Abstract
Previous studies have reported that intrahemispheric connections between area 17 (V1, striate cortex) and other cortical visual areas are not point-to-point, but instead have some degree of convergence and divergence. Many pathological conditions can interfere with the normal development of patterns of cortico-cortical connections, but there is little information regarding whether or not early pathological insults can also induce permanent changes in the convergence and divergence of cortical connections. Obtaining this information is important because loss of precision in neural projections can contribute to functional deficits and behavioral impairment. In the present study we investigated whether retinal input is required for the development of normal values of convergence and divergence in the visual callosal pathway. We found that enucleation performed at birth induced significant increases in convergence and divergence compared to control animals. In contrast, values of convergence and divergence in rats enucleated at postnatal day 7 (P7) were similar to those in controls. Previous studies have shown that retinal input during the first postnatal week is required for the specification of the overall distribution and internal topography of visual callosal pathways. Our present results therefore extend these previous finding by showing that retinal input during the first postnatal week also specifies the precision of cortico-cortical projections. These findings raise the possibility that the precision of neural connections may be reduced in other pathological conditions that affect early development of neural connections.
Keywords: Convergence, divergence, interhemispheric, corpus callosum, plasticity, enucleation, deprivation, deafferentation
INTRODUCTION
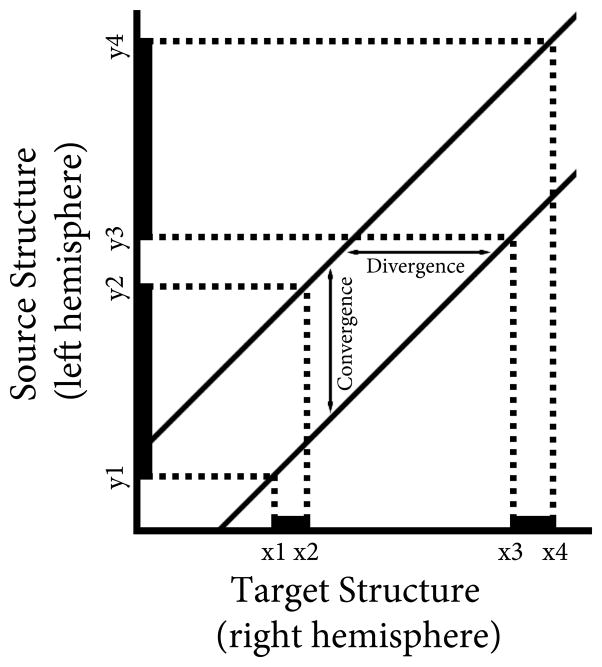
Previous studies in normal adult animals have reported that connections between area 17 (V1, striate cortex) and other cortical visual areas are not point-to-point, but instead have varying degrees of convergence and divergence [5, 7, 8, 10, 11, 20, 21, 24]. Convergence is defined here as the extent of cortex that projects to an infinitely small region of the contralateral cortex, while divergence is the extent of cortex that receives input from an infinitely small region in the contralateral cortex [24] (see Fig. 1). Several factors can contribute to convergence and divergence of connections, including the variability (scatter) of axon trajectories in white matter, the pattern of interstitial branches emerging from individual parental axons, and the size of axonal terminal arborization within gray matter. Studies of the development of neuronal projections in the visual pathway of normal kittens show decreases in convergence and divergence with age, although the magnitude of the changes depends on the neuronal system studied [11, 21].
Figure 1.
Connectivity graph constructed from the distribution of labeled cells in the left area 17 (thick black lines on the y-axis) produced by two tracer injections into the right area 17 (thick black lines on the x-axis). The graph’s origin corresponds to the most posterior point in area 17 (indicated by a star in Fig. 2), the axes indicate the distance from this point in the rostrocaudal direction. The points x1and x3 correspond to the posterior edge of the posterior and anterior tracer injections, respectively, while the points x2 and x4 correspond to the anterior edge of the posterior and anterior tracer injections, respectively. The points y1 and y3 correspond to the posterior edge of the posterior and anterior retrogradely labeled fields, respectively, while the points y2 and y4 correspond to the anterior edge of the posterior and anterior retrogradely labeled fields, respectively. The vertical distance between the line through the caudal points (x1y1, x3,y3) and the line through the rostral points (x2y2, x4y4) represents the degree of convergence, while the horizontal distance between these two lines represents the degree of divergence. Adapted from Fig. 1 in Salin, Bullier & Kennedy (1989).
