Abstract
Vitamin deficiencies are common in patients with inflammatory bowel disease (IBD). Homocysteine (Hcys) is a thrombogenic amino acid produced from methionine (Met), and its increase in patients with IBD indicates a disruption of Met metabolism; however, the role of Hcys and Met metabolism in IBD is not well understood. We hypothesized that disrupted Met metabolism from a B-vitamin-deficient diet would exacerbate experimental colitis. Mice were fed a B6-B12-deficient or control diet for 2 wk and then treated with dextran sodium sulfate (DSS) to induce colitis. We monitored disease activity during DSS treatment and collected plasma and tissue for analysis of inflammatory tissue injury and Met metabolites. We also quantified Met cycle activity by measurements of in vivo Met kinetics using [1-13C-methyl-2H3]methionine infusion in similarly treated mice. Unexpectedly, we found that mice given the B-vitamin-deficient diet had improved clinical outcomes, including increased survival, weight maintenance, and reduced disease scores. We also found lower histological disease activity and proinflammatory gene expression (TNF-α and inducible nitric oxide synthase) in the colon in deficient-diet mice. Metabolomic analysis showed evidence that these effects were associated with deficient B6, as markers of B12 function were only mildly altered. In vivo methionine kinetics corroborated these results, showing that the deficient diet suppressed transsulfuration but increased remethylation. Our findings suggest that disrupted Met metabolism attributable to B6 deficiency reduces the inflammatory response and disease activity in DSS-challenged mice. These results warrant further human clinical studies to determine whether B6 deficiency and elevated Hcys in patients with IBD contribute to disease pathobiology.
Keywords: methionine, pyridoxal phosphate, methylation, transsulfuration
inflammatory bowel disease (IBD) is thought to be attributable to an abnormal immune response in a genetically susceptible individual triggered by the gut microbial flora and environmental factors, yet the role of nutrition is not clear in its pathology. Nutritional deficiencies are common in patients with IBD as a result of inflammation of the absorptive surfaces of the small intestine, loss of absorptive surface attributable to surgical resection, and reduced nutrient intake (reviewed in Ref. 22). Furthermore, some drugs used to reduce the amount of inflammation can come at a nutritional cost. For example, methotrexate, which is often used as an immunosuppressor, is a known folate metabolism inhibitor (4). Therefore, nutritional status of patients with IBD can be compromised because of active disease and treatment (15).
Patients with IBD are at a greater risk for thrombotic episodes than the general population. The first case report of thrombotic complications in a patient with IBD was published in 1967 (14). Since then, numerous reports have been published in adults and children, examining this risk (19, 31, 32, 34). Many of these reports examine the association of thrombotic events and homocysteine (Hcys), a nonprotein amino acid that is well known for its thrombogenic role in cardiovascular diseases (reviewed in Ref. 9). Animal models have shown that elevated Hcys has a variety of effects, including neurodegeneration (40), renal disease (48), and endothelial dysfunction (11).
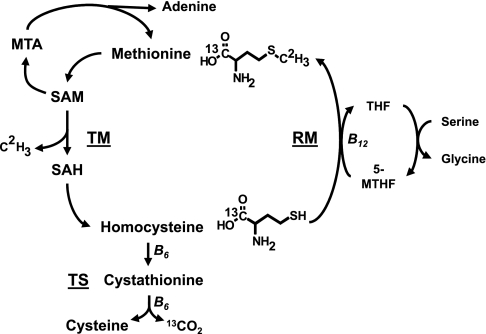
Hcys is an intermediate in the methionine (Met) metabolic cycle (Fig. 1). All cells have the ability to metabolize Met, which is converted via a series of enzymatic reactions to Hcys in a process known as transmethylation (TM). Furthermore, all cells can dispose of Hcys via remethylation (RM), where a methyl group is added to Hcys to generate more Met. However, only certain organs possess all of the enzymes necessary to dispose of Hcys via transsulfuration (TS), which ultimately results in the production of cysteine and α-ketobutyrate. Those include the liver, kidney, pancreas, and gastrointestinal tract (GIT) (30). Our group has shown that the GIT is an important site for both TM and TS, with ∼20% of dietary Met being metabolized by the GIT in piglets. This study also found that the GIT is a significant net producer of Hcys (33). Excess production of Hcys by cells can lead to an increase in circulating blood Hcys levels. Importantly, the B-vitamins folate, B6, and B12 are critical cofactors in Met metabolism. Folate is utilized as a methyl donor, and B12 is a required cofactor for RM via methionine synthase, whereas B6 is a required cofactor for two separate enzymatic reactions in the TS pathway.
Fig. 1.
Methionine (Met) metabolic cycle. Met is converted to homocysteine Hcys via a series of enzymatic reactions in a process known as transmethylation (TM). Met is first converted to S-adenosylmethionine (SAM). SAM has 2 possible fates: it is the major methyl donor for methylation reactions or it is used for polyamine synthesis. Upon its use for polyamines, SAM produces methylthioadenosine (MTA), whose disposal generates Met and adenine via a salvage pathway. When used as a methyl donor group, SAM forms S-adenosylhomocysteine (SAH). SAH is converted by a reversible reaction to Hcys, which can be disposed via 2 pathways. In remethylation (RM) via methionine synthase, 5-methyl-tetrahydrofolate (5-MTHF) donates the methyl group, and vitamin B12 is a required cofactor. Transsulfuration (TS) occurs only in a limited number of organs, and it disposes of Hcys via 2 enzymatic reactions that require vitamin B6 as a cofactor. The products of TS are cysteine and α-ketobutyrate, which enters into the tricarboxylic acid (TCA) cycle and ultimately produces CO2. This diagram also illustrates the isotopic tracer used for the infusion studies and their metabolic fate. The isotopomer used in this study was [1-13C; methyl-2H3]methionine. This is converted via TM to [1-13C]homocysteine. This labeled homocysteine can enter TS and ultimately produce 13CO2 via the TCA cycle or enter RM to form [1-13C]methionine (not shown). THF, tetrahydrofolate.
Numerous studies have found an association between elevated blood Hcys and increased risk of cardiovascular events in patients with IBD (reviewed in Ref. 6). Many of these studies also measured B-vitamin levels in patients in an attempt to link nutritional status and disrupted Met metabolism to elevated Hcys. However, the role of Hcys in IBD is still not completely understood, with reports offering conflicting results on the potential role of vitamins and therefore nutrition in the sequela of IBD. Furthermore, little is known about the kinetics of Met metabolism and how this is altered in patients with IBD. Finally, whether Hcys is a contributing factor or consequence of IBD also remains to be determined.
We hypothesized that disruption of Met metabolism as demonstrated by elevated Hcys negatively impacts the course of experimental colitis. To test this hypothesis, we utilized dietary B-vitamin deficiency to first induce elevated Hcys in mice and then subjected them to dextran sodium sulfate (DSS) to induce colitis; disease activity and severity were assessed using both macroscopic and molecular disease markers. We also used a targeted metabolomic approach and in vivo kinetic studies with a dual-labeled [1-13C-methyl-2H3]methionine tracer to quantify the Met cycle activity in response to B-vitamin deficiency and colitis.
MATERIALS AND METHODS
Animals and diets.
Six-week-old wild-type male C57BL/6 mice were purchased from Jackson Laboratory (Bar Harbor, ME) and maintained in standard group housing under a 12-h:12-h light/dark cycle. Mice were placed on one of two purified diets (Harlan Laboratories, Indianapolis, IN). The control diet (Con) was the AIN-93M mouse maintenance diet. The B-vitamin-deficient diet (Def) was a modified AIN-93M without vitamins B6 and B12 (diet composition described in Table S1; supplemental material for this article is available online at the American Journal of Physiology Gastrointestinal and Liver Physiology website); otherwise diet composition was identical. Mice were placed on diets for 2 wk before the start of the DSS regimen. The animal protocol was approved by the Animal Care and Use Committee of Baylor College of Medicine and was conducted in accordance with National Institutes of Health guidelines.
Induction of colitis and disease monitoring.
