Abstract
BACKGROUND
Key recommendations of the American Academy of Pediatrics guideline on management of severe hyperbilirubinemia in healthy infants of ≥35 weeks' gestation include predischarge screening for risk of subsequent hyperbilirubinemia, follow-up at 3 to 5 days of age, and lactation support. Little information is available on contemporary compliance with follow-up recommendations.
OBJECTIVE
To assess timing and content of the first newborn office visit after birth hospitalization in urban and suburban pediatric practices in Houston, Texas.
METHODS
We reviewed office records for the first visit within 4 weeks of birth during January through July 2006 for apparently healthy newborns with a gestational age of ≥35 weeks or birth weight of ≥2500 g seen within a pediatric provider network. For each pediatrician, we selected every fifth patient up to a total of 6.
RESULTS
Of 845 records abstracted, 698 (83%) were eligible for analysis. Infants were seen by 136 pediatricians in 39 practices. They had vaginal (64%) or cesarean (36%) deliveries at 20 local hospitals, of which 17 had routine predischarge bilirubin screening policies. Only 37% of all infants, 44% of vaginally delivered infants, and 41% of exclusively breastfed infants were seen before 6 days of age. Thirty-five percent of the infants were seen after 10 days of age. Among 636 infants seen at ≤15 days, jaundice was noted on examination in 33%; of these, 44% had bilirubin measured. Nine infants had phototherapy documented after birth hospitalization.
CONCLUSIONS
Among a large group of urban and suburban pediatricians, implementation of the American Academy of Pediatrics recommendation for follow-up was inconsistent, and delayed follow-up was common. Understanding reasons for delayed follow-up and providing guidance for jaundice management may promote a safer first week of life.
Keywords: infant, newborn, hyperbilirubinemia, practice guidelines, quality of care
The majority of healthy term and late-preterm infants develop jaundice as a result of hyperbilirubinemia during the first week of life that is usually benign.1 Severe hyperbilirubinemia can be toxic to the newborn brain and may result in kernicterus, a rare but devastating condition. Kernicterus had been thought almost extinct until a combination of increased use of exclusive breastfeeding, a relaxation of vigilance about hyperbilirubinemia, and a trend toward early postpartum hospital discharge led to its apparent resurgence during the 1990s.2,3
In 2004, the American Academy of Pediatrics (AAP) issued revised guidelines on the management of hyperbilirubinemia in healthy infants of ≥35 weeks' gestation.4 Recommendations included predischarge risk assessment for subsequent severe hyperbilirubinemia, follow-up at 3 to 5 days of age, and lactation support. According to the revised guidelines, 2 clinical options for assessing the risk of subsequent severe hyperbilirubinemia are acceptable: “predischarge measurement of the bilirubin level using total serum bilirubin or transcutaneous bilirubin and/or assessment of clinical risk factors. Whether either or both options are used, appropriate follow-up after discharge is essential.”4 Although these guidelines have been widely disseminated, little is known about compliance with the recommendations.
This study evaluates adherence to the AAP recommendation for assessment of hyperbilirubinemia after hospital discharge. We describe the timing and content of the first newborn office visit after birth hospitalization in pediatric practices associated with a large academic medical center.
METHODS
Study Design and Sample
We performed a retrospective review of pediatric office records from a cross-sectional sample of apparently healthy newborns with gestational age (GA) at birth of ≥35 weeks or birth weight (BW) of ≥2500 g who had their first ambulatory visit within 4 weeks of birth during January through July 2006. We restricted the sample to those seen within 28 days of birth to minimize the risk of falsely attributing delayed follow-up to infants who may have received medical care elsewhere. Infants were identified from a list of all newborns who were seen during this period by pediatricians at Texas Children's Pediatric Associates (TCPA), a subsidiary of Texas Children's Hospital in Houston, Texas. TCPA practices include both urban and suburban practice settings. For each pediatrician, we selected every fifth patient from a list of consecutive appointments up to a total of 6. Patients not meeting the GA, BW, and study-period criteria were excluded from the analysis. We also excluded infants with a BW of <2000 g even if their GA was ≥35 weeks, because these infants may have experienced prolonged birth hospitalizations even if their office records did not indicate it (n = 6). Infants with medical complications, first visit after 28 days of age, birth place outside the United States, and inadequate documentation were also excluded.
