Abstract
The airway epithelial surface liquid is generally considered to be composed of two layers, a periciliary layer and a continuous thick mucus layer moving in bulk. This view may not be appropriate for all areas of the lung. Our hypothesis, that mucus may form a discontinuous layer with dynamic attachments to the surface, is investigated using a culture system. We used live-cell confocal microscopy to investigate thin mucus layers and fluorescent beads and exogenous MUC5B to visualize mucus dynamics on ciliated human bronchial cultures. A continuous mucus layer was not observed. In sparsely ciliated cultures, mucus attached to ciliated cells; however, in highly ciliated cultures, mucus formed strands several hundred micrometers long. As with increases in ciliation, increases in bead concentration caused the appearance of mucus strands. We confirmed the involvement of mucins in the binding of mucus to cilia by adding labeled purified MUC5B to the cultures. These data suggest that mucins may have an intrinsic ability to form attachments to cilia. The significance of these findings is that aberrant modulation of such an intrinsic property may explain the initiation of highly adherent mucus in cystic fibrosis lung disease.
Keywords: MUC5B, cilia, beads, adhesion, airways
air we breathe contains particles that are deposited on the mucus layer covering the epithelial surfaces of the lung. This layer entraps these particles and is propelled toward the oropharynx under the action of ciliary beating. The current view of the airway surface generally considers it to comprise a periciliary layer (PCL) and an overlying continuous mucus layer (9, 13, 14, 18).
This view comes from images of fixed tissues and cultures. Fixation of excised tissue and cultures generally shows mucus as a variably thick internally heterogeneous layer (19, 20, 25). Transmission electron micrographs of cultured cells show mucus in a dense thin layer in some places and a thick layer in others (13, 18). In electron and light micrographs of fixed tracheas, the mucus layer had a much tighter mesh in continuous contact with cilia (19). This tighter mesh has also been seen in isolated mucus (16). The heterogeneity in many of these images has been confirmed by bead-tracking experiments (11).
The dynamics of mucus have also been investigated. Live microscopy has been used in vivo, in excised tissue, and in cultures (10, 12, 14, 25). These experiments investigated average mucus velocity over large areas. Only one study has reported more detailed mucus velocity measurements by differentiating between different radial positions in a circular culture system with thick mucus (14). Possibly because of this lack of data on mucus dynamics on the scale of cilia, models of mucus flow have generally assumed that mucus flows freely above cilia as a homogeneous layer (3, 22). Importantly, any interaction of the cilia with this layer has been assumed to be without an attachment.
However, the view of the mucus as a mostly passive continuous layer that moves in bulk may not be appropriate for all areas of the lung. Our hypothesis is that mucus may form a discontinuous layer with dynamic attachments to the surface. We could find only one example of mucus as a discontinuous layer: a scanning electron micrograph of mucus on the surface of a trachea was described as forming “rafts” and “strands” (17). Also, attachment of mucus to the surface has been confined to reports from pathogenic conditions such as cystic fibrosis (CF).
In early experiments to probe the PCL, we removed as much of the mucus as possible from cultures and added albumin-coated beads of various sizes. We expected these beads to diffuse freely through the PCL, limited only by their size. Instead, 20-nm beads were entrapped in mucus above the cilia before they reached the PCL. Because this mucus was attached to cilia, we developed the hypothesis that mucus binds to cilia when there is little mucus present. This is in distinction to all investigations of mucus to date, which have dealt with thick mucus layers. As there is little mucus in the deep airways, we supposed that a thin layer of mucus may be attached to the surface and may only be released when contaminants reached a critical level. In testing this hypothesis, we found that mucus dynamics were more complex. We report these dynamics in the present study.
MATERIALS AND METHODS
We aimed to determine how mucus behaves when there is little mucus present. We observed well-washed live cultures by confocal microscopy, and we recorded mucus dynamics in ∼20-s videos with a ∼100-μm field of view. We labeled the mucus nonspecifically with albumin-coated fluorescent beads. One-micrometer beads labeled the surface of the mucus, while 20-nm beads became entrapped inside mucus. We also tested antibodies to specific mucins, and most did not label mucins in the live culture. However, we successfully labeled the gel-forming mucin MUC5B after its purification from saliva and, thus, were able to add it back to the culture and observe its dynamics.
