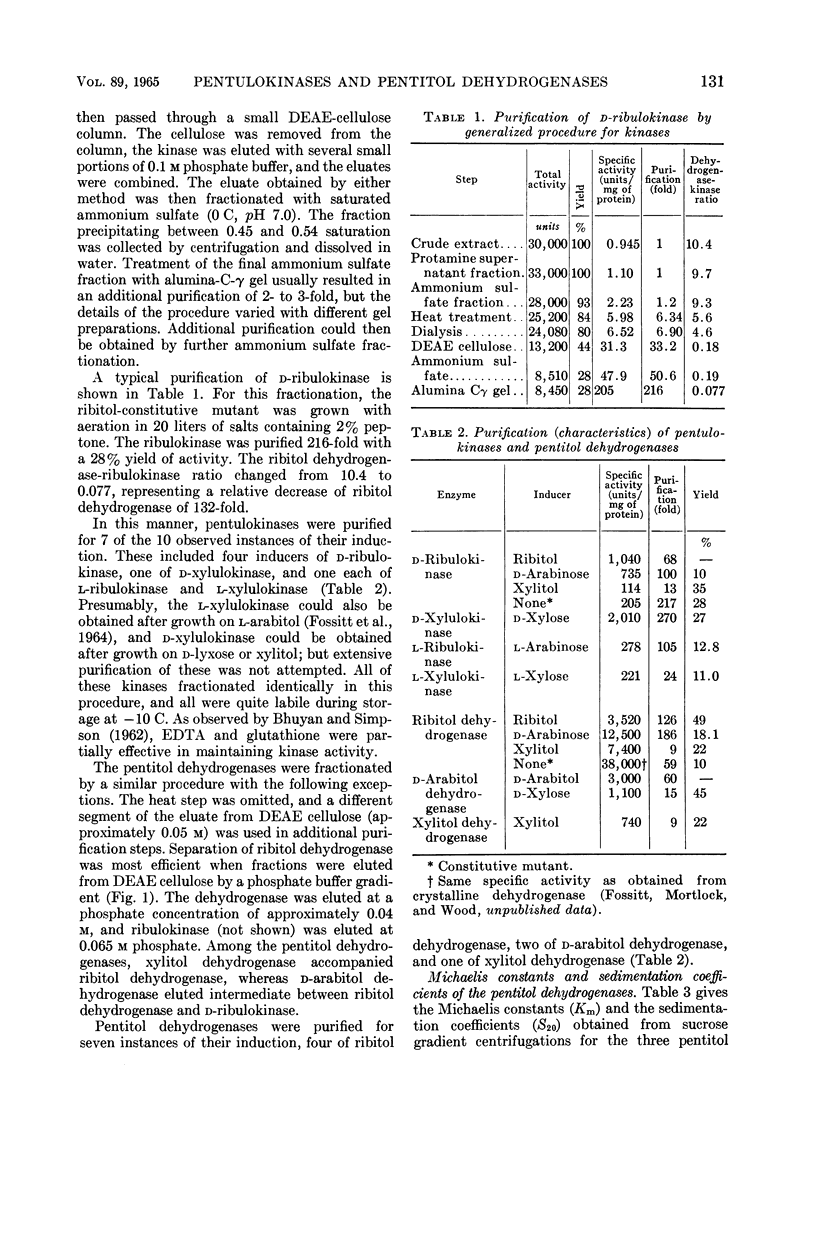
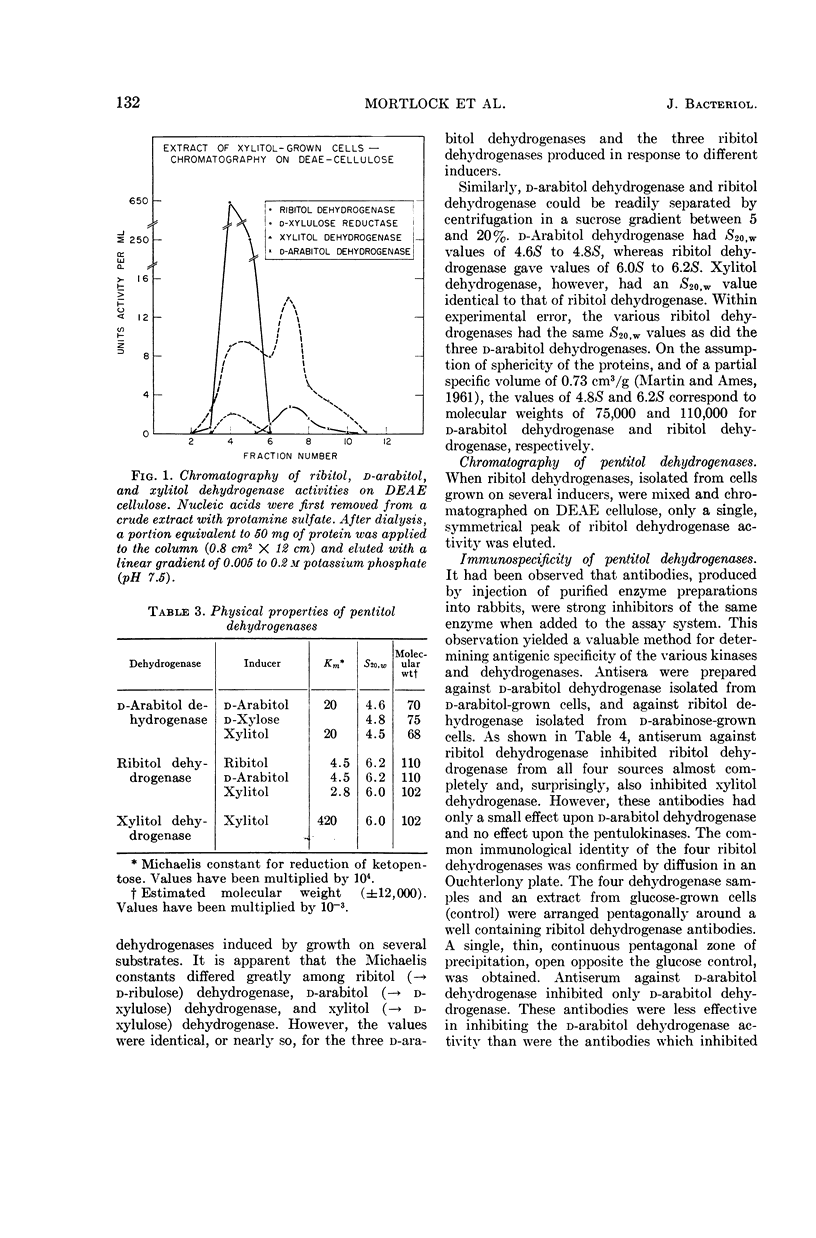
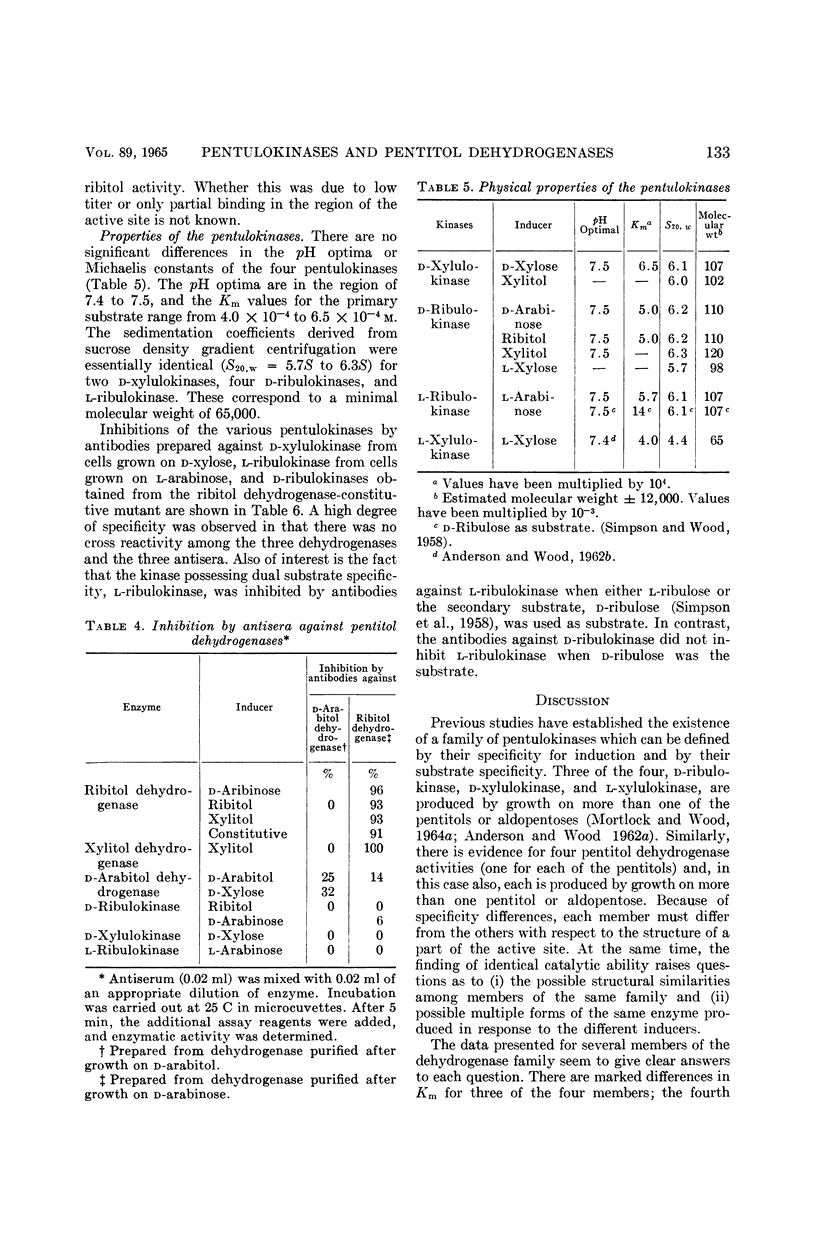
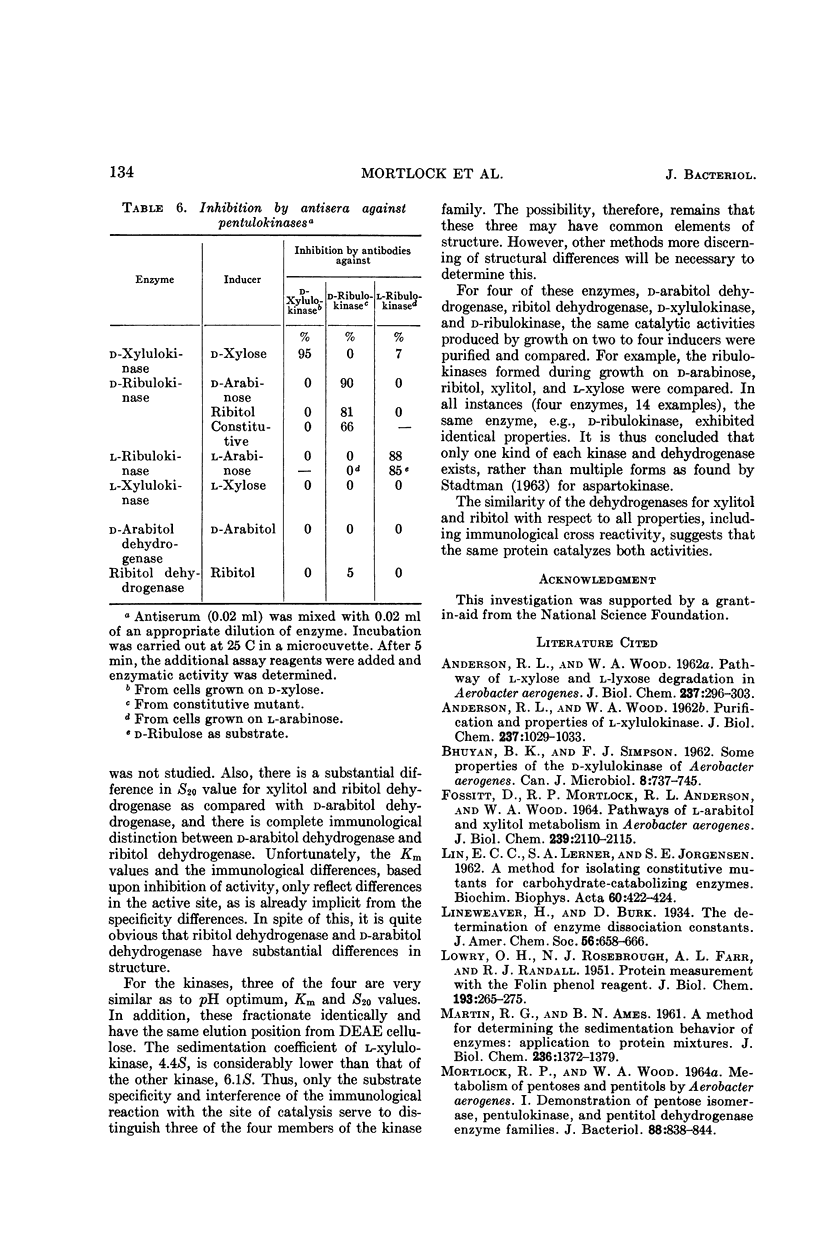
Abstract
Mortlock, R. P. (Michigan State University, East Lansing), D. D. Fossitt, D. H. Petering, and W. A. Wood. Metabolism of pentoses and pentitols by Aerobacter aerogenes. III. Physical and immunological properties of pentitol dehydrogenases and pentulokinases. J. Bacteriol. 89:129–135. 1965.—Four pentulokinases and three pentitol dehydrogenases were purified from Aerobacter aerogenes PRL-R3, and the properties of the enzymes within each family were compared. d-Ribulokinase was purified from cells grown on ribitol, d-arabitol, and xylitol, and from a mutant constitutive for ribitol dehydrogenase; d-xylulokinase, from cells grown on d-xylose and xylitol; l-ribulokinase, from cells grown on l-arabinose; and l-xylulokinase, after growth on l-xylose. Similarly, ribitol dehydrogenase was purified after growth on ribitol, d-arabinose, and xylitol, and from the ribitol dehydrogenase-constitutive mutant. d-Arabitol