Measures
Data were recorded by experienced nurse abstractors. The main outcome variable was age at first follow-up visit after birth hospitalization. We conservatively categorized the date of birth as day of life 0. Pediatricians in this study do not routinely use home visiting nurses, so that the first comprehensive medical contact after the birth hospitalization occurred at the ambulatory visit. Independent variables were collected at the practice, pediatrician, and patient levels. Practice characteristics included practice size (number of pediatricians in practice); pediatrician characteristics included years since graduation from medical school (simplified as “experience”) and US versus foreign medical graduate; and patient-level characteristics included GA, BW, mode of delivery, age at visit, chief complaint, presence of jaundice, type of feeding, and insurance status. Maternal parity was not assessed because it could not be ascertained reliably from the office medical record.
Analysis
Analyses refer to the available sample, with missing data noted as “not documented.” We conducted basic descriptive analyses to illustrate the timing and content of the first ambulatory office visit. Continuous data are presented as means, SDs, and range; categorical data are reported as proportions. For bivariate analyses, we stratified our outcome variable (age at follow-up) according to <6/≥6 days of age to reflect guideline recommendations. We used bivariate and multivariate logistic regression to test for associations between our outcome and predictor variables. Multivariate models included all variables considered for bivariate analysis. We considered 2-sided P values of <.05 as statistically significant. All analyses were performed with Microsoft Excel 2003 software (Microsoft, Redmond, WA) and SAS 9.1 (SAS Institute Inc, Cary, NC). The study was approved by the Baylor College of Medicine institutional review board.
RESULTS
The study sample consisted of 845 infants. Of these infants, 147 were excluded because they did not meet inclusion criteria or documentation was inadequate (see Fig 1), resulting in a final sample of 698 infants seen by 136 pediatricians in 39 practices. Mean number of pediatricians per practice was 3.6 (SD: 2.0; range: 1–11). Pediatricians in this study were experienced, with a mean of 21.6 (SD: 12; range: 5–56) years since graduation from medical school. Most providers graduated from US medical schools (85% [116 of 136]).
FIGURE 1.
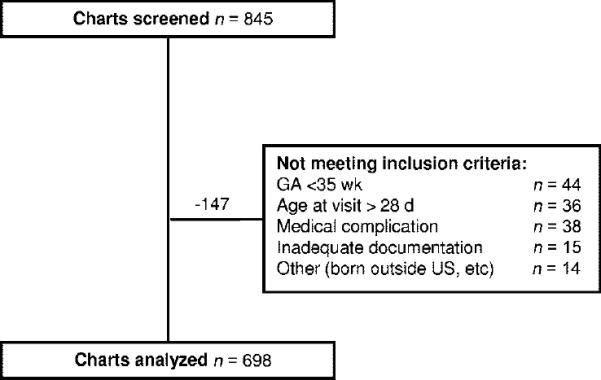
Study-sample flow chart.
Characteristics of the Birth Hospitalization
Table 1 shows the birth characteristics of our sample. Infants had vaginal (64%) or cesarean (36%) deliveries at 20 local hospitals, of which 17 had routine predischarge bilirubin screening policies. Mean BW was 3340 g (range: 2085–5273 g). Information regarding GA at birth, BW, and mode of delivery was missing in 35%, 1.6%, and 9.6% of records, respectively. In 86% of the charts there was no documentation with regard to the need for phototherapy during the birth hospitalization. Of the 95 charts in which this information was recorded, 12 (12%) infants received phototherapy.
TABLE 1.
Patient Characteristics at Birth Hospitalization (N = 698 Patients)
| Characteristic | Value | Not Documented, n |
|---|---|---|
| GA, mean (SD), wka | 38.8 (1.3) | 244 |
| BW, mean (SD), g | 3342 (463) | 11 |
| Type of delivery, n (%) | 67 | |
| Vaginal | 406 (64) | — |
| Cesarean | 225 (36) | — |
| Phototherapy, n (%) | 602 | |
| Yes | 12 (12) | — |
| No | 84 (88) | — |
We substituted a GA of 40 weeks for 95 infants whose GA was recorded as “full term.”
