parietal lobes, and the cerebellum. Among all subjects, the performance on neuropsychological tests, as indexed by a summary variable, was related to the volume of both the GM and WM. Contrary to our predictions, the CVD variables were not linked to brain volume in statistically adjusted models.
Conclusion
In the post-HAART era, having HIV infection is still linked to atrophy in both GM and WM. Secondly, advancing age, even in this relatively young cohort, is also linked to changes in GM and WM volume. Thirdly, CNS structural integrity is associated with overall cognitive functions, regardless of the HIV infection status of the study volunteers.
Keywords: MRI, Cognition, HIV, Age, Voxel-based morphometry
Introduction
The proportion of persons living with HIV/AIDS aged 50 years and older rose to 24% of all cases in 2005, up from 17.1% in 2001 (http://www.cdc.gov/hiv/topics/over50/resources/factsheets/over50.htm). While the total number of infected persons rose 20% in that time period, the increase in individuals over 50 was 58%. Factors normally associated with age-related neuropsychiatric syndromes may play an increasingly important role in understanding central nervous system (CNS) dysfunction occurring in older HIV + patients. Indeed, age-associated medical comorbidities are significant risk modifiers for HIV-associated neurocognitive disorder (HAND) [1–7]. There may be a differential effect of the presence of the APOE*4 allele in older HIV subjects [8], and diabetes is a critical comorbidity [9]. The use of highly active anti-retroviral therapy (HAART) may be associated with abnormal amyloid deposition in the brain [10], and amyloid-beta and tau found in the cerebrospinal fluid may be related to HIV-associated dementia [11].
The Multicenter AIDS Cohort Study (MACS) has compared 345 asymptomatic HIV-infected men and 237 controls on neuropsychological test performance on Symbol-Digit Modalities and Trailmaking Tests over a 5-year period [12]. There was no evidence of differences between these groups in either performance, or in year-to-year decline during the observation period. These findings indicate that psychomotor speed, a very sensitive measure of HIV-associated cognitive loss, is preserved over many years among HIV-infected individuals with controlled viremia.
We have also analyzed effects of cardiovascular factors on cognitive function in a cross-sectional study of 428 infected men and 207 uninfected controls aged 40 years and older [13]. Carotid intima-media thickness and estimated glomerular filtration rate were significantly associated with slower psychomotor speed. HIV serostatus, however, was not associated with psychomotor speed in a covariate-adjusted model. There was no association between test performance and detectable HIV RNA, CD4+ cell counts, or AIDS in the HIV+ individuals. Abnormal coronary artery calcification, however, was a risk factor for poorer performance on memory tasks. Among the HIV-infected individuals only, the presence of detectable HIV RNA in plasma significantly increased risk of poorer memory performance.
The associations between brain structure and cognition in HIV infection have been reviewed by Paul [14] and by Thurnher [15]. In addition to the well-documented findings of subcortical brain abnormalities, there is increasing evidence that the neocortex is injured in HIV infection [16, 17]. AIDS patients exhibit severe, selective gray matter thinning (10–15%) in a broad anatomic area that includes primary sensory and motor cortices of both hemispheres [18] and prefrontal and parietal cortical thinning were correlated with neuropsychological impairment. Loss of CNS structural integrity was also reflected in a 24% increase in ventricular volume in infected subjects, and these measurements also correlated with neuropsychological impairment. The volumes of the frontal horns provided good between-group discrimination [19]. A recent study [20] demonstrated a loss of gray matter secondary to HIV disease in the anterior cingulate and temporal cortices, with a loss of midbrain white matter, as well.
This research report presents the findings of a voxel-based morphometry (VBM) analysis of the brain structural images of HIV-infected and seronegative participants of the MACS [21]. We hypothesized that cardiovascular variables would be associated with increased neurological risk, as measured by effects on measures of both brain structure and cognition. We predicted that HIV-related effects in this HAART era sample would be attenuated relative to pre-HAART observations once cardiovascular risk variables were adjusted for in multivariate analyses.
Methods
Standard protocol approvals, registrations, and patient consents
This study was approved by the ethical standards committee on human experimentation at each of the MACS sites. Written informed consent was obtained from all participants prior to their undergoing research procedures.
Subjects
The MACS is a four-site study of the natural and treated history of HIV infection among men who have sex with men. Volunteers were enrolled in three waves: 1984/1985, 1987/1990, and in 2001/2003 (primarily from racial/ethnic minorities). The MACS has tracked cognitive functions among the study participants for the past 24 years using screening tools (Trail Making, Symbol-Digit Substitution Tests) and has followed a sub-cohort with more detailed testing for approximately 20 years.
The MACS cardiovascular disease (CVD) substudy included men, age ≥40 years, with no self-reported history of heart disease (heart attack, heart surgery, and other heart illness) or cerebrovascular disease, and weight <300 lb [22]; the baseline visit was completed between April 2004 and January 2006. In 2007, each site conducted magnetic resonance imaging (MRI) examinations of 40 MACS participants (total N=160), including high-resolution anatomic sequences.
The men who were approached for participation in the MRI study were all part of the CVD substudy and were matched on a 1:1 basis as a function of age, education and race (Caucasian vs. Other). At the Chicago and Baltimore sites it was possible to create 20 pairs of subjects (HIV±) within site. However, because of the distribution of cases in Los Angeles and Pittsburgh, these sites had to match cases across the centers. The resulting distribution of cases by serostatus thus differed by site. Demographic characteristics of the subjects are shown in Table 1.
Table 1.