While it is known that many pathological conditions can interfere with the normal development of patterns of cortico-cortical connections, there is little information regarding whether or not early pathological insults can also induce permanent changes in the convergence and divergence of cortical connections. Obtaining this information is important because loss of precision in neural projections can contribute to functional deficits and behavioral impairments. Increases in convergence and divergence may be associated with increases in the scatter and intercrossing of axonal trajectories in white matter. Such changes in fiber organization can potentially be evaluated and monitored by non-invasive imaging techniques, such as diffusion tensor imaging (DTI). DTI measures parameters of water diffusion anisotropy that depend on the shape and orientation of cellular elements, such as somas, axons and dendrites [1, 13]. For example, the use of DTI has revealed dramatic differences in white matter diffusion anisotropy between normally-sighted human adults and adults that lost sight early in life [25], but whether these differences are due to changes in convergence and divergence of axonal projections or to other factors is not known.
In the present study we investigated the changes induced by neonatal bilateral enucleation on the convergence and divergence of the callosal pathway. We chose this model because previous studies have shown that neonatal enucleation induces massive changes in the distribution and topography of the interhemispheric connections through the corpus callosum [3, 4, 14, 16, 17], but there is little information available on the effects of enucleation on the convergence and divergence of this pathway. We compared convergence and divergence values for the callosal pathway in striate cortex of adult normal rats and in adult rats bilaterally enucleated at birth (BEP0), and found that BEP0 induces a significant increase in convergence and divergence values. Previous studies have also shown that enucleation alters the distribution of cortical patterns of callosal connections only if it is performed before P6 [16]. To investigate whether values of convergence and divergence in the callosal pathway also become immune to enucleation by P6, we studied a group of animals enucleated at P7 (BEP7). We found no change in convergence and divergence in adult BEP7 animals compared to controls, suggesting that the retina is only required during a brief window early in development for the specification of both the normal overall callosal pattern and the level of precision in the projection that is observed in normal adult animals.
MATERIALS AND METHODS
A total of 12 Long-Evans rats were used in this study. All procedures involved were approved by the University of Washington Institutional Animal Care and Use Committee and were carried out in accordance with the NIH “Guide for the Care and Use of Laboratory Animals” (NIH publication no. 86–23, revised 1987). The births of the litters were determined to within 12 hours, and the first postnatal day was considered P0. Four rats were binocularly enucleated on postnatal day 0 (BEP0) under isoflurane anesthesia (2% in air), three were binocularly enucleated on postnatal day 7 (BEP7), and the remaining five rats were used as controls. After full recovery from anesthesia, animals were returned to their mothers.
Histological processing
Anatomical tracer injections were performed on 12 animals (five control, four BEP0, three BEP7) at adulthood (P60 or older). Animals were placed under 2% isoflurane anesthesia and a craniotomy was performed over the occipital lobe of the right hemisphere using a hand-held bone drill. The tracers injections were aimed at lateral portions of striate cortex because these regions are richly innervated by callosal connections in both normal and enucleated animals [14]. Single 0.01–0.02 μl injections of the retrograde fluorescent tracers rhodamine and green beads (RB and GB, respectively, LumaFluor, Naples, FL; concentrated stock solution) were administered through glass micropipettes (50–100 μm tip diameter) into area 17, just medial to the 17/18 border. The injections of both tracers were separated in the rostrocaudal direction by a distance ranging from 0.5 to 3.5 mm (Fig. 2). Following the injections, the bone chip was replaced and the muscle and skin layers were sutured closed.
Figure 2.