Mice within the diet groups (Con and Def) were further subdivided into with and without DSS. Orally administered DSS (molecular weight 36–50 kDa; MP Biomedicals, Solon, OH) induces a reproducible colitis and was administered ad libitum in the drinking water at 2.5–5% for 3–5 days. Mice were weighed every other day before the start of DSS and everyday during DSS administration. An established disease activity index (43) (DAI) was scored during days of DSS administration and used to monitor animal health. This index gives each mouse a score based on daily percentage of weight loss (0–3), stool consistency (0–2), presence of blood in stool (0–2), and appearance (normal, hunched, starey coat, lethargic scale, 0–3) with a max score of 10. Moribund animals or those with greater than 20% weight loss over a single day were euthanized before the end of the study.
After DSS administration, mice were anesthetized with isoflurane, and blood was collected via cardiac puncture; mice were then euthanized by isoflurane overdose. Anticoagulated blood was centrifuged to isolate plasma, which was snap frozen in liquid nitrogen. Liver was collected and snap frozen in liquid nitrogen for additional analysis as described below. Colon was collected and segmented into five equal sections. Sections 2 and 4 were fixed in 10% formalin for histological analysis. The remaining colon was collected together and snap frozen for further analysis.
Myeloperoxidase activity assay.
Myeloperoxidase (MPO) activity was measured using the method in Suzuki et al. (38) with modifications. Briefly, whole colon samples were homogenized in PBS and centrifuged at 20,000 g. The pellet fraction was then subject to an additional homogenization and centrifugation at 20,000 g in a buffer containing hexadecyltrimethylammonium bromide (Sigma-Aldrich; St. Louis, MO) to disrupt cell membranes (20). Supernatants were then assayed using a 96-well microplate reader (Molecular Devices, Sunnyvale, CA) for the colorimetric activity of tetramethylbenzidine (Sigma-Aldrich). Activity was calculated on the basis of the standard curve of human macrophage-derived MPO (Sigma-Aldrich) standards at activities of 5–100 mU/ml.
Histology.
Segments 2 and 4 of the colon were fixed in 10% formalin, embedded in paraffin, and cut into cross sections. These 4-μm-thick sections were stained with hematoxylin and eosin. Sections were scored using an established histological DAI (10) (hDAI) by a single individual blinded to the treatment groups. The hDAI gives each section a score based on the amount of inflammation (0–3), depth of injury (0–3), and amount of crypt damage (0–4). These numbers are added together and then multiplied by a number representing the percent of tissue involved (0–4) with a maximum score of 40 per section. Each animal had four to six sections, each containing 140 cm2 of mucosa scored, and these numbers were averaged to give each mouse a single hDAI score.
Tissue gene expression measurement.
Colonic RNA was isolated using a modified RNeasy protocol (Qiagen, Valencia, CA). These modifications included additional wash steps to remove DSS from the RNA sample, as DSS contamination is known to inhibit polymerase activity. The RNA was then treated with a DNase kit (Life Technologies, Carlsbad, CA) to remove any contaminating DNA. Quality and quantity were determined using a microsample UV spectrophotometer (Nanodrop; Thermo Fisher Scientific, Waltham, MA). We synthesized cDNA from 1.4 μg of RNA using the ABI High Capacity cDNA Reverse Transcription kit (Life Technologies) according to manufacturer's instructions and a PTC-200 thermocycler (Bio-Rad, Richmond, CA). Quantitative PCR was completed using a TaqMan Universal PCR Master Mix kit and TaqMan probes for TNF-α, inducible nitric oxide synthase (iNOS), IL-10, and GAPDH (Life Technologies) on an ABI 7900HT quantitative PCR system (Life Technologies). Data were normalized to GAPDH, and fold change was calculated using the ΔΔCT method.
Met cycle metabolomics.
Plasma, colon, and liver concentrations of Hcys, cysteine, and glutathione were quantified by reverse-phase HPLC with O-phthaldialdehyde derivatization using the method described previously (46, 47). Concentrations of adenosine, S-adenosylmethionine (SAM), S-adenosylhomocysteine (SAH), and methylthioadenosine (MTA) were measured by HPLC using the method described in Farrar and Clarke (12). Methionine concentrations in the plasma, colon, and liver were determined by reverse-phase HPLC of their phenyl isothiocyanate derivatives, as described by Stoll et al. (36). Measurement of vitamin B6 in its active form of pyridoxal-phosphate (PLP) in plasma and colon was performed using the method described in Ubbink et al. (41). B12 levels were not measured directly, but a panel of metabolites associated with B12 was analyzed on plasma and liver samples as described in Stabler et al. (35).
[1-13C; methyl-2H3]methionine infusion.
An additional group of 6-wk-old, male C57BL/6 mice (n = 16) subjected to a similar dietary and DSS study design were used to study the in vivo kinetics of Met metabolic pathway. Mice were singly housed in standard cages under a 12-h:12-h light/dark cycle and placed on their study diets (Con or Def, n = 8/diet). Mice were weighed every other day while in standard housing. After 19 days, mice were transferred to the comprehensive laboratory animal monitoring system (CLAMS) (Columbus Instruments, Columbus, OH). CLAMS allowed for the collection of expired 13CO2 during the infusion of labeled methionine.
After 1–2 days of acclimation in the CLAMS cages, a subset of mice in each diet group (n = 4/diet group) were given 3% DSS for 3 days. On day 4, mice were prepared for isotopic infusion by inserting a single catheter in the tail vein as described previously (27). Mice then received a 1-h priming bolus (60 μmol/kg) of [1-13C; methyl-2H3]methionine (98% APE; Cambridge Isotope Laboratories, Andover, MA) followed by a 4-h continuous infusion of [1-13C; methyl-2H3]methionine (60 μmol·kg−1·h−1). The total labeled Met given during the infusion was 1.148 mg per mouse. The Met infusate was prepared in a complete amino acid and glucose mixture and infused at a rate of 1.2 and 2.3 mg·kg−1·h−1, respectively. Total unlabeled Met given during the 4-h infusion was 2.52 mg/mouse; this provides sufficient Met to mimic the fed state in these mice. During the infusion, mice were restrained in Plexiglas restraint devices to prevent them from chewing or dislodging the catheter. Expired 13CO2 breath was collected in evacuated breath collection tubes (Labco Limited, Buckinghamshire, England) every 20 min during the entire 4-h infusion. At the end of the infusion, mice were anesthetized with isoflurane and blood was collected via cardiac puncture; mice were then euthanized by isoflurane overdose. Blood was centrifuged to isolate plasma, which was snap frozen in liquid nitrogen. Colon, small intestine, and liver were collected and snap frozen in liquid nitrogen for additional analysis as described below.
Mass spectrometry.
Plasma colon, small intestine, and liver isotopic enrichments of [1-13C;methyl-2H3]methionine, [1-13C]methionine and [1-13C]homocysteine were quantified on their heptafluorobutyric anhydride derivatives by gas chromatography-mass spectrometry (GC-MS). For plasma preparation, we used a modified method from Davis et al. (8). Briefly, plasma samples were pretreated with DTT (Sigma-Aldrich) and acidified with 10% trichloroacetic acid, and amino acids were separated by cation exchange (AG 50W-X8, 100–200 mesh, hydrogen form resin; Bio-Rad). The samples were again treated with DTT and derivatized with heptafluorobutyric acid (Pierce, Rockford, IL). Tissue samples were homogenized and deproteinized with 2 M perchloric acid (PCA), and the PCA-soluble (free pool) and acid-insoluble (protein-bound pool) fractions were prepared for mass spectrometric analysis as described previously (36).
Analysis was performed by negative chemical ionization on a GC-MS (model 6890N/5975B coupled with CTC=GC PAL autosampler; Agilent Technologies, Wilmington, DE) using a 60-m-long, 0.25-mm-ID column (0.5-μm film thickness, model HP-5ms; Agilent Technologies, Santa Clara, CA). Met and Hcys were run separately. The abundance of specific ions was determined by selected-ion monitoring at the following mass-to-charge ratios: methionine (367–368–371) and homocysteine (549–550). Isotopic enrichments were expressed as molar percent excess (MPE) of labeled to unlabeled isotopomer ratios [tracer-to-tracee ratios (TTR)] after correction for natural abundance and standard curves, where MPE = [TTR / (TTR + 1)] × 100, as defined by Wolfe and Chinkes (45). Expired breath 13CO2 enrichment was determined by gas isotope ratio mass spectrometry (44).
Whole body Met kinetics.