Subject characteristics
| HIV− | HIV+ | Statisticsa | |
|---|---|---|---|
| N | 67 | 81 | |
| Age | 57.7 (6.3) | 56.3 (3.9) | 1.57, .13 |
| Education | 16.7 (2.5) | 15.7 (2.7) | 2.47, .20 |
| CES-D | 9.80 (11) | 8.70 (85) | .636, .05 |
| Raceb | 81 (54) | 73 (59) | 1.22, .09 |
| Crack usec | 16 (11) | 22 (18) | .78, .07 |
| Cocainec | 16 (11) | 26 (21) | 1.96, .12 |
| Uppersc | 8 (5) | 16 (13) | 2.53, .13 |
| Cardiovascular health variables | |||
| Hypertensiond | 42 (28) | 42 (34) | .001, .002 |
| Diabetesd | 16 (11) | 11 (9) | .88, −.08 |
| Systolic BP | 128.3 (16) | 128.5 (13) | .936, .07 |
| Diastolic BP | 77.1 (10) | 78.0 (9) | .619, .05 |
| Total cholesterol | 192.7 (40) | 196.0 (54) | .680, .06 |
| HDL | 52.0 (15) | 48.1 (15) | .132, .01 |
| LDL | 112.2 (35) | 111.8 (34) | .07, .005 |
| Triglycerides | 15.5 (15) | 19.6 (25) | −1.05, .09 |
| Glucose | 111.5 (62) | 101.8 (19) | 1.20, .10 |
| Hemoglobin A1C | 5.99 (1.6) | 5.54 (8.4) | 2.05, .17* |
| Neuropsychological summary variables | |||
| Psychomotor speed | 0.064 (0.72) | −0.036 (0.79) | 0.81, .07 |
| Verbal memory | 0.134 (0.89) | −0.132 (0.90) | 1.79, .15 |
| Visual memory | 0.132 (0.92) | −0.150 (0.89) | 1.83, .15 |
| Verbal fluency | −0.081 (0.85) | 0.065 (0.90) | −1.00, .08 |
| NP summary score | 0.061 (0.63) | −0.058 (0.65) | 1.12, .09 |
t and r or χ2 and Phi
White/non-white (percent (N))
Never/last 5 years (percent (N))
Yes/no (percent (N))
p < .05
Neuropsychological evaluation
Beginning in 2005 the MACS instituted a new schedule of assessing cognitive function within the entire study cohort. Between 2005 and 2007, all MACS participants completed a standard neuropsychological test battery; those subjects who scored within normal limits were scheduled to return bi-annually. Those subjects whose performance fell below normal limits are retested on a semi-annual basis. In addition to these tests, each of the volunteers who were enrolled in this study completed additional tests which more closely conform to those mentioned in the revised research criteria for HAND [23]. All of the neuropsychological test data were sent to the Pittsburgh site where the raw scores from the tests were either transformed into demographically adjusted T scores [24], or to standard scores derived from published norms.
Magnetic resonance imaging
The MRI scanning sequences were taken from the protocol developed by the Alzheimer’s disease neuroimaging initiative (ADNI) for use with scanners with 3 Tesla field strengths (http://www.adni-info.org/images/stories//mritrainingmanualv1.pdf). Three of the sites housed a Siemens 3 T Trio scanner (maximum gradient slew rate: 200 mT/m/s; maximum gradient strength 40mT/m), with the Siemens phase-array head coil. One of the sites (Los Angeles) used a Siemens Allegra scanner. The sequences were (in order): localizer scan, transversal (axial) proton density, transversal (axial) T2-weighted, transversal (axial) FLAIR, coronal MP-RAGE (8–10 min), and transversal (axial) diffusion tensor imaging. The sagittal MP-RAGE sequence used for this analysis was: FOV=256 mm; slices= 160; TR=2,300 ms; TE=2.91 ms; TI=900 ms; flip angle=9 °; and thickness=1.2 mm. Only the data from the MP-RAGE will be described in this report.
In order to standardize the acquisition of the data, the subjects were required to remove any dentures, hair clips, combs, earrings, necklaces, etc. and to remove all upper body clothing with metallic trim, such as zippers, buttons, or embroideries that could cause artifact. In order to assist with the verification of scan orientation, a fiducial marker (vitamin E or fish oil capsule) was taped to the subjects’ right temple. A procedures manual, modeled after that of ADNI, was distributed to the technologists and investigators at each site. All of the de-identified MRI data were transferred from each site to a central location at the University of Pittsburgh. They were subsequently stored on local hard disks with the first author of this report. A copy of all of the MRI data was also sent to the central data repository in Baltimore.
Cardiovascular disease evaluation
Subclinical CVD was assessed using electron beam tomography or multidetector computed tomography to measure coronary artery calcium and ultrasound examination of the carotid artery to measure carotid IMT, plaque, and stiffness/distensibility. Laboratory measures included total cholesterol, low and high density lipoproteins, glucose, insulin, glycosylated hemoglobin, and standardized blood pressure and heart rate measures. GFR was estimated using a standard protocol. The CVD variables are shown in Table 1.
Data reduction
The CVD variables were reduced to categorical variables (present/absent, normal/abnormal) based on standard criteria or the distribution of values within the HIV− group [13]. These included the presence of hypertension (resting BP >130/90, or self-report of HTN, or use of anti-hypertensive medications) and diabetes (self-report or use of anti-diabetic medications). The CES-D score was calculated for each subject, and those scoring above 16 were classified as “depressed” for the purpose of this analysis. Education was classified as high school or less, 13–15 years, and college or greater. We classified each participant with reference to current use, or any use within the past 5 years of illicit drugs.