Characteristics of the First Office Visit
There is a bimodal distribution in the timing of first office visits with a broad peak around 3 to 7 days of life and a narrow peak at 14 days of age (Fig 2). Table 2 shows the timing and patient characteristics at the first office visit. First follow-up visits occurred at day of age <6 in 36%. Most visits were routine, scheduled follow-up; jaundice or feeding concerns were noted as the chief compliant in 17% and 6%, respectively. Recorded feeding was by breast (51%), bottle (22%), or combined breast/bottle (27%). Most infants had commercial insurance (85%). Only 2 infants were readmitted, and 1 presented to the emergency department. Information regarding intercurrent readmission or emergency department visits was not recorded in 196 charts. Only 44% of the vaginally delivered infants, 41% of exclusively breastfed infants, and 48% of vaginally delivered and exclusively breastfed infants were seen before day 6 (the latter is shown in Fig 3). Additional analyses showed that among 636 infants seen at ≤15 days, jaundice was noted on follow-up examination in 33%; of these, 46% had serum bilirubin measured. Nine infants had phototherapy documented after birth hospitalization.
FIGURE 2.
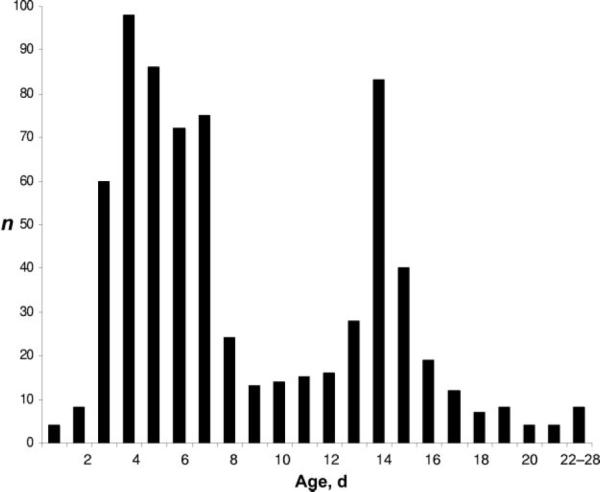
Timing of first office visits. Note the bimodal distribution with a broad peak around 4 and a narrow peak around 14 days of life. The broad peak is a reflection of a combination of guideline-recommended visits by 5 days of life and the customary 1-week follow-up. The narrow peak reflects the “customary” 2-week follow-up.
TABLE 2.
Patient Characteristics at First Office Visit
| n (%) | Not Documented, n | |
|---|---|---|
| Timing of visit | ||
| <6 d | 256 (37) | — |
| ≥6 d | 442 (63) | — |
| Chief complaint | ||
| None or not recorded | 507 (73) | — |
| Jaundice | 119 (17) | — |
| Feeding relateda | 41 (6) | — |
| Jaundice on examinationb | 47 | |
| Yes | 206 (31) | — |
| No | 434 (68) | — |
| Phototherapy | 195 | |
| Yes | 9 (2) | — |
| No | 494 (98) | — |
| Feeding | 45 | |
| Exclusive breast | 333 (51) | — |
| Breast and bottle | 175 (27) | — |
| Bottle | 145 (22) | — |
| Insurance | 4 | |
| Commercial | 591 (85) | — |
| Medicaid | 91 (13) | — |
| Self-pay | 10 (1) | — |
This category includes concerns about feeding, gastro-esophageal reflux, vomiting, and weight gain.
Infants seen at <15 days of age, n = 635.
FIGURE 3.