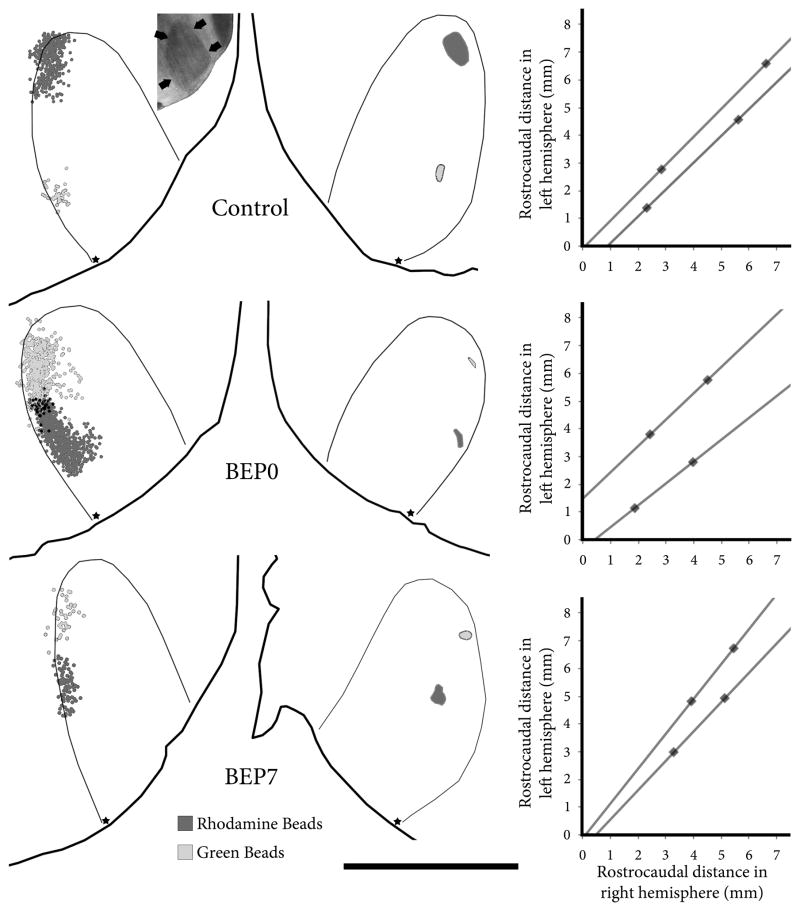
Injection sites, labeled callosal cell bodies, and connectivity graphs for control (top row), BEP0 (middle row) and BEP7 (bottom row) animals. The left column shows the labeled cells bodies in the left hemisphere plotted using Neurolucida 2.0; dark gray dots indicate cell bodies labeled with rhodamine beads and light gray dots indicate cell bodies labeled with green beads. Black dots result from overlap of the two fields (not double labeled cells). Inset in top row, left column shows the profile of area 17 as revealed by scanning wet, unstained tissue. Arrows in inset indicate border of area 17. The middle column shows the injection sites in the right hemisphere; dark gray indicates the rhodamine beads injection site and light gray indicates the green beads injection site. The thick black lines in the left and middle columns indicate the border of the tissue sections, and the thin black lines indicate the border of area 17. Stars in the left and middle column indicate the most posterior point in area 17 from which measurements were made. The right column shows the connectivity graphs that result from the data shown in the left and middle columns. Scale bar = 5 mm.
After a recovery period of 2 days, animals were deeply anesthetized with pentobarbital sodium (100 mg/kg i.p.) and perfused through the heart with 0.9% saline followed by 2% paraformaldehyde in 0.1 M phosphate buffer (PB, pH 7.4). The brains were removed, the cortices from each hemisphere were flattened between glass slides and left overnight in 0.1M PB, and the thalamus was left overnight in 30% sucrose in 0.1M PB. The following day cortices were placed in 30% sucrose in 0.1M PB for one hour, 60 μm thick sections were cut tangential to the cortical surface [15], and the thalamus was cut into 60 μm thick coronal sections with a freezing microtome. Previous studies have shown that myelin patterns can be readily revealed by scanning unstained sections [22], and we used this procedure to identify the border of area 17 (see Fig. 2 inset). The locations of labeled cell bodies in the left hemisphere, injection sites in the right hemisphere and labeled cell bodies within the ipsilateral dorsal lateral geniculate nucleus (dLGN) were plotted using a fluorescence microscope equipped with a motorized stage (LEPCO) controlled by a Dell XPS T500 computer running the graphic System Neurolucida (MicroBright-Field, Colchester, VT). Injections sites appeared as brightly fluorescent regions and borders were drawn at the sharply defined edges of these regions.