The neuropsychological test data were reduced to z scores based on the distribution of the entire study sample. The data were then recombined into four summary scores representing psychomotor speed (Symbol-Digit Substitution, Trailmaking Part A), Visual Memory (Recall of Visual Reproductions), Verbal Memory (Logical Memory Recall), and Verbal Fluency (letter and category word generation). The signs were adjusted in each case so that positive scores represented better performance. A summary score was computed by taking the mean of the four composite variables (see Table 1).
Voxel-based morphometry
The MRI data were first processed through a non-parametric non-uniform intensity normalization [25] to reduce between scan and between-site differences in the images. This was followed by a bias correction (within SPM2) in order to help improve spatial registration. We then used a recursive implementation of the brain extraction tool [26] from the FMRIB Software Library (http://www.fmrib.ox.ac.uk/fsl/) to strip off the skull and other extraneous tissue.
We created a normal template image using the seronegative control subjects, and estimated the prior probabilities of each tissue class (i.e., gray, white, and CSF) for use in the segmentation routines. We then used the VBM2 script (http://dbm.neuro.uni-jena.de/vbm/vbm2-for-spm2/) for normalization and segmentation of the data. The resulting maps of gray matter had been modulated to render the values in each of the 1×1×1 mm voxels as a volume, which were then smoothed using a 10×10×10 mm Gaussian filter to reduce the effects of registration error and render the data more amenable to parametric analysis. At this stage, 12 of the initial 160 scans (7.5%) did not pass our quality control checks, and these subjects were excluded from further analysis.
For all of the VBM analyses, total intra-cranial volume was entered as a covariate. In addition, because the subjects were scanned at different sites (and on two different model scanners), we also entered four binary dummy variables to adjust for possible between-site differences. For all analyses, the default threshold for reporting statistical significance was set at a False discovery rate of P<.05, with an extent threshold of 100 voxels [27]. The mean whole brain image created from the seronegative control subjects was used to project all study findings.
Results
There are three main findings from this analysis. Firstly, age has a significant effect on both GM and WM volume at the voxel level. Secondly, HIV disease had an independent effect on GM and WM volume at the voxel level. Thirdly, among all subjects, the performance on neuropsychological tests, as indexed by a summary variable, was related to the volume of both the GM and WM. Contrary to our predictions, the CVD variables were not linked to brain volume in statistically adjusted models.
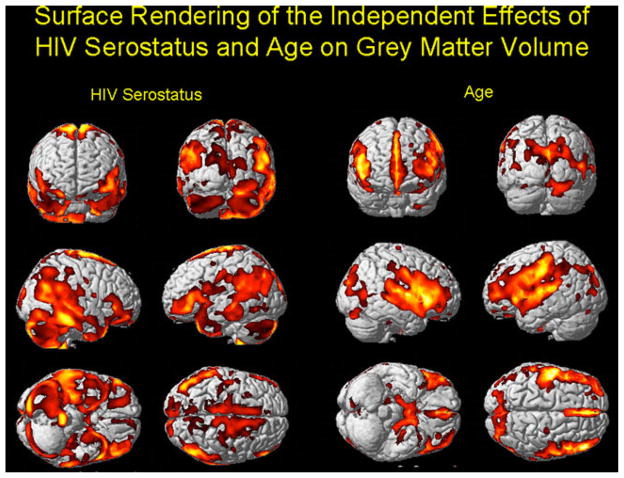
Figure 1 shows the results of the VBM analysis of gray matter projected onto a single-subject template of the cortical surface. The effects of age are shown in the right-hand columns, and the effects of HIV disease in the left-hand columns. Both variables were entered into the same analysis simultaneously, with total intra-cranial volume and study site as covariates. There was no significant interaction between age and HIV group. The age-related GM atrophy is focused primarily in the superior temporal and inferior frontal regions, with additional tissue loss in the medial temporal and cingulate cortices (cf. [28]). By contrast, the HIV-related GM loss was more widely distributed. It includes the posterior and inferior temporal lobes (right > left), the parietal lobes, and the cerebellum (see Fig. 1).
Fig. 1.
The results of the VBM analysis of gray matter projected onto the single-subject template of the cortical surface from SPM2. The effects of age are shown in the right-hand columns, and the effects of HIV disease in the left-hand columns. Both variables were entered into the same analysis simultaneously, with total intra-cranial volume as a covariate. False discovery rate=p<.05, with an extent threshold of 100 voxels. The regions with the hotter colors are those with the greatest amount of atrophy attributable to age or HIV status
White matter atrophy was significantly associated with advancing age (see Fig. 2), but much less so with HIV status. Older subjects, regardless of their serostatus had atrophy in the peri-ventricular white matter and frontal and temporal regions.
Fig. 2.