dehydrogenase was obtained after growth on d-arabitol or d-xylose, and xylitol dehydrogenase was obtained after growth on xylitol. Except for l-xylulokinase, which also had a different S20 value, the pentulokinases had identical pH optima, Km for the ketopentose, and sedimentation constants, and fractionated identically by a number of procedures. These kinases could be distinguished only by their substrate and immunological specificity. Ribitol dehydrogenase and d-arabitol dehydrogenase could be distinguished by several properties, but the properties of xylitol dehydrogenase were always similar to ribitol dehydrogenase. In all cases, individual kinases or dehydrogenases produced by different inducers had identical properties. These data constitute evidence against multiple forms of the same enzyme being produced by different inducers and against the dehydrogenase family containing essentially identical proteins differing only at the active site. For the kinases, three of the four appear to differ only at the active site.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON R. L., WOOD W. A. Pathway of L-xylose and L-lyxose degradation in Aerobacter aerogenes. J Biol Chem. 1962 Feb;237:296–303. [PubMed] [Google Scholar]
- ANDERSON R. L., WOOD W. A. Purification and properties of L-xylulokinase. J Biol Chem. 1962 Apr;237:1029–1033. [PubMed] [Google Scholar]
- FOSSITT D., MORTLOCK R. P., ANDERSON R. L., WOOD W. A. PATHWAYS OF L-ARABITOL AND XYLITOL METABOLISM IN AEROBACTER AEROGENES. J Biol Chem. 1964 Jul;239:2110–2115. [PubMed] [Google Scholar]
- LIN E. C., LERNER S. A., JORGENSEN S. E. A method for isolating constitutive mutants for carbohydrate-catabolizing enzymes. Biochim Biophys Acta. 1962 Jul 2;60:422–424. doi: 10.1016/0006-3002(62)90423-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- MORTLOCK R. P., WOOD W. A. METABOLISM OF PENTOSES AND PENTITOLS BY AEROBACTER AEROGENES. I. DEMONSTRATION OF PENTOSE ISOMERASE, PENTULOKINASE, AND PENTITOL DEHYDROGENASE ENZYME FAMILIES. J Bacteriol. 1964 Oct;88:838–844. doi: 10.1128/jb.88.4.838-844.