Materials.
Cell culture medium was purchased from GIBCO BRL (Gaithersburg, MD) and its supplements from Collaborative Research (Bedford, MA). DMEM-Ham's F-12 nutrient mixture (DMEM-F12) was also purchased from GIBCO BRL. Transwell-Col (T-Col) and Transwell-Clear (T-Clear) membrane supports were obtained from Costar. Beads were obtained from Invitrogen (Carlsbad, CA). Antibodies were obtained from Sigma-Aldrich (St. Louis, MO).
Cell culture.
Human bronchial epithelial (HBE) cells were obtained from normal human bronchi, as previously described (4). Briefly, HBE cells were isolated and grown on plastic culture dishes in bronchial epithelial cell growth medium (BEGM) and passaged at ∼70% confluence, and first-passage cells were seeded onto collagen-coated, 12-mm T-Col or T-Clear permeable supports at 250,000 cells per support. After confluence, the cells were maintained under air-liquid interface (ALI) conditions in ALI culture medium [BEGM modified as described elsewhere (4)], which was changed at the basolateral surface three times a week. HBE cell cultures were used for experiments 4–10 wk after confluence, when the columnar cells are well differentiated.
Bead and MUC5B preparation.
Beads were 20-nm or 1-μm-diameter and were coated with albumin to decrease bead clumping at physiological salt concentrations. Beads were diluted from their stock solutions into a solution containing 1 mg/ml albumin in PBS. A microscope was used to check the final solutions for clumping. The 1-μm beads were centrifuged, and the precipitate was brought up in the experimental working buffer. The 20-nm beads were always used at a dilution that reduced any sodium azide levels, having no effect on ciliary beat frequency. MUC5B was purified from saliva by density gradient centrifugation and gel filtration chromatography, as previously described (15, 24). MUC5B was labeled with a rabbit primary antibody to MUC5B and then with a FITC-labeled anti-rabbit secondary antibody (F-4890, Sigma). After gel filtration, several size fractions were pooled and used within 2 days.
Handling of cultures before and during microscopy.
The apical surfaces of the cultures were soaked in PBS for 30 min in an incubator at every feeding. In addition, 4 days before the experiment, the cultures were washed with PBS containing 1 mM DTT to remove as much surface-attached mucus as possible. During the 3 days preceding the experiment and on the morning of the experiment, cultures were washed without DTT. Before they were placed on the microscope stage, the cultures were altered to be used with a high-numerical-aperture objective and to control evaporation. First, any protrusions were removed from the underside of the culture. Then a small circular plastic ridge surrounding the underside of the culture was carefully trimmed off with a scalpel. Side openings were plugged with putty. Finally, the culture was placed on a #1 cover glass on the microscope stage, buffer was added to the apical surface, and a large #2 cover glass was placed on top. The volume of added buffer ensured that there was ≥200 μm of fluid above the cells in the center of the culture, with one exception (see Fig. 1). Once the cultures were on the microscope stage, the top cover glass was lifted, and the solution was gently pipetted down and up several times, so that the new solution was diluted into the apical fluid without approaching the ALI to the culture surface. Unless otherwise mentioned, the test solution containing the beads, antibody, or exogenous MUC5B was added at the start of the experiment, then the solution was changed at a rate of one-half volume every 20 min.
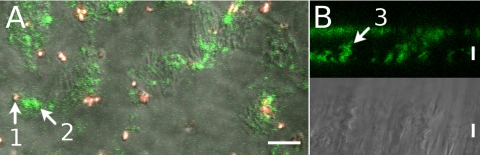
Fig. 1.
Sparsely ciliated cultures in 2 views. A: confocal x,y scan at the level of cilia. Red-fluorescent beads were 1 μm diameter; green-fluorescent beads were 20 nm diameter. B: x,z scan for fluorescence and differential interference contrast (DIC) in which only 20-nm green-fluorescent beads were present. One-micrometer beads (arrow 1) remained outside the structures labeled by 20-nm beads (arrow 2). Structures often extended for >10 μm above the cell surface (arrow 3). See Supplemental Material for 1 movie for each view (Movies 1A and 1B). Scale bars, 10 μm.