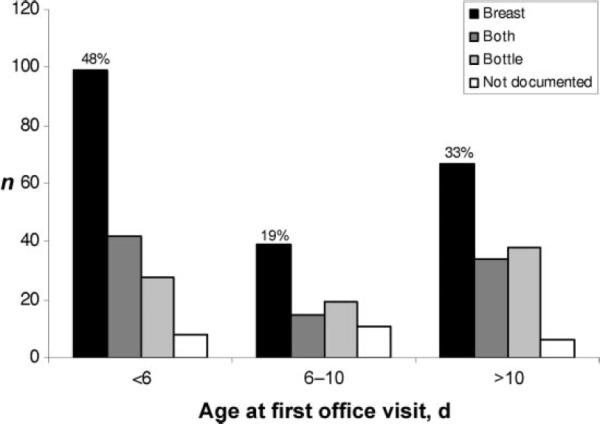
Many vaginally delivered and breastfed infants experience delayed follow-up.
Bivariate and logistic regression analyses showed that follow-up on or after 6 days of age was associated with cesarean delivery, bottle feeding (when compared with exclusive breastfeeding), Medicaid insurance coverage, and being seen in a smaller practice (see Table 3). Neither provider experience nor location of medical school was associated with compliance with recommended follow-up timing.
TABLE 3.
Association Between Timing of Visit After 6 Days of Age and Infant and Physician Characteristics
| Variable | Age at Visit |
Unadjusted OR |
Adjusted OR |
|||
|---|---|---|---|---|---|---|
| <6 d | ≥6 d | OR (CI) | P | OR (CI) | P | |
| Type of delivery, n | ||||||
| Vaginal | 177 | 229 | Reference | Reference | ||
| Cesarean | 62 | 163 | 2.03 (1.43–2.89) | <.0001 | 2.48 (1.70–3.62) | <.0001 |
| Feeding, n | ||||||
| Breast | 137 | 196 | Reference | Reference | ||
| Breast and bottle | 65 | 110 | 1.14 (0.79–1.65) | .49 | 0.95 (0.63–1.42) | .79 |
| Bottle | 39 | 106 | 1.83 (1.20–2.78) | .005 | 1.60 (1.00–2.55) | .05 |
| Insurance, n | ||||||
| Commercial | 232 | 359 | Reference | Reference | ||
| Medicaid | 18 | 73 | 1.85 (1.21–2.84) | .005 | 1.89 (1.16–3.10) | .01 |
| US medical graduate, n | ||||||
| Yes | 211 | 365 | Reference | Reference | ||
| No | 37 | 59 | 1.09 (0.70–1.69) | .72 | 0.98 (0.60–1.60) | .94 |
| Physicians per practice, mean | 4.1 | 3.6 | 0.91 (0.85–0.97) | .003 | 0.93 (0.86–1.00) | .04 |
| Experience, mean, y | 22 | 21 | 1.00 (0.98–1.01) | .55 | 1.00 (0.98–1.01) | .46 |
Adjusted odds ratios were derived from multivariate models that include all variables considered for bivariate analysis. OR indicates odds ratio; CI, confidence interval.
DISCUSSION
In this study we evaluated the extent of implementation of the 2004 AAP guideline for the management of hyperbilirubinemia in the newborn infant of ≥35 weeks of gestation. The key findings from this study are that implementation of recommendation for follow-up remains incomplete and that many infants may be exposed to the risk of severe hyperbilirubinemia. Although most birth hospitals in the geographic area studied have policies to perform predischarge risk assessment that includes bilirubin measurement, more than half of vaginally delivered and breastfed infants did not receive timely follow-up. The results of our study are similar to those from assessments of newborn follow-up patterns published before the revised AAP guidelines of 2004. Maisels and Kring5 found that despite physician education regarding the importance of evaluating infants within 2 to 3 days of discharge if the hospital stay was <48 hours, 38% of short-stay infants were scheduled to be seen ≥4 days after discharge. Among a national sample of >4000 healthy term infants, Bernstein et al6 found that only 54% of first office visits actually occurred within 7 days after discharge.
Barriers to compliance with guidelines include provider knowledge, attitude, and behavior.7 Although publication of the 2004 AAP guideline for management of hyperbilirubinemia was accompanied by substantial education, some providers may remain unaware. It is possible that some providers may be reassured by a predischarge bilirubin level in a zone that does not predict a high risk for subsequent severe hyperbilirubinemia8 and, thus, delay a follow-up visit. This prioritization may not be appropriate, because some infants at lower risk will develop significant hyperbilirubinemia, and there are additional advantages to an early visit, including lactation support.9 Providers may disagree with the guidelines10 by perceiving them as too stringent or not cost-effective, thinking they are unable to implement the required changes in their practice, or, because kernicterus is a rare event, considering the effect on outcomes too negligible to change their practice. Providers may also experience external barriers to performing recommendations such as financial disincentives, difficulties with care coordination during off hours and weekends, and inadequate access to laboratory services.