Plasma Met kinetics were calculated on the basis of the model of Storch et al. (37) using Hcys isotopic enrichments to correct methionine fluxes, as described by MacCoss et al. (23). Met carboxyl flux (Qc) refers to the rate of appearance of the Met carboxyl group from protein breakdown and the tracer infusion. However, plasma [1-13C]homocysteine enrichment reflects the intracellular Met enrichment because Met is transmethylated to Hcys within the cell compartment only. Thus whole body Qc (expressed in μmol·kg−1·h−1) was calculated using [1-13C]homocysteine enrichment as the intracellular [1-13C]methionine enrichment, as follows: Qc = IR × [(Etracer/EHcys) − 1], where IR is the [1-13C;methyl-2H3]methionine tracer infusion rate (μmol·kg−1·h−1) and Etracer and EHcys are the isotopic enrichments (expressed in MPE) of the infusate and [1-13C]homocysteine in the plasma, respectively. Met methyl flux (Qm) includes the same Met sources described for Qc, as well as the production of Met via RM of Hcys. Qm cannot be calculated using directly [1-13C]homocysteine enrichment for intracellular enrichment of [1-13C;methyl-2H3]methionine because the methyl group is released during TM and is not present in Hcys. However, the intracellular enrichment of the methyl group of Met with [1-13C]homocysteine enrichment can be estimated to calculate Qm (μmol·kg−1·h−1) as follows: Qm = IR × [(Etracer/Emethyl-Met) − 1] with Emethyl-Met = E4 × [EHcys/(E1 + E4)], where IR is the [1-13C;methyl-2H3]methionine tracer infusion rate (μmol·kg−1·h−1) and Etracer, Emethyl-Met, EHcys, E1, and E4 represent the isotopic enrichment (expressed in MPE) of the infusate, the methyl group of Met, [1-13C]homocysteine, [1-13C]methionine, and [1-13C;methyl-2H3]methionine in the plasma, respectively, as described by Jahoor et al. (18).
The following calculations were used for RM, TS, TM, and protein synthesis (PS) (μmol·kg−1·h−1): RM = Qm − Qc; TS = V13CO2 × [1/EHcys − 1/Etracer]; TM = RM + TS; PS = Qc − TS, where V13CO2 represents the 13CO2 produced from the oxidation of the Met tracer via the TS pathway.
Statistical analysis.
All data are expressed as means ± SE. Comparison among all groups was performed using two-way ANOVA to compare both diet and DSS effects with the exception of the weight change and DAI, which used a two-way ANOVA for diet and time. Survival curves were analyzed using Kaplan Meier. All analyses were completed using Minitab software (State College, PA). Statistical significance was defined as P < 0.05.
RESULTS
B-vitamin deficiency improved survival and reduced morbidity in DSS-treated mice.
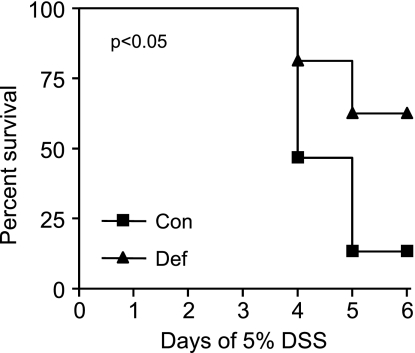
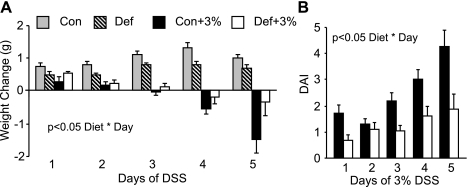
The first study conducted examined the impact of the B-vitamin-deficient diet and acute treatment with 5% DSS to induce colitis. By day 4, we observed mortality in DSS-treated mice in both the control and deficient diet groups, and we euthanized all mice on day 6. However, significantly more mice given the deficient diet survived compared those given the control diet (Fig. 2). We then repeated this study using an acute dose (5 days) of 3% DSS. This is the dose and length of DSS administration used for all remaining studies except the infusion study (3 days of 3% DSS). Figure 3A shows that control-diet mice given DSS (Con+3%) lost significantly more weight than the deficient-diet mice given DSS (Def+3%) when compared over all days. Calculation of the DAI showed that the Con+3% mice had significantly higher DAI scores than Def+3% mice when compared over all days (Fig. 3B).
Fig. 2.
Survival of mice treated with 5% dextran sodium sulfate (DSS) on control and deficient diets. Mice were given a control (n = 15) or vitamin B6-B12-deficient (n = 16) diet for 2 wk followed by 4 days of 5% DSS administered in the drinking water. Survival was measured until day 6 when all remaining animals were euthanized. A significantly greater proportion of the deficient-diet animals (62.5%) survived until day 6 compared with those given the control diet (13.3%) (P < 0.05).
Fig. 3.
Weight change and disease activity index (DAI) during 3% DSS. Mice were given the control (Con) or B6-B12-deficient diet (Def) for 2 wk followed by 5 days of 3% DSS. (Con n = 16, Def n = 10, Con+3% n = 20, Def+3% n = 20). A: daily weight loss was significantly greater in the Con+3% mice compared with Def+3% over all days (P < 0.05 Diet * Day). B: DAI was significantly lower in Def+3% compared with Con+3% (P < 0.05 Diet * Day).
Inflammation is reduced in deficient mice.
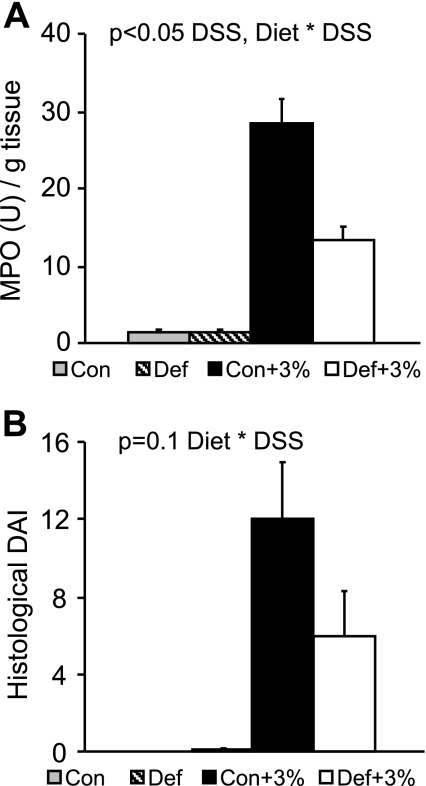
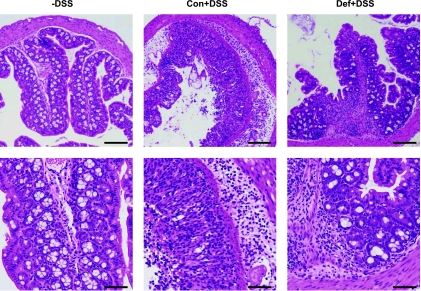
Colonic MPO was measured as an indicator of neutrophil infiltration and subsequent inflammation. As expected, we found that MPO was increased in animals treated with DSS (Con+3% and Def+3%) compared with untreated animals. However, MPO was significantly lower in the Def+3% mice compared with Con+3% (Fig. 4A). We scored histological damage using an established index (10) and found that Con and Def mice had no histological abnormalities, as expected (Fig. 4B). Administration of DSS caused a significant increase in hDAI scores, with the Def+3% mice tending to be lower (P = 0.1) compared with Con+3%. Histopathological images at low and high magnification are presented in Fig. 5.
Fig. 4.
Myeloperoxidase (MPO) and histological scoring for 3% DSS. Mice were given the control or B6-B12-deficient diet for 2 wk followed by 5 days of 3% DSS. After euthanasia, colons were collected for analysis of MPO activity (A) (Con n = 16, Def n = 10, Con+3% n = 20, Def+3% n = 19) and histological damage (B) (Con n = 10, Def n = 10, Con+3% n = 10, Def+3% n = 10). As expected, both MPO activity and histological scores were significantly higher in the DSS-treated groups than in the non-DSS controls (P < 0.05 DSS). For MPO, Def+3% was significantly lower compared Con+3% (P < 0.05 Diet * DSS). For histology, Def+3% tended to be lower than Con+3% (P = 0.1 Diet * DSS).
Fig. 5.