Preparation of cultures for addition of exogenous MUC5B.
For experiments involving the addition of exogenous MUC5B, the cultures were washed and allowed to stand in PBS with 1 mM DTT and 100 μM ATP prior to the microscopy. ATP depleted the mucin stores, while DTT helped remove as much of the mucus as possible and also temporarily interfered with new mucin synthesis.
Labeling of cultures with low concentrations of 20-nm beads.
For experiments involving the addition of low concentrations of 20-nm beads, a working solution of beads was diluted to 1:10,000 from their stock concentration of 2% solids. While the culture on the microscope was monitored, solution changes were made by addition of 50 μl of bead solution to a 200-μl volume on the culture surface followed by removal of 50 μl to maintain constant surface volume. A total of five additions were made. Several regions of the culture surface were videotaped between each solution change.
Two-color sequential bead addition.
The sequential addition of 20-nm beads of two different colors was done, such that the addition of the second color (green) occurred after all the beads of the first color (red) were entrapped in mucus. Addition of red-fluorescent 20-nm beads was followed by four solution changes using buffer with no beads. The solution changes changed half the surface fluid volume and were done every 10 min. Then addition of green-fluorescent 20-nm beads was followed by four more solution changes. With four solution changes, the final bead concentration would be 1/16th of the original bead concentration, if there was no mucus to entrap beads. With mucus present, the free red bead concentration was negligible at the time of green bead addition. This allowed new mucus to be labeled exclusively with green beads.
Microscopy.
Imaging was done in the Michael Hooker Microscopy Facility at the University of North Carolina at Chapel Hill. For the fluorescence measurements, a Zeiss 510 Meta laser scanning confocal microscope with a ×40/1.2 NA C-Apochromat water immersion objective was used. For determination of the level and heterogeneity of ciliation in cultures, a Nikon Eclipse TE-2000 microscope equipped with a ×40 objective was used. A MegaPlus ES-310 T camera (Redlake, Tucson, AZ) was used to record differential interference contrast images of ciliary activity at 125 frames per second, under the control of the Sisson-Ammons video analysis system (Ammons Engineering, Mt. Morris, MI) (21).
RESULTS
To easily refer to mucus structures we observed, we introduce the following three terms. We will use “plumes” for ∼10-μm-wide mucus structures that taper as they extend directly away from the culture for ∼30 μm, “strands” for structures that are much longer than wide and lie horizontally on the culture, and “clumps” for other structures that do not fit well into the other two categories.
Sparsely ciliated cultures.
In sparsely ciliated cultures (<60% of the surface), 20-nm beads became entrapped in mucus structures on ciliated cells (Fig. 1). These structures extended upward between 10 and 30 μm and waved as the cilia beat, so we named them plumes. The 1-μm beads bound to these plumes but did not enter them. We do not know if the plumes were present before addition of the beads, but, once formed, they were only removed by vigorous washing, after which they reformed within minutes.
Mucus behavior on fully ciliated cultures.
Upon further investigation of mucus plumes, we found that they did not form on fully ciliated cultures. Instead, small clumps of mucus visible on cilia immediately after addition of beads (Fig. 2A; see Supplemental Movie 2A, available online at the Journal website) coalesced into long structures that we named strands (Fig. 2B; see Supplemental Movie 2B). The 20-nm and 1-μm beads had this effect, although the mass concentration of 1-μm beads required was higher, since they interacted much less with the mucus. These strands extended for several hundred micrometers and flowed, remaining in contact with the culture surface. While these strands flowed (∼1 h), most of the culture remained free of fluorescence, and plumes were never seen. Occasionally, small clumps of fluorescence were seen on cilia, but these were quickly removed by the passage of strands. Eventually, the strands developed strong attachments to some spots on the culture surface and stopped flowing (Fig. 2B, inset). So it appeared that, in the presence of beads, mucus attached to cilia (small clumps and plumes) but that this attachment was delicate enough that vigorous flow or larger flowing mucus structures (strands) could clean the surface. Furthermore, highly ciliated areas generated the strands needed to clean the surface.