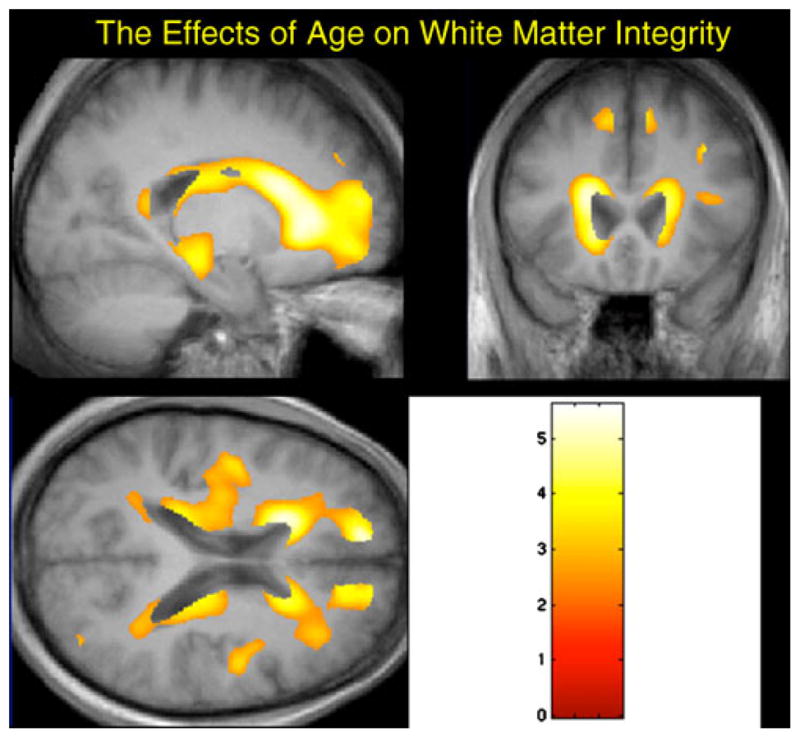
The results of the VBM analysis of the white matter atrophy projected onto the mean whole brain image created from the seronegative subjects. For all study participants, atrophy was significantly associated with advancing age, but not with HIV status. False discovery rate=p<.05, with an extent threshold of 100 voxels. The regions with the hotter colors are those with the greatest amount of white matter atrophy attributable to age
We then analyzed the associations between the cognitive summary score and GM and WM volumes. Instead of entering HIV status into the SPM model, we instead entered the Global Impairment Rating for each participant and the resulting analysis was similar to a multiple regression model, with total intra-cranial volume and study site as covariates. We found a significant link between GM and test performance in the right temporal lobes, predominantly. Consequently, we created a regional mask using PickAtlas (http://www.fmri.wfubmc.edu/cms/software#PickAtlas) and focused our analysis on the right temporal pole (i.e., we excluded the remainder of the brain from the search space). We found extensive regions of the anterior temporal lobe that were linked to performance on the neuropsychological tests, as indexed by the cognitive summary score (see Fig. E-1 in the Electronic supplementary materials). Analysis of WM also revealed that atrophy was associated with lower scores on the neuropsychological tests (see Fig. 3). The regions were in the same WM areas as those observed for the aging effect, but were less extensive.
Fig. 3.
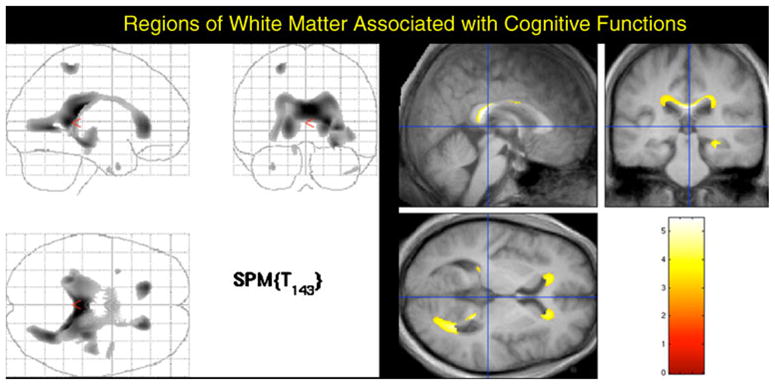
WM atrophy associated with lower scores on the neuropsychological tests. These regions were in the same WM regions as those observed for the aging effect, but with less spatial extent. The left-hand image shows a “glass brain” view in which all voxels are visible in each of the three perspectives. The images on the right show the areas of significant association between white matter volume and test performance
We tested the effects of time since infection, CD4+ cell counts, and viral load on GM and WM integrity among the seropositive men in the study. None of the effects was significant at an FDR of p<.05. The same was true when we tested the effects of drug use, diabetes, and hypertension on structural integrity among all study participants.
Discussion
These data make several important points. Firstly, in the post-HAART era, having HIV infection is still linked to atrophy in GM, and to a lesser extent WM. Secondly, advancing age, even in this relatively young cohort, is also linked to changes in GM and WM volume. Thirdly, CNS structural integrity is associated with overall cognitive functions, regardless of the HIV infection status of the study volunteers.
Our findings are fully consistent with the accumulating data that the neocortex is affected in HIV disease, even among individuals with mild or no cognitive dysfunction [20]. We have previously identified, in a separate non-MACS study, those brain regions that were selectively vulnerable to HIV disease by computing 3D maps of cortical gray matter thickness [18]. Prefrontal and parietal cortical thinning in both brain hemispheres was linked with neuropsychological impairment. This loss of CNS structural integrity was also reflected in an expansion of the lateral ventricles, estimated as a 24% increase in size of the ventricles in the HIV-infected participants [19, 29].
Chiang and colleagues [30] used tensor-based morphometry to visualize the brain regional atrophy in that same group of HIV-infected individuals. Significant atrophy was found bilaterally in the primary and association sensorimotor areas, basal and medial frontal lobes (~15–20% deficit), corpus callosum, cingulum, putamen, globus pallidus, and thalamus (~10–15% deficit). Atrophy of these regions, particularly in the white matter, correlated with cognitive impairment and CD4+ cell counts. Leporé [31] developed a more sensitive method of analysis of these data, retaining the full deformation tensors and applying a manifold version of Hotelling’s test. With this approach, consistent but more extensive patterns (relative to the standard methods) of structural abnormalities were detected.
We [32] and others [17, 20] have shown that the extent of GM atrophy in HIV-infected individuals is related to the duration of infection. One interpretation of these findings is that it is due to the “legacy effect”—that is, the individuals with long-term HIV infection (like many of the men in this study) were also the ones with no exposure to treatment, use of only monotherapy, and use of HAART only after significant immune suppression and rampant viral replication. HIV viral proteins are neurotoxic, the GM and WM volumetric differences may reflect neuronal loss, reduced dendritic complexity, synaptic loss [33], and associated white matter degeneration. Because HAART (and other) medications often fail to significantly permeate the blood brain barrier [34], brain degeneration still may proceed even in patients treated with anti-retroviral medication. Thus, we may be seeing the residual effects of uncontrolled HIV infection.