Data analysis
Using Adobe Photoshop CS2 (Adobe Systems), digital images of histological sections were carefully aligned with each other using the edges of the sections, blood vessels and other fiducial marks, and scored Neurolucida images were superimposed to reconstruct the tracer injections in the right hemisphere and the distribution of labeled callosal cells in the left hemisphere (Fig. 2).
The degree of convergence and divergence of callosal connections in area 17 was determined following methods described by Salin, Bullier, & Kennedy [24] (see Fig. 1). Connectivity graph were constructed based on the extent and location of the injections sites (plotted along the x-axis, representing the target structure) and the extent and location of the retrogradely labeled fields in the contralateral hemisphere (plotted along the y-axis, representing the source structure). Values plotted corresponded to distances measured from the most caudal point in area 17 (marked with a star in Fig. 2) to the caudal and rostral extents of the injections and labeled fields. The caudal boundary of the caudal injection (x1) is paired with the caudal boundary of the corresponding labeled field (y1), the rostral boundary of the caudal injection (x2) is paired with the rostral boundary of the caudal labeled field (y2), and so on until four points are plotted in this graph (x1y1, x2y2, x3y3, x4y4). The connectivity graph is made up of two lines, one connecting the caudal boundaries x1y1 and x3y3, and another connecting the rostral boundaries x2y2 and x4y4. When the connection between these two structures is point-to-point, the two lines overlap completely. However, when there is a degree of convergence and divergence between these two structures, the two lines are displaced in the y-plane by the degree of convergence and in the x-plane by the degree of divergence. A point 3 mm from the caudal most point in area 17, roughly corresponding to the middle of area 17 in the rostrocaudal direction, was chosen to calculate the degree of convergence and divergence in each animal. Statistic comparison between groups was performed using an ANOVA with α set at 0.05; post hoc comparisons were made using Scheffe’s method. The assumption of homogeneity of variance was met for all analyses.
RESULTS
In normal rats, callosal fibers in striate cortex connect cortical loci in both hemispheres that are in retinotopic, but not anatomic, correspondence [12]. Thus, as the injection sites move away from the border of area 17, the labeled fields move closer to the 17/18a border in the contralateral hemisphere. Moreover, it has been shown that bilateral enucleation induces the development of topographically mismatched, mirror-symmetric callosal connections, but only if performed before P6 [16, 17]. Our present results are in agreement with these previous studies, and confirm that retinal input specifies the topography of callosal maps during the first postnatal week. They also extend these previous studies by showing that neonatal enucleation increases the convergence and divergence of callosal connections. We studied changes in convergence and divergence by analyzing the patterns of callosal connections that were labeled within striate cortex of one hemisphere following restricted injections of two different tracers into contralateral striate cortex. The distance between the centers of tracer injections ranged from 0.65 to 3.55 mm in the rostrocaudal direction, with an average interinjection distance of 1.56 mm.