Histological images confirm human DAI scores. Hematoxylin and eosin-stained sections confirm that non-DSS animals have normal histological architecture and cytology and are not inflamed (Con and Def without DSS were identical and both are represented by the -DSS image); Con+3% had severe and diffuse destruction of the epithelial layer, neutrophil infiltration in both epithelium and lamina propria along with edema, affecting most of the tissue. Def+3% had focal inflammation in both the epithelium and lamina propria, but the tissue damage was much less severe. Scale bar = 100 μM.
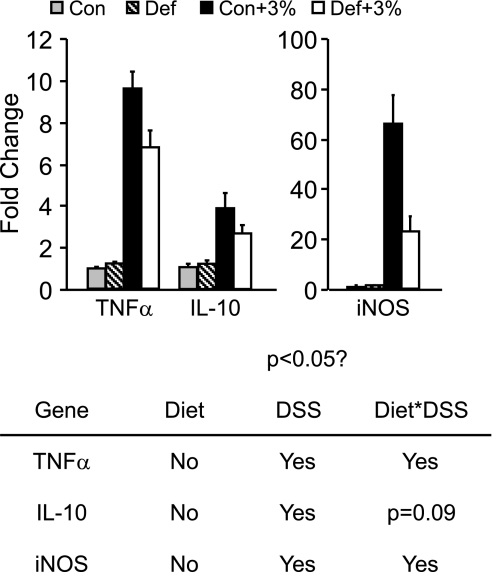
Deficient mice have blunted gene expression changes.
For the three genes measured, we found the expected increase of expression in DSS-treated groups compared with Con and Def (Fig. 6). For TNF-α, Con+3% mice had a 10-fold increase in expression compared with non-DSS-treated mice. Although we did see an increase in the Def+3% mice, this was 30% less than Con+3% mice. We saw the same results with iNOS: Con+3% mice had a 66-fold increase in message expression compared with non-DSS groups, whereas the Def+3% was 65% lower than the Con+3% group. Although IL-10 expression was significantly higher in both groups with DSS, its expression tended to be lower in the Def+3% compared with Con+3% (P = 0.09).
Fig. 6.
Gene expression changes. Mice were given the control or B6-B12-deficient diet for 2 wk followed by 5 days of 3% DSS. After euthanasia, colonic RNA was extracted for gene expression changes using TaqMan probes for real-time PCR (Con n = 10, Def n = 10, Con+3% n = 9, Def+3% n = 8). As expected, expression of TNF-α, inducible nitric oxide synthase (iNOS), and IL-10 were significantly increased in DSS mice compared with non-DSS controls (P < 0.05 DSS). Furthermore, TNF-α and iNOS were significantly lower in Def+3% compared with Con+3% (P < 0.05 Diet * DSS). IL-10 tended to be lower in the Def+3% compared with Con+3% (P = 0.09 Diet * DSS).
Metabolomic assessment of Met metabolism indicated that only B6 deficiency was achieved.
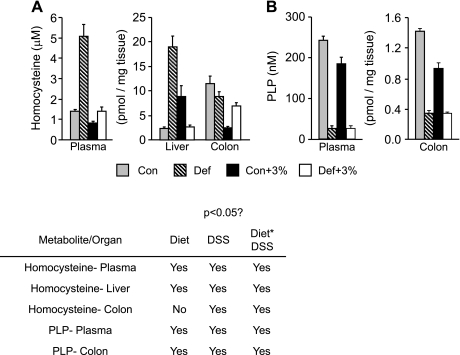
To assess the efficacy of our B-vitamin-deficient diet, we conducted targeted metabolomic profiling of a series of metabolites and vitamins associated with the Met cycle. As expected, plasma Hcys was significantly elevated in Def and Def+3% compared with Con and Con+3%, respectively (Fig. 7A). Similarly, liver Hcys showed marked elevation in Def mice compared with Con.
Fig. 7.
Changes in homocysteine and pyridoxal-phosphate (PLP) in plasma and tissues. Mice were given the control or B6-B12-deficient diet for 2 wk followed by 5 days of 3% DSS. A: Hcys levels were analyzed in plasma, colon, and liver (Con n = 10, Def n = 10, Con+3% n = 10, Def+3% n = 10). In the plasma, we saw a significant increase in Hcys in Def and Def+3% compared with Con and Con+3% (P < 0.05 Diet). In the liver, Hcys was significantly elevated in Def mice compared with Con (P < 0.05 Diet). In the colon, Hcys was similar in both Con and Def mice, and Def+3% was significantly higher than Con+3% (P < 0.05 Diet * DSS). B: PLP levels were analyzed in the plasma and colon (Con n = 10, Def n = 10, Con+3% n = 10, Def+3% n = 10). In both plasma and colon, we found a significant decrease in PLP in Def and Def+3% groups compared with controls (Con and Con+3%) (P < 0.05, Diet, DSS, Diet * DSS).
The enzymatic conversion of SAH to Hcys via SAH hydrolase is a reversible reaction (Fig. 1). Under normal conditions, the removal of Hcys is highly efficient, so the reaction favors hydrolysis to form Hcys; however, accumulation of excess Hcys can reverse the flux of this reaction to favor accumulation of SAH. Shown in Table 1, we found in the colon and liver that SAH was significantly higher in Def and Def+3% compared with Con and Con+3%. In plasma, SAH was below the detection limits of our method. Because SAM is the major methyl donor in the body, the ratio of SAM to SAH (SAM:SAH) is an indicator of methylation potential. We found a significant reduction in the SAM:SAH ratio in Def and Def+3% compared with Con and Con+3% in the colon and the liver.
Table 1.
Concentration of methionine metabolites in tissue
| Colon |
Liver |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Con | Def | Con+3% | Def+3% | P < 0.05 | Con | Def | Con+3% | Def+3% | P < 0.05 | |
| Methionine | 615 ± 24 | 472 ± 28 | 723 ± 30 | 785 ± 47 | †‡ | |||||
| SAM | 25.9 ± 2.6 | 24.3 ± 1.1 | 21.8 ± 1.4 | 26.4 ± 2.9 | n/s | 47.1 ± 2.4 | 26.4 ± 2.5 | 55.6 ± 2.4 | 41.0 ± 3.7 | *† |
| SAH | 2.8 ± 0.2 | 6.3 ± 0.7 | 2.8 ± 0.2 | 4.8 ± 0.6 | * | 46.0 ± 4.2 | 93.6 ± 4.5 | 29.0 ± 1.9 | 53.2 ± 5.0 | *†‡ |
| Cystathionine | 16.5 ± 1.9 | 87.8 ± 33.9 | 17.9 ± 1.2 | 83.6 ± 11.0 | * | |||||
| Methylthioadenosine | 3.0 ± 0.1 | 2.5 ± 0.2 | 4.3 ± 0.1 | 3.1 ± 0.1 | *†‡ | 3.9 ± 0.2 | 3.8 ± 0.1 | 3.8 ± 0.2 | 4.0 ± 0.1 | n/s |
| Adenosine | 135 ± 16 | 125 ± 15 | 109 ± 21 | 107 ± 27 | n/s | 158 ± 10 | 181 ± 10 | 156 ± 10 | 183 ± 4 | * |
| Methyl malonic acid | 4.1 ± 0.3 | 2.8 ± 0.4 | 2.1 ± 0.3 | 3.2 ± 0.2 | †‡ | |||||
| SAM:SAH | 9.5 ± 1.3 | 4.2 ± 0.5 | 8.1 ± 0.8 | 6.0 ± 0.8 | *‡ | 1.2 ± 0.2 | 0.3 ± 0.04 | 2.0 ± 0.2 | 0.9 ± 0.2 | *† |
Values are means ± SE (n = 8–10/group) and pmol/mg tissue. SAM, S-adenosylmethionine; SAH, S-adenosylhomocysteine; Con, control diet; Def, deficient diet; Con+3%, control animals treated with dextran sodium sulfate (DSS); Def+3%, deficient-diet animals treated with DSS. Colonic methionine concentration: nmol/mg tissue. P < 0.05.
Diet,
DSS,
Diet×DSS.