Care transitions, such as discharge from birth hospitalization and transfer of care to a medical home in the ambulatory care setting, have been shown to be associated with adverse outcomes. In adults, the potential patient safety risks of care transitions include inaccurate or incomplete transfer of information between providers and from provider to patient, as well as ambivalence about care responsibilities.11–14 Care transitions for newborns exhibit many of the same risks, including incompletely resolved medical conditions such as jaundice or breastfeeding competency, the need for caregiver education and participation, and an often-prolonged time between the birth hospitalization and ambulatory follow-up. In fact, the bimodal distribution of follow-up that we have documented seems to reflect partial compliance with the AAP guidelines but also a continued influence of traditional 1- and 2-week scheduled follow-up visits. In addition, unclear lines of responsibility for the patient15 and deficits in transfer of information between providers may put infants at risk. In our study, the pediatrician who saw the patient at the follow-up visit often did not provide care during the birth hospitalization and, therefore, may not have had complete or accurate information about the patient's condition. As a result, the transition period between the inpatient and outpatient settings is an environment in which deficits may be common and can have potentially severe consequences.
At the local, regional, and national levels, the formidable obstacles to safe newborn care during the first week after birth may be overcome through a combination of regulatory policies and alignment of provider incentives with desired care quality. Maisels and Kring5,16 have demonstrated that local institutional policies promulgated by a local champion, provider education, and reminder systems can significantly improve compliance with early newborn follow-up. The replication of such successes on a national scale requires a comprehensive approach that targets different levels of the health care delivery system (parents, doctor, team practice, hospital, payers of health care) and is tailored to the needs of the newborn as well as the existing practice environment.17 Such interventions are more successful if they actively engage providers and provide tools that facilitate implementation.18–21 The need for such a systems approach has been recognized by the pediatric community.22,23 The AAP's Safe and Healthy Beginnings project, which includes development of a tool kit, is working to facilitate implementation of the 2004 guideline for management of hyperbilirubinemia.24,25 However, tool kits may not be sufficient to drive further improvement in the face of insufficient incentives for provider change.
Payers of health care can support the effectiveness of local quality-improvement efforts by aligning financial incentives and regulatory frameworks with quality objectives.26 Aligning financial incentives with quality means that providers should not be penalized but, rather, encouraged to provide high-quality care. Yet, in many states Medicaid does not encourage early newborn follow-up by adding this visit as a sixth reimbursable well-child visit during the first year of life. Instead, early newborn follow-up visits can be assigned a lower-paying sick-visit reimbursement code. It is possible that financial disincentives contributed to our finding of lower compliance with guidelines for early newborn follow-up for Medicaid-insured patients, leaving the most vulnerable infants poorly protected. Redesigning financial reimbursement policies to encourage guideline-recommended care by paying a premium for high-value visits, such as the early newborn follow-up visit, might invite safer practice.
Our findings must be interpreted within the framework of the study design. The large variation in practice styles may reflect care practices in a pediatric practice association that has not developed system-wide clinical care pathways. Therefore, our findings may not generalize to more tightly managed practice settings or provider networks. However, given that most providers practice outside of tightly managed settings, this study likely provides a realistic picture of practice variation across the country. Indeed, because our study population is largely non-Hispanic white and commercially insured, our results may represent an underestimate of the true quality gap.
A limitation of our study is that data collection relied solely on office records. We did not have access to records for the birth hospitalization for validation. Therefore, we do not know the precise length of birth hospitalization for an individual infant, which might affect the timing of follow-up. We have addressed this weakness in our subgroup analyses of vaginally delivered infants, who are customarily discharged from the hospital within 48 hours of age. Such infants should be followed within 2 days of discharge, particularly if they are breastfed. However, a substantial proportion was seen after the first week of age.