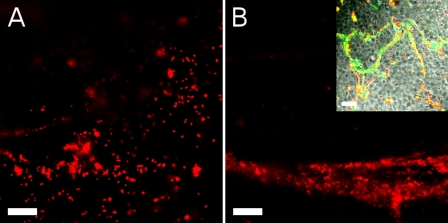
Fig. 2.
Mucus behavior on fully ciliated cultures shown as fluorescence on the culture surface from red-fluorescent 1-μm beads. A: fluorescence just after bead addition (also see Supplemental Movie 2A). B: fluorescence a few minutes later, when thick strands were moving across the field of view (also see Supplemental Movie 2B). Inset: 20-nm (green) and 1-μm (red) beads much later, when strands have stopped flowing. Scale bars, 30 μm.
Effect of bead concentration on mucus dynamics.
To determine how 20-nm beads changed mucus dynamics, we slowly increased the bead concentration in highly ciliated cultures. Figure 3 shows a culture at bead concentrations much lower than those that caused the development of strands (see Supplemental Movies 3AB, 3CD, and 3EF). When fluorescence from beads first became visible, it appeared as a thin layer of small clumps attached to cilia (Fig. 3, C and D, arrowhead in C; compare with Fig. 3, A and B, with no beads). These small clumps were attached to cilia and aligned with the flow. As the bead concentration increased (Fig. 3, E and F; see Supplemental Movie 3EF), the attached clumps extended lengthwise, and occasional detachment and flow were followed by reattachment. So, in summary, low concentrations of 20-nm beads labeled highly ciliated areas, and this thin fluorescence only started flowing as the bead concentration increased. Because the labeling of highly ciliated areas at the lowest concentration was so thin, it may only represent the labeling of tethered mucins, but the longer structures and intermittent flow that developed later suggest the involvement of gel-forming mucins, even at bead concentrations only slightly above our limit of detection.
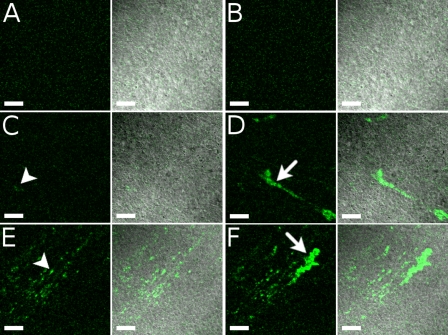
Fig. 3.
Effect of bead concentration on mucus dynamics shown in 3 stages during a slow increase in concentration of 20-nm beads. Each row of panels and each movie in Supplemental Material (Supplemental Movies 3AB, 3CD, and 3EF) show a different bead concentration. Each pair of images shows fluorescence (green on black) and merged fluorescence-DIC (green on gray). Areas imaged in the 3 rows are not identical but are highly ciliated. A, C, and E: movie frame in which no fluorescence was moving across the field of view. Frame represents attached mucus. B, D, and F: frame for which the most fluorescence was moving. A and B (Supplemental Movie 3AB): culture before addition of beads. The same movie frame was used for A and B. Some autofluorescence can be seen. C and D (Supplemental Movie 3CD): culture after second addition of beads, at which point an increase in fluorescence was first seen. E and F (Supplemental Movie 3EF): culture after last addition of beads. Some attached clumps are marked with arrowheads (C and E), and some larger flowing clumps are shown with arrows (D and F). Fluorescent images of E and F were taken using a slightly lower gain. All images were processed in exactly the same way to enhance contrast. Scale bars, 40 μm.
Two-color sequential addition of beads.
We showed the attachment of mucus to cilia but not the source of this mucus. As reported previously (7), mucins undergo a maturation process upon secretion. So, mucus that we labeled may not be representative of newly secreted mucus. We were able to show that the beads did label newly secreted mucus by first adding red 20-nm beads and then green 20-nm beads [Fig. 4 (profile view); see Supplemental Movie 4 (top-down view)]. As usual, the mucus formed strands that flowed over the culture. Most importantly, there were distinct red- and green-labeled regions with little combined labeling, suggesting that the green-labeled mucus was newly secreted.