The fact that we did not see extensive WM changes as a consequence of HIV infection is also consistent with the more recent neuropathological findings that white matter pallor and demyelination are now much less common than in the early days of the epidemic in the USA. However, prior to the widespread use of HAART, structural MRI studies consistently revealed the presence of white matter lucencies [35]. White matter abnormalities could be diffuse or focal, and seen as signal increases on T2-weighted images [36], by T1 relaxation times, or using diffusion tensor imaging [37, 38].
One question that arises from this analysis that is particularly important for clinical neuroradiologists as well as researchers is why there was a correlation between volume of white matter and cognitive function. White matter lesions are more common in older individuals (e.g., Fig. 2) and correspond to loss of myelinated axons, gliosis, enlarged perivascular spaces, and small vessel disease [39–41]. White matter damage is also linked to the presence of hypertension, and may precede the subsequent development of stroke and dementia [42, 43]. There is a range of data documenting the relationship between white matter damage and cognition (e.g., [44–47]), and data from the Cardiovascular Health Study suggest that this association is at least partially mediated by damage to the GM (Raji et al., personal communication). Specifically, WM damage can be considered as a marker of small vessel disease, which has an impact on GM integrity and subsequently impairs cognition. In the present case, because markers of subclinical vascular disease were unrelated to GM volume, we would predict (although cannot prove) that the majority of the association between WM atrophy and cognition is due to the direct link, with less of the variance accounted for by the indirect pathways through GM damage secondary to small vessel disease. However, this will need to be tested directly, preferably with longitudinal imaging data.
The results of this analysis build on the growing body of evidence that among long-term HIV-infected individuals, control of viral replication and of immunological competence appears to protect against the CNS abnormalities and cognitive dysfunction that were so common prior to the advent of HAART. Although HIV disease still has an impact on brain structure, the effects appear to be less extensive than those associated with age. As the risk factors for HAND continue to change [48], and we develop a better understanding of the multiple factors that can affect brain structure and function, being able to place HAND in the context of normal age-related processes becomes increasingly important.
Supplementary Material
Acknowledgments
The authors are grateful to the volunteers and the staff of the Multicenter AIDS Cohort Study for the additional time and effort that they contributed towards the successful completion of this project. This study was supported in part by funds from the National Institute for Allergy and Infectious Diseases to the collaborating MACS sites: UO1-AI-35042, 5-MO1-RR-00052 (GCRC), UO1-AI-35043, UO1-AI-37984, UO1-AI-35039, UO1-AI-35040, UO1-AI-37613, and UO1-AI-35041.
Footnotes
Conflict of interest Dr. Miller is the author of the CalCAP reaction time program and has a financial interest in this software.
Electronic supplementary material The online version of this article (doi:10.1007/s00234-011-0854-2) contains supplementary material, which is available to authorized users.
The Multicenter AIDS Cohort Study (MACS) includes the following: Baltimore: The Johns Hopkins University Bloomberg School of Public Health: Joseph B. Margolick (Principal Investigator), Haroutune Armenian, Barbara Crain, Adrian Dobs, Homayoon Farzadegan, Joel Gallant, John Hylton, Lisette Johnson, Shenghan Lai, Ned Sacktor, Ola Selnes, James Shepard, Chloe Thio. Chicago: Howard Brown Health Center, Feinberg School of Medicine, Northwestern University, and Cook County Bureau of Health Services: John P. Phair (Principal Investigator), Joan S. Chmiel (Co-Principal Investigator), Sheila Badri, Bruce Cohen, Craig Conover, Maurice O’Gorman, David Ostrow, Frank Palella, Daina Variakojis, Steven M. Wolinsky. Los Angeles: University of California, UCLA Schools of Public Health and Medicine: Roger Detels (Principal Investigator), Barbara R. Visscher (Co-Principal Investigator), Aaron Aaronow, Robert Bolan, Elizabeth Breen, Anthony Butch, Thomas Coates, Rita Effros, John Fahey, Beth Jamieson, Otoniel Martínez-Maza, Eric N. Miller, John Oishi, Paul Satz (deceased), Harry Vinters, Dorothy Wiley, Mallory Witt, Otto Yang, Stephen Young, Zuo Feng Zhang. Pittsburgh: University of Pittsburgh, Graduate School of Public Health: Charles R. Rinaldo (Principal Investigator), Lawrence A. Kingsley (Co-Principal Investigator), James T. Becker, Ross D. Cranston, Jeremy J. Martinson, John W. Mellors, Anthony J. Silvestre, Ronald D. Stall. Data Coordinating Center: The Johns Hopkins University Bloomberg School of Public Health: Lisa P. Jacobson (Principal Investigator), Alvaro Munoz (Co-Principal Investigator), Stephen R. Cole, Christopher Cox, Gypsyanber D’Souza, Stephen J. Gange, Janet Schollenberger, Eric C. Seaberg, Sol Su. NIH: National Institute of Allergy and Infectious Diseases: Robin E. Huebner; National Cancer Institute: Geraldina Dominguez; National Heart, Lung and Blood Institute: Cheryl McDonald; National Institute of Mental Health: Pim Brouwers.
Contributor Information
James T. Becker, Email: beckerjt@upmc.edu, Department of Psychiatry, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA. Department of Neurology, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA. Department of Psychology, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA. Neuropsychology Research Program, Suite 830, 3501 Forbes Avenue, Pittsburgh, PA 15213, USA
Victoria Maruca, Spalding University, Louisville, KY, USA.