Elevated cystathionine levels are indicative of B6 deficiency. Cystathionine was significantly higher in the deficient diet groups compared with control diet groups in liver and plasma (Table 2). To directly assess vitamin B6, we measured PLP (the active form of B6) and found that the PLP levels were significantly reduced in Def and Def+3% compared with Con and Con+3% in colon and plasma (Fig. 7B). Instead of measuring B12 directly, we measured a panel of metabolites influenced by B12, which included methylmalonic acid (MMA). MMA is formed via an enzymatic reaction that converts methylmalonyl-CoA to succinyl-CoA and requires B12 as a cofactor. When B12 is deficient, methylmalonyl-CoA is converted to MMA that is released into the circulation. In our samples, we found that plasma MMA was slightly but significantly higher in the Def and Def+3% mice compared with Con and Con+3%. MMA results in the liver were inconsistent. Glycine is produced as a result of the donation of serine of a methyl group to folate in RM (Fig. 1). We found glycine concentrations in the plasma elevated in Def and Def+3% mice compared with Con and Con+3% (Supplemental Table S2).
Table 2.
Concentration of methionine metabolites in plasma
| Plasma |
|||||
|---|---|---|---|---|---|
| Con | Def | Con +3% | Def +3% | P < 0.05 | |
| Methionine, μmol/l | 69.3 ± 4.8 | 61.6 ± 6.5 | 59.9 ± 2.8 | 61.4 ± 6.3 | n/s |
| Cystathionine, μmol/l | 0.7 ± 0.04 | 6.5 ± 0.6 | 0.7 ± 0.04 | 2.2 ± 0.2 | *†‡ |
| Methyl malonic acid, nmol/l | 421 ± 12 | 461 ± 25 | 415 ± 12 | 453 ± 16 | * |
| Methylthioadenosine, μmol/l | 11.1 ± 0.4 | 9.9 ± 0.4 | 7.2 ± 0.2 | 8.4 ± 0.6 | †‡ |
Values are means ± SE (n = 10/group). P < 0.05
Diet,
DSS,
Diet×DSS.
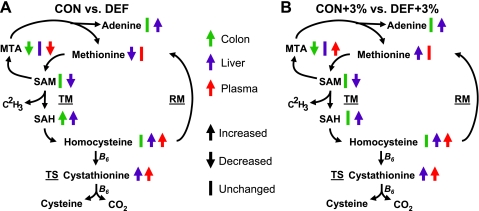
Colonic Met was significantly higher in the Def mice compared with Con; however, this effect was lost in the mice given DSS; liver Met was significantly higher in Con+3% and Def+3% compared with Con and Def. Plasma Met was not different among the groups. Adenosine was significantly higher in Def and Def+3% mice compared with Con and Con+3% in the liver and showed no significant changes in the colon. In the colon, MTA was significantly higher in Con and Con+3% compared with Def and Def+3% and higher in Con+3% and Def+3% compared with non-DSS controls (Con and Def). Liver MTA was unchanged among the groups, whereas DSS significantly reduced plasma MTA in Con+3% and Def+3%. Other Met metabolites and compounds related to vitamins B6 and B12 showed mixed results and are detailed in Supplemental Tables S2 and S3. A summary of the changes in Met cycle metabolites in all four treatment groups is summarized in Figure 8 (8).
Fig. 8.
Summary of metabolomic results. An abbreviated version of the Met metabolic cycle representing the changes seen in colon (green), liver (purple), and plasma (red) with our deficient-diet studies. Arrows represent significant changes between control and deficient (A) or untreated and DSS (B) conditions.
The B-vitamin-deficient diet had marginal effects on Met metabolic cycling.
We calculated the Met flux in the whole body and target tissues in mice under the various treatment conditions (Diet, DSS) using a 4-h, continuous intravenous infusion of [1-13C;methyl-2H3]methionine (M +4 Met). [1-13C]homocysteine (M + 1 Hcys) is formed by the TM of the parent isotopomer [1-13C;methyl-2H3]methionine. In addition, the [1-13C]methionine (M + 1 Met) is formed by the RM of M + 1 Hcys. In the plasma pool, we found no change in the isotopic enrichment of M + 4 Met or the M + 1 Hcys (Table 3). The M + 1 Met was significantly higher in the Def and Def+3% mice compared with Con and Con+3%. In the colon, we found a significant reduction in the M + 4 Met in both groups treated with DSS (Con+3% and Def+3%). Similar to the plasma, the M + 1 Met was higher in the Def and Def+3%, with both DSS-treated groups (Con+3% and Def+3%) significantly less than the untreated groups (Con and Def). Colonic M + 1 Hcys was unchanged. In the liver, M + 4 Met was higher in Def and Def+3% compared with Con and Con+3%. M + 1 Met was again higher in the Def and Def+3% mice compared with Con and Con+3%, and M + 1 Hcys was not different among the groups. In the small intestine, M + 4 and M + 1 Met were unchanged among the groups. M + 1 Hcys was significantly higher in Con and Con+3% compared with Def and Def+3%.
Table 3.
Steady-state isotopic enrichment in plasma and tissue
| Con | Def | Con +3% | Def +3% | P < 0.05 | |
|---|---|---|---|---|---|
| Plasma | |||||
| [1-13C;methyl-2H3]methionine (M +4 Met) | 19.3 ± 1.2 | 21.0 ± 0.6 | 19.3 ± 0.4 | 19.8 ± 0.7 | |
| [1-13C]methionine (M +1 Met) | 2.9 ± 0.3 | 3.6 ± 0.2 | 2.4 ± 0.4 | 3.4 ± 0.1 | * |
| [1-13C]homocysteine (M +1 Hcys) | 21.5 ± 1.1 | 22.1 ± 0.9 | 22.7 ± 0.6 | 22.5 ± 0.7 | |
| Colon | |||||
| [1-13C;methyl-2H3]methionine (M +4 Met) | 7.4 ± 0.4 | 8.2 ± 0.6 | 5.8 ± 0.7 | 5.8 ± 0.5 | † |
| [1-13C]methionine (M +1 Met) | 1.2 ± 0.02 | 1.7 ± 0.1 | 1.1 ± 0.1 | 1.4 ± 0.1 | *† |
| [1-13C]homocysteine (M +1 Hcys) | 10.2 ± 0.4 | 10.3 ± 0.5 | 9.4 ± 0.5 | 9.5 ± 0.4 | |
| [1-13C]Met/[1-13C]Hcys | 0.1 ± 0.01 | 0.2 ± 0.01 | 0.1 ± 0.01 | 0.3 ± 0.01 | * |
| Liver | |||||
| [1-13C;methyl-2H3]methionine (M +4 Met) | 5.2 ± 0.2 | 7.8 ± 0.7 | 4.4 ± 0.2 | 5.9 ± 0.5 | *† |
| [1-13C]methionine (M +1 Met) | 2.2 ± 0.2 | 3.0 ± 0.1 | 2.3 ± 0.1 | 3.2 ± 0.3 | * |
| [1-13C]homocysteine (M +1 Hcys) | 8.1 ± 0.5 | 8.5 ± 0.7 | 7.8 ± 0.6 | 8.8 ± 0.6 | |
| [1-13C]Met/[1-13C]Hcys | 0.3 ± 0.04 | 0.4 ± 0.04 | 0.3 ± 0.1 | 0.4 ± 0.03 | † |
| Small Intestine | |||||
| [1-13C;methyl-2H3]methionine (M +4 Met) | 2.6 ± 0.2 | 4.6 ± 1.1 | 2.9 ± 0.6 | 3.2 ± 1.3 | |
| [1-13C]methionine (M +1 Met) | 0.5 ± 0.2 | 0.9 ± 0.3 | 0.4 ± 0.03 | 0.7 ± 0.2 | |
| [1-13C]homocysteine (M +1 Hcys) | 9.2 ± 0.7 | 6.8 ± 0.8 | 8.4 ± 0.7 | 7.7 ± 0.7 | * |
| [1-13C]Met / [1-13C]Hcys | 0.06 ± 0.02 | 0.1 ± 0.03 | 0.1 ± 0.01 | 0.1 ± 0.03 | * |
Data are means ± SE (n = 3–4/group). Met, methionine; Hcys, homocysteine. P < 0.05.
Diet,
DSS.
By comparing the ratio of the isotopic enrichment of M + 1 Met/M + 1 Hcys in the tissues, we estimate an index of the fractional RM rate. In the colon and small intestine, Def and Def+3% had a significantly higher fractional RM rate than Con and Con+3%, regardless of DSS status. A similar trend was seen in the liver, but it was not significant.
Whole body RM was higher in deficient-diet animals.