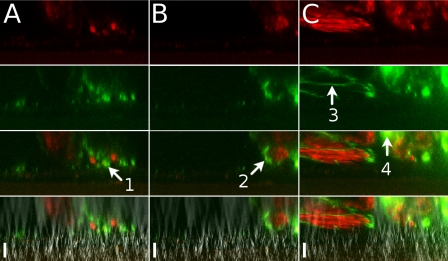
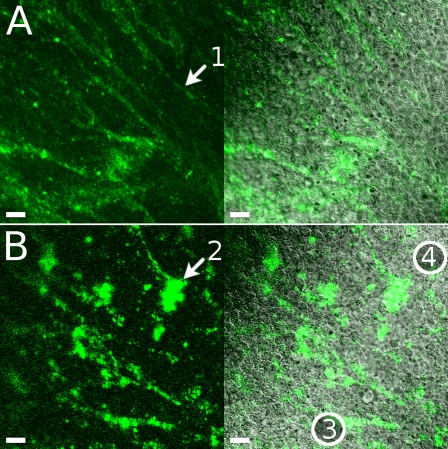
Fig. 4.
Two-color sequential addition of beads. Red- followed by green-fluorescent 20-nm beads were added to a culture seen here in profile. From top to bottom: red fluorescence, green fluorescence, merged red and green, and merged fluorescence and DIC. At 3 min after the final wash (A), green and red fluorescence was seen in separate areas, with some green fluorescence closer to the culture surface (arrow 1). At 20 min (B), green fluorescence was often seen circumscribing areas of red fluorescence (arrow 2). At 36 min (C), some areas of fluorescence appeared thin, as if stretched (arrow 3), and other areas were starting to show combined red and green fluorescence (arrow 4). Scale bars, 23 μm.
Exogenous MUC5B.
We hypothesized that mucins themselves were largely responsible for the dynamics we observed with beads. To test this notion, we applied exogenous purified FITC-labeled MUC5B to a culture that had been treated with DTT and ATP to remove as much endogenous mucus as possible and to empty mucin stores. The culture was washed with plain buffer before the addition of MUC5B, which then coalesced into strands and flowed along the culture surface (Fig. 5A, see Supplemental Movie 5A). Focusing high above the cells showed that MUC5B was not arranged into strands until it made contact with the surface. Later, these strands became shaped more like clumps and attached intermittently to ciliated cells (Fig. 5B; see Supplemental Movie 5B). These attachments were particularly evident in areas of low ciliation. So, generally, MUC5B was able to reproduce the mucus dynamics observed with bead-labeled mucus and reinforced the unusually adhesive nature of isolated ciliated cells.
Fig. 5.
Exogenous MUC5B. FITC-labeled exogenous MUC5B was added to a culture. Left: fluorescence; right: merged fluorescence and DIC. A: highly ciliated area a few minutes after MUC5B addition. Arrow 1, thin MUC5B strand. B: images obtained 2 h after MUC5B addition showing an area that is highly ciliated, except for a band running from bottom left (circled 3) diagonally up and to the right (circled 4). In this band, MUC5B (green) could be seen to temporarily attach to ciliated cells (gray DIC motion in Supplemental Movie 5B). Arrow 2, one of the thicker clumps that showed temporary attachment to a ciliated cell. Scale bars, 20 μm.
DISCUSSION
We have shown that mucus can form discontinuous layers with temporary attachments to the surface. More specifically, we have shown that beads on cultures label specific structures that we have called plumes and strands and that the formation of these structures depends on cilia. We do not know the extent to which beads cause mucus to form these structures. Most importantly, the mucus interacts with cilia, as opposed to nonciliated surfaces, and newly secreted mucus, as well as the gel-forming mucin MUC5B, does the same. Attachments are dynamic, in the sense that they are short-lived when the culture is well ciliated.
Model of mucociliary interactions.