Lawrence A. Kingsley, Department of Infectious Diseases and Microbiology, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA, USA
Joanne M. Sanders, Department of Epidemiology, Bloomberg School of Public Health, The Johns Hopkins University, Baltimore, MD, USA
Jeffery R. Alger, Department of Neurology, University of California Los Angeles, Los Angeles, CA, USA
Peter B. Barker, Department of Radiology, The Johns Hopkins University School of Medicine, Baltimore, MD, USA
Karl Goodkin, Neuropsychiatric Institute, University of California Los Angeles, Los Angeles, USA.
Eileen Martin, Department of Psychiatry, University of Illinois at Chicago, Chicago, IL, USA.
Eric N. Miller, Neuropsychiatric Institute, University of California Los Angeles, Los Angeles, CA, USA
Ann Ragin, Department of Neurology, Feinberg School of Medicine, Northwestern University, Chicago, USA.
Ned Sacktor, Department of Neurology, The Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Ola Selnes, Department of Neurology, The Johns Hopkins University School of Medicine, Baltimore, MD, USA.
References
- 1.Janssen RS, Nwanyanwu OC, Selik RM, Stehr-Green JK. Epidemiology of human immunodeficiency virus encephalopathy in the United States. Neurology. 1992;42:1472–1476. doi: 10.1212/wnl.42.8.1472. [DOI] [PubMed] [Google Scholar]
- 2.Chiesi A, Vella S, Dally LG, Pedersen C, Danner S, Johnson AM, Schwander S, Goebel FD, Glauser M, Antunes F, Lundgren JD. Epidemiology of AIDS dementia complex in Europe. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;11:39–44. doi: 10.1097/00042560-199601010-00005. [DOI] [PubMed] [Google Scholar]
- 3.Stoff DM. Mental health research in HIV/AIDS and aging: problems and prospects. AIDS. 2004;18(Suppl 1):3–10. [PubMed] [Google Scholar]
- 4.Valcour V, Shikuma C, Shiramizu B, Watters M, Puff P, Selnes O, Holck P, Grove J, Sacktor N. Higher frequency of dementia in older HIV-1 individuals. Neurology. 2004;63:822–827. doi: 10.1212/01.wnl.0000134665.58343.8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valcour CG, Shikuma CM, Watters MR, Sacktor NC. Cognitive impairment in older HIV1-seropositive individuals: prevalence and potential mechanisms. AIDS. 2004;18(Suppl 1):79–86. doi: 10.1097/00002030-200401001-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Justice A, McGinnis K, Atkinson JH, Heaton R, Young C, Sadelk J, Madenwald T, Becker JT, Conigliaro J, Brown S, Rimland D, Crystal S, Simberkoff J. Psychiatric and neurocognitive disorders among HIV-positive and negative veterans in care: Veterans Aging Cohort Five-Site Study. AIDS. 2004;18(Suppl 1):49–59. [PubMed] [Google Scholar]
- 7.Cherner M, Ellis R, Lazzaretto D, Young C, Mindt MR, Atkinson JH, Grant I, Heaton RK. Effects of HIV-1 infection and aging on neurobehavioral functioning: preliminary findings. AIDS. 2004;18(Suppl 1):35–42. [PubMed] [Google Scholar]
- 8.Valcour V, Shikuma C, Shiramizu B, Watters M, Poff P, Selnes OA, Grove J, Liu Y, Abdul-Majid KB, Gartner S, Sacktor N. Age, apolipoprotein E4, and the risk of HIV dementia: the Hawaiian Aging with HIV Cohort. J Neuroimmunol. 2004;157(1–2):197–202. doi: 10.1016/j.jneuroim.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 9.Valcour VG, Shikuma CM, Shiramiza BT, Williams AE, Watters MR, Puff PW, Grove JS, Selnes OA, Sacktor NC. Diabetes, insulin resistance, and dementia among HIV-1 infected patients. J Acquir Immune Defic Syndr. 2005;38(1):31–36. doi: 10.1097/00126334-200501010-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Green DA, Masliah E, Vinters HV, Beizai P, Moore DJ, Achim CL. Brain Deposition of beta-amyloid is a common pathologic feature in HIV positive patients. AIDS. 2005;19(4):407–411. doi: 10.1097/01.aids.0000161770.06158.5c. [DOI] [PubMed] [Google Scholar]
- 11.Brew BJ, Pemberton L, Blennow K, Wallin A, Hagberg L. CSF amyloid Beta-42 and tau levels correlate with AIDS dementia complex. Neurology. 2005;65:1490–1492. doi: 10.1212/01.wnl.0000183293.95787.b7. [DOI] [PubMed] [Google Scholar]
- 12.Cole MA, Margolick JB, Cox C, Li X, Selnes OA, Martin EM, Becker JT, Aronow HA, Cohen B, Sacktor N, Miller EN. Longitudinally preserved psychomotor performance in long-term asymptomatic HIV-infected individuals. Neurology. 2007;69(24):2213–2220. doi: 10.1212/01.WNL.0000277520.94788.82. [DOI] [PubMed] [Google Scholar]
- 13.Becker JT, Kingsley L, Mullen J, Cohen B, Martin E, Miller EN, Ragin A, Sacktor N, Selnes OA, Visscher BR. Vascular risk factors, HIV serostatus, and cognitive dysfunction in gay and bisexual men. Neurology. 2009;73(16):1292–1299. doi: 10.1212/WNL.0b013e3181bd10e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paul R, Cohen R, Navia B, Tashima K. Relationships between cognition and structural neuroimaging findings in adults with human immunodeficiency virus type-1. Neurosci Biobehav Rev. 2002;26:353–359. doi: 10.1016/s0149-7634(02)00006-4. [DOI] [PubMed] [Google Scholar]