Whole body Met kinetics were calculated using Hcys enrichments as the precursor for Met metabolism. There was no difference in either methyl (Qm) or carboxyl (Qc) group fluxes among the groups (Table 4). We found a significant reduction in the rates of TM and TS in the Def and Def+3% mice compared with Con and Con+3%. Consistent with the changes we saw in the tissues, we found whole body RM was increased in Def and Def+3% compared with Con and Con+3%. We also found a significantly higher PS rate in Def and Def+3% mice compared with Con and Con+3% (Table 4).
Table 4.
Whole body methionine kinetics
| Con | Def | Con +3% | Def +3% | P < 0.05 | |
|---|---|---|---|---|---|
| Qm | 259 ± 18 | 257 ± 12 | 234 ± 8 | 242 ± 5 | n/s |
| Qc | 218 ± 15 | 210 ± 11 | 202 ± 7 | 197 ± 6 | n/s |
| TM | 124 ± 3 | 94 ± 3 | 104 ± 1 | 98 ± 2 | *†‡ |
| TS | 83 ± 3 | 48 ± 4 | 71 ± 6 | 53 ± 3 | * |
| RM | 41 ± 4 | 47 ± 3 | 33 ± 5 | 45 ± 2 | * |
| TM/Qm | 0.5 ± 0.03 | 0.4 ± 0.02 | 0.4 ± 0.01 | 0.4 ± 0.02 | † |
| TS/Qm | 0.3 ± 0.02 | 0.2 ± 0.02 | 0.3 ± 0.03 | 0.2 ± 0.02 | * |
| RM/TM | 0.3 ± 0.03 | 0.5 ± 0.03 | 0.3 ± 0.1 | 0.5 ± 0.02 | * |
| TS/TM | 0.7 ± 0.03 | 0.5 ± 0.03 | 0.7 ± 0.1 | 0.5 ± 0.02 | * |
| PS | 135 ± 15 | 162 ± 11 | 130 ± 8 | 145 ± 2 | * |
Values (μmol·kg−1·h−1) are means ± SE (n = 3–4/group). Rates are calculated using Hcys isotopic enrichments. Qm, methionine methyl flux; Qc, methionine carboxyl flux; TM, transmethylation; TS, transsulfuration; RM, remethylation; PS, protein synthesis. P < 0.05.
Diet,
DSS,
Diet×DSS.
The fractional rate of TM compared with Met whole body flux (TM/Qm) was reduced significantly in the DSS-treated animals (Con+3% and Def+3%) compared with non-DSS groups (Con and Def). The fractional rate of TS compared with whole body Met flux (TS/Qm) was significantly lower in Def and Def+3% mice compared with Con and Con+3%, regardless of their DSS status. Of the Met metabolized by TM, the fraction remethylated to form additional Met (RM/TM) was significantly higher in Def and Def+3% mice compared with Con and Con+3%. The fraction metabolized by TS (TS/TM) is significantly lower in the Def and Def+3% mice compared with Con and Con+3%.
DISCUSSION
Many groups have reported increased risk of thrombotic complications in both adult and pediatric patients with IBD (19, 31, 32, 34). This increased risk is associated with increased plasma and tissue Hcys levels (7). Hcys is a nonprotein amino acid formed during the Met metabolic cycle and is known to have thrombotic effects in cardiovascular disease (9). Elevated Hcys levels suggest that disruption of Met metabolism is occurring in patients with IBD. However, the role of Hcys and disrupted Met metabolism changes in IBD are not well understood. Therefore, we hypothesized that disruption of Met metabolism via elevated Hcys will negatively impact the course of experimental colitis. Using a diet-induced model of elevated Hcys, we administered DSS to induce colitis. Unexpectedly, mice given a B6/B12-vitamin-deficient diet experienced a significantly lower mortality than those fed the control diet when colitis was induced acutely with 5% DSS. We further observed that, during acute 3% DSS treatment, deficient-diet mice experienced less weight loss and lower DAI scores compared with control mice, indicating that B-vitamin deficiency was protective in this model of colitis. Thus our study indicates that, at least in mice, elevated Hcys was not associated with increased tissue injury, but rather with improved clinical outcome. These results do not support our hypothesis and suggest that increased Hcys levels reported in patients with IBD compared with control subjects may not contribute to the inflammatory response (7).
Our clinical and histopathological measurements of disease activity were consistent and confirmed that DSS predictably induced morbidity and colitis in control-diet mice and that B6 deficiency was protective against colitis. Colonic MPO activity was markedly increased by DSS treatment in both diet groups, but this increase was reduced by nearly half in Def+3% compared with Con+3%, indicating that deficient diet led to reduced neutrophil infiltration and subsequent inflammation. Similar results were seen in our histopathology; however, these results were not statistically significant (P = 0.10) because of a large variation in hDAI scores. Likewise, we found that proinflammatory cytokine gene expression (TNF-α and iNOS) was significantly increased by DSS treatment and blunted in the Def+3% mice compared with Con+3%. Thus clinical and tissue endpoints of inflammation suggest that the deficient diet reduced the inflammatory response to the DSS challenge.
We performed metabolomic profiling of plasma and tissue samples to assess whether the B-vitamin-deficient diet caused a measurable perturbation in Met metabolism. Plasma and liver Hcys were elevated in the Def-diet mice compared with Con. As the reaction that forms Hcys from SAH is readily reversible, SAH will also accumulate when Hcys is in excess. In both colon and liver, the deficient-diet animals had significantly higher SAH concentrations than animals given the control diet, regardless of their DSS status; there was also a significant decrease in the SAM:SAH ratio in deficient-diet mice. Cystathionine is an intermediate in Hcys disposal by TS. Cystathionine was elevated in both plasma and liver in the deficient-diet mice compared with controls regardless of DSS status; all of these data indicate B6 deficiency. Our direct measure of PLP (the active form of B6) confirmed this, with the deficient-diet mice having significantly lower PLP compared with control-diet animals in both the colon and plasma. We also measured a panel of B12-associated metabolites to assess B12 deficiency and found that MMA was significantly higher in the plasma but not in the liver. Furthermore, the increase in glycine we measured indicates an increase in RM in deficient-diet mice. These results suggest that our diet induced robust B6 but not B12 deficiency.
The B6-B12-deficient diet we used was a modified version of the diet described in Troen et al. (40). Their diet was B6-B12-folate deficient and included antibiotics to eliminate the production of vitamins from the microbiota. We decided to include folate in our diet to avoid the pleiotropic effects of folate deficiency. Furthermore, we did not include antibiotics because the present etiology for IBD includes a role for the microbiota, and we felt this might also confound our results. Interestingly, reports in the literature using this diet indicate a wide range of effectiveness. Groups have administered this diet (B6-B12-folate) with and without antibiotics for 6–8 wk in mice and reported Hcys levels ranging from 2.4 μM to 250 μM (13, 17, 21, 39, 40). The variation seems to be explainable by the inclusion of antibiotic in the diet, highlighting the importance of the microbiota in micronutrient production. It is possible that, when faced with a shortage of B12 in the diet, the absorption of resident microbial-derived B12 increases. We may have seen a greater effect of the diet if it had been administered longer or we had included antibiotics. However, our results show that the B6 deficiency perturbed Met metabolism, and this provided protection against DSS colitis.
Our measurements of Met kinetics confirmed that the deficient diet caused disruption of Met cycle activity via B6 deficiency. The whole body TS rate was significantly lower in the deficient-diet mice compared with controls, where only 50% of the Hcys produced was metabolized via TS in the deficient mice compared with nearly 70% in control mice. In agreement with our B12 analysis, we discovered that whole body RM was significantly higher in deficient-diet animals compared with controls. We examined the ratio of M + 1 Met to M + 1 Hcys in the tissues as an indicator of Hcys disposed via RM. In the colon and small intestine, we found that significantly more Hcys was metabolized via RM in deficient-diet animals compared with controls. Given the robust deficiency of B6 and minimal effect on B12, it is not surprising that RM was increased to compensate for the loss of Hcys disposal via TS.
Another interesting observation was that whole body TS was not increased in DSS-treated mice on either diet group. We expected that TS would be increased in the Con+3% animals to produce more cysteine and glutathione to combat oxidative stress, on the basis of previous reports in animals subjected to inflammation (24–26). We did find increased colonic cysteine concentration in both groups given DSS, but this must be due to protein breakdown because the rate of methionine TS (measured by TS/TM) was unchanged with DSS in both of the diet groups. However, DSS-induced colitis did markedly reduce the whole body TM rate, indicating that inflammation decreased Met catabolism via the Met cycle, making more Met available for protein synthesis.