These observations lead us to the following model of mucociliary interactions. Gel-forming mucins are secreted into the mucus layer, where they are responsible for its weak interactions with cilia (the labeling of the surface in Fig. 3E is thin). Upon the deposition of contaminants on this layer, the mucus collapses around these contaminants. The interactions with the cilia (Fig. 1) are strengthened by this collapse, but this leads to the bundling of the collapsed mucus into strands by the cilia (Fig. 2) and its transport in a packaged form. The increased mucociliary interactions resulting from the collapse of the mucus cause further mucus secretion (Fig. 4), which will help bundle the contaminants and regenerate the layer of mucus in its less sticky form.
Full model of the airway liquid layers.
We believe that the two layers of the airway liquid work together to modulate the mucociliary interactions according to the level of contamination. The last layer of defense would be the tethered mucins, MUC1, MUC4, and MUC16, which are known to be present in our cultures (6). We were able to detect MUC1 (data not shown) at the level of the microvilli and shed into the layer above the cilia, where it has been found in exosomes (8). The larger MUC4 and MUC16 mucins have been identified in the mucus layer and on cilia by conventional staining methods. On cilia, they provide a barrier that may also carry a small amount of beads (Fig. 3C). So a full model would include the shedding of these mucins from the PCL into the mucus layer as cilia bundle contaminants. The gel-forming mucin MUC5AC will also be important in mucociliary interactions, although in our cultures, the mucus is composed of 5–10 times more MUC5B than MUC5AC (2, 6, 23). The investigation of the mucociliary interactions when exogenous MUC5AC, instead of MUC5B, is present may explain the need for two gel-forming mucins in the airways. Finally, surfactant may also modulate these interactions, since it has been observed between cilia and the mucus layer (5).
Significance of mucociliary interactions.
Our model presumes the appropriate modulation of the mucociliary interactions. Pathological mucus may be permanently shifted toward stronger interactions. This would explain highly adherent mucus as an early sign of disease in the CF lung. It may also explain why CF lung disease originates in the deepest airways (1), where there are fewer ciliated cells, since we observed the strongest interactions on sparsely ciliated surfaces.
GRANTS
The project was supported by National Heart, Lung, and Blood Institute Grant HL-084934-04.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the members of the Cystic Fibrosis Center Tissue Culture Core at University of North Carolina at Chapel Hill.
REFERENCES
- 1. Armstrong DS, Hook SM, Jamsen KM, Nixon GM, Carzino R, Carlin JB, Robertson CF, Grimwood K. Lower airway inflammation in infants with cystic fibrosis detected by newborn screening. Pediatr Pulmonol 40: 500–510, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Bernacki SH, Nelson AL, Abdullah L, Sheehan JK, Harris A, Davis CW, Randell SH. Mucin gene expression during differentiation of human airway epithelia in vitro. Muc4 and Muc5b are strongly induced. Am J Respir Cell Mol Biol 20: 595–604, 1999 [DOI] [PubMed] [Google Scholar]
- 3. Dillon RH, Fauci LJ, Omoto C, Yang X. Fluid dynamic models of flagellar and ciliary beating. Ann NY Acad Sci 1101: 494–505, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Fulcher ML, Gabriel S, Burns KA, Yankaskas JR, Randell SH. Well-differentiated human airway cultures. Methods Mol Med 107: 183–206, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Gehr P, Green FH, Geiser M, Hof VI, Lee MM, Schürch S. Airway surfactant, a primary defense barrier: mechanical and immunological aspects. J Aerosol Med 9: 163–181, 1996 [DOI] [PubMed] [Google Scholar]