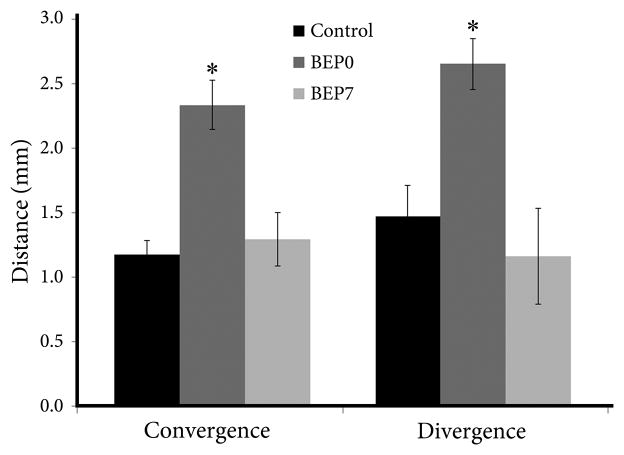
The connectivity graphs constructed from control animals yielded convergence values of 0.89–1.52 mm (Mean (M) = 1.18 mm, SD = 0.26) and divergence values of 0.91–2.24 mm (M = 1.47 mm, SD = 0.26) (Figs. 2, 3). We found that, compared to controls, enucleation at birth induced significant increases in both convergence (range 1.78–2.77 mm, M = 2.41 mm, SD = 0.44; p=0.003) and divergence (range 2.14–3.11 mm, M = 2.76 mm, SD = 0.46; p=0.033) of callosal connections (Figs. 2, 3). In contrast, in BEP7 animals the values of convergence (range 0.95–1.79 mm, M = 1.30 mm, SD = 0.44; p = 0.902) and divergence (range 0.60–2.06 mm, M = 1.17 mm, SD = 0.78; p = 0.787) were not significantly different from controls (Figs. 2, 3). We found that the diameters of injections sites measured in the rostrocaudal direction were not significantly (p = 0.283) different between control (M = 0.61mm, SD = 0.24), BEP0 (M = 0.43 mm, SD = 0.16) and BEP7 (M = 0.50 mm, SD = 0.20) animals.
Figure 3.
Histograms showing average values of convergence and divergence for control, BEP0 and BEP7 animals. Asterisks indicate a significant increase in convergence and divergence in BEP0 animals compared to control and BEP7 animals. Errors bars indicate standard error of the mean.
DISCUSSION
Previous studies have shown that retinal input specifies the normal distribution and internal topography of the visual callosal pathway [3, 4, 14, 16, 17]. The goal of the present study was to determine the effect of neonatal enucleation on the precision of visual callosal projections, as assessed my measuring convergence and divergence [24]. We found that callosal connections in area 17 of normal adult rats are not point-to-point, but rather display a degree of convergence and divergence. These results are in agreement with studies in the cat reporting that, with the exception of connections from the LGN, all intrahemispheric connections with area 17 display a degree of convergence [5, 7, 8, 10, 11, 21, 24]. Analysis of adult rats enucleated at birth showed a significant increase in the convergence and divergence of these connections, indicating that lack of retinal input during development induces a permanent reduction in the precision of the callosal projection. Our results are consistent with a study by Caric and Price [6], who reported that thalamic lesions at birth lead to an increase in the convergence and divergence of the 17–18 projection in cats studied at one month of age. However, we did not observe increases in convergence and divergence in adult rats bilaterally enucleated at P7, an age when previous studies have shown that the distribution and internal topography of the callosal pathway become immune to enucleation [14, 16]. Together, these observations suggest that retinal input specifies the overall distribution of callosal connections and the precision of this projection through common mechanisms.
Our approach was to determine the distribution of cells labeled retrogradely in striate cortex of one hemisphere following restricted tracer injections in lateral striate cortex in the opposite hemisphere. While this approach provides accurate overall values of convergence and divergence [24], it does not determine the relative contribution of factors such as variability (scatter) of axon trajectories in white matter, the pattern of interstitial branches emerging from individual parental axons, and the size of axonal terminal arborizations within gray matter. Evidence that lack of retinal input leads to larger axonal terminal arborizations comes from studies in rats [19] and hamsters [9] reporting that a proportion of callosal arbors in striate cortex of neonatally enucleated animals are more widespread than callosal arbors in normal animals. Additional information about the contribution of the size of terminal arbors to the increase in convergence and divergence in the callosal pathway of enucleated rats is likely to come from experiments analyzing the location and incidence of double-labeled cells resulting from similarly-spaced injections of two different retrograde tracers in adult control and enucleated animals. Olavarria and Safaeian [18] analyzed the effect of neonatal enucleation on the development of visual callosal connections at P6–7, just as interstitial branches of simple architecture emerge from their parental axons and grow into superficial cortical layers to form terminal arbors. They observed that restricted injections of anterograde or retrograde tracers in normal pups produced focused fields of anterogradely labeled fibers or retrogradely labeld cell bodies at topographically correct places in contralateral gray matter, whereas similar injections in enucleated pups produced diffuse fields of labeled fibers or cell bodies. However, from the observation in both control and enucleated pups of a low proportion of double labeled neurons following contralateral