The results of this study were surprising, as our diet-induced B6 deficiency would not be expected to be beneficial, especially in an individual with IBD. In models of alcoholic liver disease and multiple sclerosis, studies have shown that the Met metabolite MTA is capable of reducing the inflammatory insult initiated after disease onset (1, 16, 28). Indeed, we did see small increases in MTA production in the colon of DSS-treated mice in both diet groups compared with their respective non-DSS controls. In liver disease models, MTA has been shown to provide anti-inflammatory effects via changes in methylation of histones associated with proinflammatory genes (2). Perhaps the increase in MTA secondary to Met cycle derangement is changing histone methylation patterns. Our gene expression data support this hypothesis, as TNF-α and iNOS were significantly reduced in Def+3% compared with Con+3%. However, it is also known that SAH is an inhibitor of methyltransferase activity (42), so the elevated SAH concentration and decreased SAM:SAH ratio we observed in our deficient mice may alter DNA or protein methylation. Furthermore, SAH can be disposed of via an alternative pathway in which it is converted to ribosyl-l-homocysteine, releasing water and adenine, which can form adenosine. This is of interest because adenosine accumulation is thought to be the primary mediator of the anti-inflammatory effects of methotrexate (5). Others have shown that both adenosine and methotrexate have similar anti-inflammatory actions, such as reducing the adhesion of leukocytes to endothelial cells and mediating neutrophil superoxide production (3). We did see a significant increase in liver adenosine in the deficient diet mice compared with controls, as well as the reduction in MPO activity, a measure of neutrophil infiltration.
One limitation of this study is the use of the DSS model. DSS induces a chemical injury in the colon in a reproducible and predictable manner, but this inflammatory response only involves the innate immune system. Human IBD is a chronic, relapsing condition that involves both B and T cell responses as well as the innate immune system. Therefore, the DSS model does not mimic the physiological responses seen in human IBD. However, the model is useful for initial investigations such as the one described here. It is unclear whether similar benefit would be seen in other mouse models of IBD. As this report is the first to examine B-vitamin deficiency in the context of IBD, the use of the DSS model was an acceptable first step. It would be interesting and relevant to repeat this study in other models to confirm our findings, which could have significant impact on patient management. One final consideration is that the affect seen in the deficient mice may be attributable to changes in wound severity, not inflammation. We did find a nonsignificant reduction in histological scores in the deficient mice compared with controls. Studies of the recovery phase after withdrawal of DSS would provide further insight as to the mechanism of action.
In conclusion, we found that short-term feeding of a B-vitamin-deficient diet in mice induced B6 deficiency, which provided protection against DSS-induced morbidity and mortality. This was demonstrated clinically by 1) reduced mortality during acute 5% DSS, 2) improved weight maintenance, and 3) reduced DAI scores during acute 3% DSS. Molecular analysis confirmed our clinical results, with Def+3% mice having lower MPO activity as well as blunted upregulation of the proinflammatory genes TNF-α and iNOS. Together, these results indicate a reduction in the inflammatory response in these deficient-diet mice compared with controls. We confirmed the effect of our deficient diet by measuring Met cycle metabolites. Specifically, Hcys, SAH, and cystathionine were significantly elevated in deficient-diet mice, whereas PLP was significantly reduced; MMA was only changing slightly. Isotopic studies found that most of the disruption occurred in the TS pathway, with RM higher in deficient-diet mice compared with controls. These results agreed with our HPLC results and confirmed that our mice had robust B6 but not B12 deficiency. Perhaps with longer administration of our diet or use of an antibiotic to eliminate the resident microbiota, we could have achieved a more robust B12 deficiency. Furthermore, the mechanism by which this protective effect occurs is unknown. Our metabolite analysis indicates three possible mechanisms of action, all of which warrant further exploration. This paper highlights the need for further study into the relationship between B-vitamin status and IBD in physiologically relevant models.
GRANTS
This work was supported by federal funds from the U.S. Department of Agriculture, Agricultural Research Service under Cooperative Agreement Number 58-6250-6-001, and the Texas Medical Center Digestive Diseases Center (NIH Grant P30 DK-56338).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
Supplementary Material
ACKNOWLEDGMENTS
The authors acknowledge Li Wei Cui, Xiaoyan Chang, Inka Cajo-Didelija, Patrycja Puiman, Caroline Bauchart-Thevret, and the staff of the Gulf Coast Digestive Disease Center Cellular and Molecular Morphology Core for technical assistance. This work is a publication of the USDA/ARS Children's Nutrition Research Center, Department of Pediatrics, Baylor College of Medicine and Texas Children's Hospital, Houston, TX. The contents of this publication do not necessarily reflect the views or policies of the U. S. Department of Agriculture, nor does mention of trade names, commercial products, or organizations imply endorsement by the U. S. Government.
REFERENCES
- 1. Ansorena E, Garcia-Trevijano ER, Martinez-Chantar ML, Huang ZZ, Chen L, Mato JM, Iraburu M, Lu SC, Avila MA. S-adenosylmethionine and methylthioadenosine are antiapoptotic in cultured rat hepatocytes but proapoptotic in human hepatoma cells. Hepatology 35: 274–280, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Ara AI, Xia M, Ramani K, Mato JM, Lu SC. S-adenosylmethionine inhibits lipopolysaccharide-induced gene expression via modulation of histone methylation. Hepatology 47: 1655–1666, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Asako H, Wolf RE, Granger DN. Leukocyte adherence in rat mesenteric venules: effects of adenosine and methotrexate. Gastroenterology 104: 31–37, 1993 [DOI] [PubMed] [Google Scholar]
- 4. Chan ES, Cronstein BN. Molecular action of methotrexate in inflammatory diseases. Arthritis Res Ther 4: 266–273, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cronstein BN. Low-dose methotrexate: a mainstay in the treatment of rheumatoid arthritis. Pharmacol Rev 57: 163–172, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Danese S, Papa A, Saibeni S, Repici A, Malesci A, Vecchi M. Inflammation and coagulation in inflammatory bowel disease: The clot thickens. Am J Gastroenterol 102: 174–186, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Danese S, Sgambato A, Papa A, Scaldaferri F, Pola R, Sans M, Lovecchio M, Gasbarrini G, Cittadini A, Gasbarrini A. Homocysteine triggers mucosal microvascular activation in inflammatory bowel disease. Am J Gastroenterol 100: 886–895, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Davis SR, Stacpoole PW, Williamson J, Kick LS, Quinlivan EP, Coats BS, Shane B, Bailey LB, Gregory JF., 3rd Tracer-derived total and folate-dependent homocysteine remethylation and synthesis rates in humans indicate that serine is the main one-carbon donor. Am J Physiol Endocrinol Metab 286: E272–E279, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Di Minno MN, Tremoli E, Coppola A, Lupoli R, Di Minno G. Homocysteine and arterial thrombosis: Challenge and opportunity. Thromb Haemost 103: 942–961, 2010 [DOI] [PubMed] [Google Scholar]