- 6. Kesimer M, Kirkham S, Pickles RJ, Henderson AG, Alexis NE, Demaria G, Knight D, Thornton DJ, Sheehan JK. Tracheobronchial air-liquid interface cell culture: a model for innate mucosal defense of the upper airways? Am J Physiol Lung Cell Mol Physiol 296: L92–L100, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kesimer M, Makhov AM, Grith JD, Verdugo P, Sheehan JK. Unpacking a gel-forming mucin: a view of MUC5B organization after granular release. Am J Physiol Lung Cell Mol Physiol 298: L15–L22, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kesimer M, Scull M, Brighton B, DeMaria G, Burns K, O'Neal W, Pickles RJ, Sheehan JK. Characterization of exosome like vesicles released from human tracheobronchial ciliated epithelium: a possible role in innate defense. FASEB J 23: 1858–1868, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Knowles MR, Boucher RC. Mucus clearance as a primary innate defense mechanism for mammalian airways. J Clin Invest 109: 571–577, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kurosawa H, Wang CG, Dandurand RJ, King M, Eidelman DH. Mucociliary function in the mouse measured in explanted lung tissue. J Appl Physiol 79: 41–46, 1995 [DOI] [PubMed] [Google Scholar]
- 11. Lai SK, O'Hanlon DE, Harrold S, Man ST, Wang YY, Cone R, Hanes J. Rapid transport of large polymeric nanoparticles in fresh undiluted human mucus. Proc Natl Acad Sci USA 104: 1482–1487, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ledowski T, Manopas A, Lauer S. Bronchial mucus transport velocity in patients receiving desurane and fentanyl vs. sevourane and fentanyl. Eur J Anaesthesiol 25: 752–755, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Luchtel DL. Mucociliary interactions in rabbit intrapulmonary airways. Prog Clin Biol Res 80: 77–81, 1982 [DOI] [PubMed] [Google Scholar]
- 14. Matsui H, Randell SH, Peretti SW, Davis CW, Boucher RC. Coordinated clearance of periciliary liquid and mucus from airway surfaces. J Clin Invest 102: 1125–1131, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mehrotra R, Thornton DJ, Sheehan JK. Isolation and physical characterization of the MUC7 (MG2) mucin from saliva: evidence for self-association. Biochem J 334: 415–422, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Olmsted SS, Padgett JL, Yudin AI, Whaley KJ, Moench TR, Cone RA. Diffusion of macromolecules and virus-like particles in human cervical mucus. Biophys J 81: 1930–1937, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rogers DF. Physiology of airway mucus secretion and pathophysiology of hypersecretion. Respir Care 52: 1134–1149, 2007 [PubMed] [Google Scholar]
- 18. Sanderson MJ, Sleigh MA. Ciliary activity of cultured rabbit tracheal epithelium: beat pattern and metachrony. J Cell Sci 47: 331–347, 1981 [DOI] [PubMed] [Google Scholar]
- 19. Sims DE, Horne MM. Heterogeneity of the composition and thickness of tracheal mucus in rats. Am J Physiol Lung Cell Mol Physiol 273: L1036–L1041, 1997 [DOI] [PubMed] [Google Scholar]
- 20. Sims DE, Westfall JA, Kiorpes AL, Horne MM. Preservation of tracheal mucus by nonaqueous fixative. Biotech Histochem 66: 173–180, 1991 [DOI] [PubMed] [Google Scholar]
- 21. Sisson JH, Stoner JA, Ammons BA, Wyatt TA. All-digital image capture and whole-field analysis of ciliary beat frequency. J Microsc 211: 103–111, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Smith DJ, Lubkin DJ, Ganey EA, Blake JR. A viscoelastic traction layer model of muco-ciliary transport. Bull Math Biol 69: 289–327, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Thornton DJ, Gray T, Nettesheim P, Howard M, Koo JS, Sheehan JK. Characterization of mucins from cultured normal human tracheobronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 278: L1118–L1128, 2000 [DOI] [PubMed] [Google Scholar]
- 24. Thornton DJ, Khan N, Mehrotra R, Howard M, Veerman E, Packer NH, Sheehan JK. Salivary mucin MG1 is comprised almost entirely of different glycosylated forms of the MUC5B gene product. Glycobiology 9: 293–302, 1999 [DOI] [PubMed] [Google Scholar]
- 25. Zahm JM, Gaillard D, Dupuit F, Hinnrasky J, Porteous D, Dorin JR, Puchelle E. Early alterations in airway mucociliary clearance and inflammation of the lamina propria in CF mice. Am J Physiol Cell Physiol 272: C853–C859, 1997 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.