- 15.Thurnher MM, Post MJD. The uses of structural neuroimaging in the brain in HIV1-infected patients. In: Goodkin K, Shapshak P, Verma A, editors. The spectrum of neuro-AIDS disorder: pathophysiology, diagnosis and treatment. ASM Press; Washington: 2008. pp. 247–272. [Google Scholar]
- 16.Cysique LA, Maruff P, Brew BJ. Prevalence and pattern of neuropsychological impairment in human immunodeficiency virus-infected/acquired immunodeficiency syndrome (HIV/AIDS) patients across pre- and post-highly active antiretroviral therapy eras: a combined study of two cohorts. J Neurovirol. 2004;10(6):350–357. doi: 10.1080/13550280490521078. [DOI] [PubMed] [Google Scholar]
- 17.Cohen RA, Harezlak J, Schifitto G, Hana G, Clark U, Gongvatana A, Paul R, Taylor M, Thompson P, Alger J, Brown M, Zhong J, Campbell T, Singer E, Daar E, McMahon D, Tso Y, Yiannoutsos CT, Navia B. Effects of nadir CD4 count and duration of human immunodeficiency virus infection on brain volumes in the highly active antiretroviral therapy era. J Neurovirol. 2010 doi: 10.3109/13550280903552420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson PM, Dutton RA, Hayaski KM, Toga AW, Lopez OL, Aizenstein HJ, Becker JT. Thinning of the cerebral cortex visualized in HIV/AIDS reflects CD4+ T-lymphocyte decline. Proc Natl Acad Sci USA. 2005;102(43):15647–15652. doi: 10.1073/pnas.0502548102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson PM, Dutton RA, Hayashi KM, Lu A, Lee SE, Lee JY, Lopez OL, Aizenstein HJ, Toga AW, Becker JT. 3D mapping of ventricular and corpus callosum abnormalities in HIV/AIDS. Neuroimage. 2006;31(1):12–23. doi: 10.1016/j.neuroimage.2005.11.043. [DOI] [PubMed] [Google Scholar]
- 20.Kuper M, Rabe K, Esser S, Gizewski ER, Husstedt IW, Maschke M, Obermann M. Structural gray and white matter changes in patients with HIV. J Neurol. 2011 doi: 10.1007/s00415-010-5883-y. [DOI] [PubMed] [Google Scholar]
- 21.Ashburner J, Friston KJ. Voxel-based morphometry—the methods. Neurology. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 22.Kingsley LA, Cuervo-Rojas J, Munoz A, Palella FJ, Post W, Witt MD, Budoff M, Kuller L. Subclinical coronary atherosclerosis, HIV infection and antiretroviral therapy: multicenter AIDS Cohort Study. AIDS. 2008;22(13):1589–1599. doi: 10.1097/QAD.0b013e328306a6c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Clifford DB, Cinque P, Epstein LG, Goodkin K, Gisslen M, Grant I, Heaton RK, Joseph J, Marder K, Marra CM, McArthur JC, Nunn M, Price RW, Pulliam L, Robertson KR, Sacktor N, Valcour V, Wojna VE. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heaton RK, Taylor MJ. Revised comprehensive norms for an expanded Halstead–Reitan battery: demographically adjusted neuropsychological norms for African American and Caucasian adults. Psychological Assessment Resources; Odessa, FL: 2004. [Google Scholar]
- 25.Boyes RG, Gunter JL, Frost C, Janke AL, Yeatman T, Hill DL, Bernstein MA, Thompson PM, Weiner MW, Schuff N, Alexander GE, Killiany RJ, DeCarli C, Jack CR, Fox NC. Intensity non-uniformity correction using N3 on 3-T scanners with multichannel phased array coils. Neuroimage. 2008;39(4):1752–1762. doi: 10.1016/j.neuroimage.2007.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fagiolo G, Waldman A, Hajnal JV. A simple procedure to improve FMRIb Software Library Brain Extraction Tool performance. Br J Radiol. 2008;81(963):250–251. doi: 10.1259/bjr/12956156. [DOI] [PubMed] [Google Scholar]
- 27.Genovese CR, Lazar NA, Nichols TE. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15(4):870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- 28.Raji CA, Lopez OL, Kuller LH, Carmichael OT, Becker JT. Age, Alzheimer disease, and brain structure. Neurology. 2009;73 (22):1899–1905. doi: 10.1212/WNL.0b013e3181c3f293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y, Zhang J, Gutman B, Chan TF, Becker JT, Aizenstein HJ, Lopez OL, Tamburo RJ, Toga AW, Thompson PM. Multivariate tensor-based morphometry on surfaces: application to mapping ventricular abnormalities in HIV/AIDS. Neuroimage. 2009;49(3):2141–2157. doi: 10.1016/j.neuroimage.2009.10.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chiang MC, Dutton RA, Hayashi KM, Toga AW, Lopez OL, Aizenstein HJ, Becker JT, Thompson PM. 3D pattern of brain atrophy in HIV/AIDS visualized using tensor-based morphometry. Neuroimage. 2007;34(1):44–60. doi: 10.1016/j.neuroimage.2006.