- 10. Dieleman LA, Palmen MJ, Akol H, Bloemena E, Pena AS, Meuwissen SG, Van Rees EP. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol 114: 385–391, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eberhardt RT, Forgione MA, Cap A, Leopold JA, Rudd MA, Trolliet M, Heydrick S, Stark R, Klings ES, Moldovan NI, Yaghoubi M, Goldschmidt-Clermont PJ, Farber HW, Cohen R, Loscalzo J. Endothelial dysfunction in a murine model of mild hyperhomocyst(e)inemia. J Clin Invest 106: 483–491, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Farrar C, Clarke S. Altered levels of S-adenosylmethionine and S-adenosylhomocysteine in the brains of L-isoaspartyl (D-Aspartyl) O-methyltransferase-deficient mice. J Biol Chem 277: 27856–27863, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Fuso A, Nicolia V, Cavallaro RA, Ricceri L, D'Anselmi F, Coluccia P, Calamandrei G, Scarpa S. B-vitamin deprivation induces hyperhomocysteinemia and brain S-adenosylhomocysteine, depletes brain S-adenosylmethionine, and enhances PS1 and BACE expression and amyloid-beta deposition in mice. Mol Cell Neurosci 37: 731–746, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Harrison MJ, Truelove SC. Cerebral venous thrombosis as a complication of ulcerative colitis. Am J Dig Dis 12: 1025–1028, 1967 [DOI] [PubMed] [Google Scholar]
- 15. Hartman C, Eliakim R, Shamir R. Nutritional status and nutritional therapy in inflammatory bowel diseases. World J Gastroenterol 15: 2570–2578, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hevia H, Varela-Rey M, Corrales FJ, Berasain C, Martinez-Chantar ML, Latasa MU, Lu SC, Mato JM, Garcia-Trevijano ER, Avila MA. 5'-Methylthioadenosine modulates the inflammatory response to endotoxin in mice and in rat hepatocytes. Hepatology 39: 1088–1098, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Hofmann MA, Lalla E, Lu Y, Gleason MR, Wolf BM, Tanji N, Ferran LJ, Jr, Kohl B, Rao V, Kisiel W, Stern DM, Schmidt AM. Hyperhomocysteinemia enhances vascular inflammation and accelerates atherosclerosis in a murine model. J Clin Invest 107: 675–683, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jahoor F, Badaloo A, Reid M, Forrester T. Sulfur amino acid metabolism in children with severe childhood undernutrition: methionine kinetics. Am J Clin Nutr 84: 1400–1405, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Koutroubakis IE. Role of thrombotic vascular risk factors in inflammatory bowel disease. Dig Dis 18: 161–167, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Krawisz JE, Sharon P, Stenson WF. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology 87: 1344–1350, 1984 [PubMed] [Google Scholar]
- 21. Lalonde R, Barraud H, Ravey J, Gueant JL, Bronowicki JP, Strazielle C. Effects of a B-vitamin-deficient diet on exploratory activity, motor coordination, and spatial learning in young adult Balb/c mice. Brain Res 1188: 122–131, 2008 [DOI] [PubMed] [Google Scholar]
- 22. Lucendo AJ, De Rezende LC. Importance of nutrition in inflammatory bowel disease. World J Gastroenterol 15: 2081–2088, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. MacCoss MJ, Fukagawa NK, Matthews DE. Measurement of intracellular sulfur amino acid metabolism in humans. Am J Physiol Endocrinol Metab 280: E947–E955, 2001 [DOI] [PubMed] [Google Scholar]
- 24. Malmezat T, Breuille D, Capitan P, Mirand PP, Obled C. Glutathione turnover is increased during the acute phase of sepsis in rats. J Nutr 130: 1239–1246, 2000 [DOI] [PubMed] [Google Scholar]
- 25. Malmezat T, Breuille D, Pouyet C, Buffiere C, Denis P, Mirand PP, Obled C. Methionine transsulfuration is increased during sepsis in rats. Am J Physiol Endocrinol Metab 279: E1391–E1397, 2000 [DOI] [PubMed] [Google Scholar]
- 26. Malmezat T, Breuille D, Pouyet C, Mirand PP, Obled C. Metabolism of cysteine is modified during the acute phase of sepsis in rats. J Nutr 128: 97–105, 1998 [DOI] [PubMed] [Google Scholar]
- 27. Marini JC, Lee B, Garlick PJ. In vivo urea kinetic studies in conscious mice. J Nutr 136: 202–206, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Moreno B, Hevia H, Santamaria M, Sepulcre J, Munoz J, Garcia-Trevijano ER, Berasain C, Corrales FJ, Avila MA, Villoslada P. Methylthioadenosine reverses brain autoimmune disease. Ann Neurol 60: 323–334, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Morgenstern I, Raijmakers MT, Peters WH, Hoensch H, Kirch W. Homocysteine, cysteine, and glutathione in human colonic mucosa: elevated levels of homocysteine in patients with inflammatory bowel disease. Dig Dis Sci 48: 2083–2090, 2003 [DOI] [PubMed] [Google Scholar]
- 30. Mudd SH, Finkelstein JD, Irreverre F, Laster L. Transsulfuration in mammals. Microassays and tissue distributions of three enzymes of the pathway. J Biol Chem 240: 4382–4392, 1965 [PubMed] [Google Scholar]
- 31. Nakano E, Taylor CJ, Chada L, McGaw J, Powers HJ. Hyperhomocystinemia in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr 37: 586–590, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Oldenburg B, Van Tuyl BA, van der GR, Fijnheer R, Berge Henegouwen GP. Risk factors for thromboembolic complications in inflammatory bowel disease: the role of hyperhomocysteinaemia. Dig Dis Sci 50: 235–240, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Riedijk MA, Stoll B, Chacko S, Schierbeek H, Sunehag AL, van Goudoever JB, Burrin DG. Methionine transmethylation and transsulfuration in the piglet gastrointestinal tract. Proc Natl Acad Sci USA 104: 3408–3413, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Romagnuolo J, Fedorak RN, Dias VC, Bamforth F, Teltscher M. Hyperhomocysteinemia and inflammatory bowel disease: prevalence and predictors in a cross-sectional study. Am J Gastroenterol 96: 2143–2149, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Stabler SP, Sampson DA, Wang L, Allen RH. Elevations of serum cystathionine and total homocysteine in pyridoxine-, folate-, and cobalamin deficient rats. Nutrit Biochem 8: 279–289, 1997 [Google Scholar]
- 36. Stoll B, Chang X, Fan MZ, Reeds PJ, Burrin DG. Enteral nutrient intake level determines intestinal protein synthesis and accretion rates in neonatal pigs. Am J Physiol Gastrointest Liver Physiol 279: G288–G294, 2000 [DOI] [PubMed] [Google Scholar]
- 37. Storch KJ, Wagner DA, Burke JF, Young VR. [1–13C; methyl-2H3]methionine kinetics in humans: methionine conservation and cysteine sparing. Am J Physiol Endocrinol Metab 258: E790–E798, 1990 [DOI] [PubMed] [Google Scholar]
- 38. Suzuki K, Ota H, Sasagawa S, Sakatani T, Fujikura T. Assay method for myeloperoxidase in human polymorphonuclear leukocytes. Anal Biochem 132: 345–352, 1983 [DOI] [PubMed] [Google Scholar]
- 39. Troen AM, Lutgens E, Smith DE, Rosenberg IH, Selhub J. The atherogenic effect of excess methionine intake. Proc Natl Acad Sci USA 100: 15089–15094, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Troen AM, Shea-Budgell M, Shukitt-Hale B, Smith DE, Selhub J, Rosenberg IH. B-vitamin deficiency causes hyperhomocysteinemia and vascular cognitive impairment in mice. Proc Natl Acad Sci USA 105: 12474–12479, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ubbink JB, Serfontein WJ, de Villiers LS. Stability of pyridoxal-5-phosphate semicarbazone: applications in plasma vitamin B6 analysis and population surveys of vitamin B6 nutritional status. J Chromatogr 342: 277–284, 1985 [DOI] [PubMed] [Google Scholar]
- 42. Ueland PM. Pharmacological and biochemical aspects of S-adenosylhomocysteine and S-adenosylhomocysteine hydrolase. Pharmacol Rev 34: 223–253, 1982 [PubMed] [Google Scholar]
- 43. van der SM, De Koning BA, De Bruijn AC, Velcich A, Meijerink JP, van Goudoever JB, Buller HA, Dekker J, Van SI, Renes IB, Einerhand AW. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology 131: 117–129, 2006 [DOI] [PubMed] [Google Scholar]
- 44. van Goudoever JB, Stoll B, Henry JF, Burrin DG, Reeds PJ. Adaptive regulation of intestinal lysine metabolism. Proc Natl Acad Sci USA 97: 11620–11625, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wolfe R, Chinkes D. Isotope Tracer in Metabolic Research: Principles and Practice of Kinetic Analysis. Hoboken, NJ: Wiley, 2005 [Google Scholar]
- 46. Wu G, Davis PK, Flynn NE, Knabe DA, Davidson JT. Endogenous synthesis of arginine plays an important role in maintaining arginine homeostasis in postweaning growing pigs. J Nutr 127: 2342–2349, 1997 [DOI] [PubMed] [Google Scholar]
- 47. Wu G, Knabe DA. Free and protein-bound amino acids in sow's colostrum and milk. J Nutr 124: 415–424, 1994 [DOI] [PubMed] [Google Scholar]
- 48. Yi F, dos Santos EA, Xia M, Chen QZ, Li PL, Li N. Podocyte injury and glomerulosclerosis in hyperhomocysteinemic rats. Am J Nephrol 27: 262–268, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.