Finally, some pediatricians in our study may have used predischarge bilirubin measurement results to prioritize follow-up. Indeed, infants with a predischarge bilirubin measurement below the 40th percentile (low risk) are unlikely to develop subsequent severe hyperbilirubinemia. However, such cases have occurred22 and have contributed to the current recommendations for routine early follow-up for all infants discharged before 72 hours.
CONCLUSIONS
In this retrospective cross-sectional study, we found significant noncompliance with AAP recommendations for early follow-up after birth hospitalization. Efforts to improve adherence with the 2004 AAP guideline for management of hyperbilirubinemia are underway and will need to be evaluated in intervention trials for their effectiveness. In addition, qualitative research may add to our understanding regarding barriers to follow-up recommendations.
WHAT'S KNOWN ON THIS SUBJECT.
To decrease the incidence of newborns with kernicterus, the AAP issued revised guidelines on management of severe hyperbilirubinemia in 2004. Compliance with these guidelines is unknown.
WHAT THIS STUDY ADDS.
Among a large group of urban and suburban pediatricians, implementation of the AAP recommendations for management of severe hyperbilirubinemia was inconsistent. Outpatient follow-up after birth hospitalization was commonly delayed.
ACKNOWLEDGMENT
This work is supported in part by National Institutes of Health grant K23 HD056298-01 (to Dr Profit, principal investigator).
ABBREVIATIONS
- AAP
American Academy of Pediatrics
- GA
gestational age
- BW
birth weight
Footnotes
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
REFERENCES
- 1.Maisels MJ. Neonatal jaundice. Pediatr Rev. 2006;27(12):443–454. doi: 10.1542/pir.27-12-443. [DOI] [PubMed] [Google Scholar]
- 2.American Academy of Pediatrics. Subcommittee on Neonatal Hyperbilirubinemia Neonatal jaundice and kernicterus. Pediatrics. 2001;108(3):763–765. doi: 10.1542/peds.108.3.763. [DOI] [PubMed] [Google Scholar]
- 3.Bhutani VK, Johnson LH. Kernicterus: lessons for the future from a current tragedy. NeoReviews. 2003;4(2):e30–e32. [Google Scholar]
- 4.American Academy of Pediatrics. Subcommittee on Hyperbilirubinemia Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation [published correction appears in Pediatrics. 2004;114(4):1138] Pediatrics. 2004;114(1):297–316. doi: 10.1542/peds.114.1.297. [DOI] [PubMed] [Google Scholar]
- 5.Maisels MJ, Kring E. Early discharge from the newborn nursery: effect on scheduling of follow-up visits by pediatricians. Pediatrics. 1997;100(1):72–74. doi: 10.1542/peds.100.1.72. [DOI] [PubMed] [Google Scholar]
- 6.Bernstein H, Spino C, Finch S, et al. Inadequate attention given to jaundice in healthy term infants during the first postpartum week [abstract] Pediatr Res. 2004;55(2):282A. [Google Scholar]
- 7.Cabana MD, Rand CS, Powe NR, et al. Why don't physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282(15):1458–1465. doi: 10.1001/jama.282.15.1458. [DOI] [PubMed] [Google Scholar]
- 8.Bhutani VK, Johnson L, Sivieri EM. Predictive ability of a predischarge hour-specific serum bilirubin for subsequent significant hyperbilirubinemia in healthy term and near-term newborns. Pediatrics. 1999;103(1):6–14. doi: 10.1542/peds.103.1.6. [DOI] [PubMed] [Google Scholar]
- 9.Maisels MJ, Kring EA. Routine transcutaneous bilirubin (TcB) measurements in the nursery predict the risk of subsequent hyperbilirubinemia. E-PAS. 2006;59 doi: 10.1038/jp.2009.43. 5575.479. [DOI] [PubMed] [Google Scholar]
- 10.Worsley JB. Hyperbilirubinemia guidelines and unintended harms. Pediatrics. 2004;114(4):1134–1135. doi: 10.1542/peds.2004-1584. [DOI] [PubMed] [Google Scholar]