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORTLOCK R. P., WOOD W. A. METABOLISM OF PENTOSES AND PENTITOLS BY AEROBACTER AEROGENES. II. MECHANISM OF ACQUISITION OF KINASE, ISOMERASE, AND DEHYDROGENASE ACTIVITY. J Bacteriol. 1964 Oct;88:845–849. doi: 10.1128/jb.88.4.845-849.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIMPSON F. J., WOLIN M. J., WOOD W. A. Degradation of L-arabinose by Aerobacter aerogenes. I. A pathway involving phosphorylated intermediates. J Biol Chem. 1958 Jan;230(1):457–472. [PubMed] [Google Scholar]
- SIMPSON F. J., WOOD W. A. Degradation of L-arabinose by Aerobacter aerogenes. II. Purification and properties of L-ribulokinase. J Biol Chem. 1958 Jan;230(1):473–486. [PubMed] [Google Scholar]
- STADTMAN E. R. Symptosium on multiple forms of enzymes and control mechanisms. II. Enzyme multiplicity and function in the regulation of divergent metabolic pathways. Bacteriol Rev. 1963 Jun;27:170–181. doi: 10.1128/br.27.2.170-181.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOD W. A., McDONOUGH M. J., JACOBS L. B. Rihitol and D-arabitol utilization by Aerobacter aerogenes. J Biol Chem. 1961 Aug;236:2190–2195. [PubMed] [Google Scholar]