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lepore N, Brun CA, Chou YY, Chiang MC, Dutton RA, Hayashi KM, Lu A, Lopez OL, Aizenstein HJ, Toga AW, Becker JT, Thompson PM. Generalized tensor-based morphometry of HIV/AIDS usingmultivariate statistics on strain matrices and their application to HIV/AIDS. IEEE Trans Med Imaging. 2008;27(1):129–141. doi: 10.1109/TMI.2007.906091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Becker JT, Sanders J, Madsen SK, Ragin A, Kingsley L, Maruca V, Cohen B, Goodkin K, Martin E, Miller EN, Sacktor N, Alger JR, Barker PB, Saharan P, Carmichael OT, Thompson PM. Subcortical brain atrophy persists even in HAART-regulated HIV disease. Brain Imaging Behav. 2011 doi: 10.1007/s11682-011-9113-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wiley CA, Masliah E, Morey M, Lemere C, DeTeresa R, Grafe M, Hansen L, Terry R. Neocortical damage during HIV infection. Ann Neurol. 1991;29:651–657. doi: 10.1002/ana.410290613. [DOI] [PubMed] [Google Scholar]
- 34.Cysique LAJ, Maruff P, Brew BJ. Antiretroviral therapy in HIV infection. Arch Neurol. 2004;61:1699–1704. doi: 10.1001/archneur.61.11.1699. [DOI] [PubMed] [Google Scholar]
- 35.Navia BA, Gonzalez RG. Neuroimaging of AIDS II: functional imaging of the AIDS Dementia Complex and the metabolic pathology of the HIV1-infected brain. Neuroimaging Clin N Am. 1997;7(3):431–445. [PubMed] [Google Scholar]
- 36.Olsen WL, Longo FM, Mills CM, Norman D. White matter disease in AIDS: findings at MR imaging. Radiology. 1988;169:445–448. doi: 10.1148/radiology.169.2.3174991. [DOI] [PubMed] [Google Scholar]
- 37.Marcus CD, Taylor-Robinson SD, Sargentoni J, Ainsworth JG, Frize G, Easterbrook PJ, Shaunak S, Bryant DJ. 1H MR spectroscopy of the brain in HIV-1-seropositive subjects: evidence for diffuse metabolic abnormalities. Metab Brain Dis. 1998;13(2):123–136. doi: 10.1023/a:1020609213664. [DOI] [PubMed] [Google Scholar]
- 38.Pomara N, Crandall DT, Choi SJ, Johnson G, Lim KO. White matter abnormalities in HIV-1 infection: a diffusion tensor imaging study. Psychiatry Res. 2001;106:15–24. doi: 10.1016/s0925-4927(00)00082-2. [DOI] [PubMed] [Google Scholar]
- 39.Grafton ST, Sumi SM, Stimac GK, Alvord EC, Shaw C, Nochlin D. Comparison of postmortem magnetic resonance imaging and neuropsthologic findings in the cerebral white matter. Arch Neurol. 1991;48:293–298. doi: 10.1001/archneur.1991.00530150061019. [DOI] [PubMed] [Google Scholar]
- 40.Kobari M, Meyer JS, Ichijo M, Oravez WT. Leukoaraiosis: correlation of MR and CT findings with blood flow, atrophy, and cognition. Am J Neuroradiol. 1990;11:273–281. [PMC free article] [PubMed] [Google Scholar]
- 41.Scheltens P, Barkhof F, Leys D, Wolters E, Ravid R, Kamphorst W. Histopathologic correlates of white matter changes on MRI in Alzheimer’s disease. Neurology. 1995;45:883–888. doi: 10.1212/wnl.45.5.883. [DOI] [PubMed] [Google Scholar]
- 42.Kuller LH, Lopez OL, Newman A, Beauchamp NJ, Burke G, Dulberg C, Fitzpatrick A, Fried L, Haan MN. Risk factors for dementia in the cardiovascular health cognition study. Neuroepidemiology. 2003;22(1):13–22. doi: 10.1159/000067109. [DOI] [PubMed] [Google Scholar]
- 43.Kuller LH, Longstreth WT, Arnold AM, Bernick C, Bryan N, Beauchamp NJ. White matter hyperintensity on cranial magnetic resonance imaging. Stroke. 2004;35:1821–1825. doi: 10.1161/01.STR.0000132193.35955.69. [DOI] [PubMed] [Google Scholar]
- 44.Bartzokis G, Cummings JL, Syltzer D, Henderson VM, Nuechterlein KH, Mintz J. White matter structural integrity in healthy aging adults and patients with Alzheimer disease. Arch Neurol. 2003;60:393–398. doi: 10.1001/archneur.60.3.393. [DOI] [PubMed] [Google Scholar]
- 45.Vernooij MW, Ikram MA, Vrooman HA, Wielopolski PA, Krestin GP, Hofman A, Niessen WJ, Van der Lugt A, Breteler MM. White matter microstructural integrity and cognitive function in a general elderly population. Arch Gen Psychiatry. 2009;66(5):545–553. doi: 10.1001/archgenpsychiatry.2009.5. 66/5/545[pii]10.1001/archgenpsychiatry.2009.5. [DOI] [PubMed] [Google Scholar]
- 46.Longstreth WT, Manolio TA, Arnold A, Burke GL, Bryan N, Jungreis CA, Enright PL, O’Leary D, Fried L. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. Stroke. 1996;27:1274–1282. doi: 10.1161/01.str.27.8.1274. [DOI] [PubMed] [Google Scholar]
- 47.Pantoni L, Leys D, Fazekas F, Longstreth WT, Inzitari D, Wallin A, Filippi M, Scheltens P, Erkinjuntti T, Hachinski V. Role of white matter lesions in cognitive impairment of vascular origin. Alzheimer Dis Assoc Disord. 1999;13(Suppl 3):S49–S54. [PubMed] [Google Scholar]
- 48.McArthur JC, Haughey N, Gartner S, Conant K, Pardo C, Nath A, Sacktor N. Human immunodeficiency virus-associated dementia: an evolving disease. J NeuroVirology. 2003;9:205–221. doi: 10.1080/13550280390194109. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.