- 11.Coleman EA, Smith JD, Raha D, Min SJ. Posthospital medication discrepancies: prevalence and contributing factors. Arch Intern Med. 2005;165(16):1842–1847. doi: 10.1001/archinte.165.16.1842. [DOI] [PubMed] [Google Scholar]
- 12.Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–167. doi: 10.7326/0003-4819-138-3-200302040-00007. [DOI] [PubMed] [Google Scholar]
- 13.Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. Adverse drug events occurring following hospital discharge. J Gen Intern Med. 2005;20(4):317–323. doi: 10.1111/j.1525-1497.2005.30390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McMillan TE, Allan W, Black PN. Accuracy of information on medicines in hospital discharge summaries. Intern Med J. 2006;36(4):221–225. doi: 10.1111/j.1445-5994.2006.01028.x. [DOI] [PubMed] [Google Scholar]
- 15.Salem-Schatz S, Peterson LE, Palmer RH, et al. Barriers to first-week follow-up of newborns: findings from parent and clinician focus groups. Jt Comm J Qual Saf. 2004;30(11):593–601. doi: 10.1016/s1549-3741(04)30070-5. [DOI] [PubMed] [Google Scholar]
- 16.Maisels MJ, Kring E. Follow up (FU) following early discharge: it is possible to change pediatricians' practices [abstract] Pediatr Res. 2001;49(2):139A. [Google Scholar]
- 17.Grol R, Grimshaw J. From best evidence to best practice: effective implementation of change in patients' care. Lancet. 2003;362(9391):1225–1230. doi: 10.1016/S0140-6736(03)14546-1. [DOI] [PubMed] [Google Scholar]
- 18.Davis D, O'Brien MA, Freemantle N, Wolf FM, Mazmanian P, Taylor-Vaisey A. Impact of formal continuing medical education: do conferences, workshops, rounds, and other traditional continuing education activities change physician behavior or health care outcomes? JAMA. 1999;282(9):867–874. doi: 10.1001/jama.282.9.867. [DOI] [PubMed] [Google Scholar]
- 19.Grimshaw JM, Shirran L, Thomas R, et al. Changing provider behavior: an overview of systematic reviews of interventions. Med Care. 2001;39(8 suppl 2):II2–II45. [PubMed] [Google Scholar]
- 20.Jamtvedt G, Young JM, Kristoffersen DT, O'Brien MA, Oxman AD. Audit and feedback: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2006;(2) doi: 10.1002/14651858.CD000259.pub2. CD000259. [DOI] [PubMed] [Google Scholar]
- 21.Margolis PA, Lannon CM, Stuart JM, Fried BJ, Keyes-Elstein L, Moore DE., Jr. Practice based education to improve delivery systems for prevention in primary care: randomised trial. BMJ. 2004;328(7436):388. doi: 10.1136/bmj.38009.706319.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson LH, Bhutani VK, Brown AK. System-based approach to management of neonatal jaundice and prevention of kernicterus. J Pediatr. 2002;140(4):396–403. doi: 10.1067/mpd.2002.123098. [DOI] [PubMed] [Google Scholar]
- 23.Palmer RH, Clanton M, Ezhuthachan S, et al. Applying the “10 simple rules” of the Institute of Medicine to management of hyperbilirubinemia in newborns. Pediatrics. 2003;112(6 pt 1):1388–1393. doi: 10.1542/peds.112.6.1388. [DOI] [PubMed] [Google Scholar]
- 24.Lannon C, Stark AR, Thiessen K. Quality improvement methods facilitate strategies to improve perinatal care practices. E-PAS. 2008;63 5120.1. [Google Scholar]
- 25.Lannon C, Stark AR. Closing the gap between guidelines and practice: ensuring safe and healthy beginnings. Pediatrics. 2004;114(2):494–496. doi: 10.1542/peds.114.2.494. [DOI] [PubMed] [Google Scholar]
- 26.Petersen LA, Woodard LD, Urech T, Daw C, Sookanan S. Does pay-for-performance improve the quality of health care? Ann Intern Med. 2006;145(4):265–272. doi: 10.7326/0003-4819-145-4-200608150-00006. [DOI] [PubMed] [Google Scholar]