injections of two tracers placed close to each other, Olavarria and Safaeian [18] concluded that callosal axons in white matter commonly give rise to only one interstitial branch, and, when more than one branch originated from one axon, these tended to be close to each other. A similar result was recently reported for the development of projections from area 17 to neighboring area 18a in the rat [23]. These previous findings therefore rule out exuberant growth of interstitial branches from single parental axons as an important contributor to the increase in divergence and convergence observed in enucleated rats in the present study. Instead, they suggest that retinal input specifies the sites on the parental axons from which interstitial branches will grow to invade gray matter at topographically correct places. In contrast, in enucleated animals, the distribution of emerging interstitial branches would be guided by non-retinal cues exerting only a weak control on the topographical location of sites giving rise to interstitial branches, resulting in diffuse and scattered patterns of fiber ingrowth (see Fig. 12 in [18]). In turn, diffuse patterns of interstitial fiber ingrowth would lead to increases in the convergence and divergence of the callosal pathway in neonatally enucleated animals, in agreement with the results of the present study. Finally, the increases in convergence and divergence we found in neonatally enucleated rats may be associated at least in part with increases in the scatter and intercrossing of axonal trajectories in white matter. Measuring these changes is difficult with current anatomical techniques, but, if they occur, they may produce detectable changes in water diffusion anisotropy in white matter at adulthood. As in the rat, neonatal enucleation in the ferret induces marked changes in the distribution of visual callosal connections [3], and DTI measurements show a reduction in white mater water diffusion anisotropy in visual cortex of these animals [2]. It will be important to determine the extent to which these changes in white matter DTI reflect increases in the intercrossing of axonal projections.
In summary, earlier studies have shown that retinal input during the first postnatal week is required for the specification of the overall distribution and internal topography of visual callosal pathways. Our present results extend the role that retinal influences have on cortico-cortical development by showing that during this same postnatal period retinal input is also required for the development of callosal connections with normal values of convergence and divergence. These results raise the possibility that circuit specificity may be reduced in other pathological conditions that affect early development of neural connections. If this were the case, behavioral and cognitive impairment resulting from early pathological insults may reflect, at least in part, reductions in convergence and divergence in central nervous circuits.
Highlights.
Normal visual cortico-cortical projections show a degree of convergence/divergence.
We studied whether enucleation changes convergence/divergence in callosal projections.
Enucleation at birth induces a significant increase in convergence/divergence.
Enucleation at postnatal day 7 has no significant effect on convergence/divergence.
Retinal input during first week specifies precision of cortico-cortical projections.
Acknowledgments
This work was supported in part by National Institutes of Health grant EY016045 (to J.F.O.), and by an award from The Royalty Research Fund, University of Washington (to J.F.O.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994;66:259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bock AS, Kroenke CD, Taber EN, Olavarria JF. Critical period for the effects of neonatal enucleation on callosal connectivity and water diffusion anisotropy in the ferret visual system. Program No. 580.16. Society for Neuroscience Annual Meeting; 2010; San Diego, CA. 2010. [Google Scholar]
- 3.Bock AS, Kroenke CD, Taber EN, Olavarria JF. Retinal input influences the size and corticocortical connectivity of visual cortex during postnatal development in the ferret. Journal of Comparative Neurology. 2011 doi: 10.1002/cne.22738. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bock AS, Olavarria JF, Leigland LA, Taber EN, Jespersen SN, Kroenke CD. Diffusion tensor imaging detects early cerebral cortex abnormalities in neuronal architecture induced by bilateral neonatal enucleation: an experimental model in the ferret. Front Syst Neurosci. 2010;4:149. doi: 10.3389/fnsys.2010.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bullier J, Kennedy H, Salinger W. Branching and laminar origin of projections between visual cortical areas in the cat. J Comp Neurol. 1984;228:329–341. doi: 10.1002/cne.902280304. [DOI] [PubMed] [Google Scholar]
- 6.Carić D, Price DJ. Evidence that the lateral geniculate nucleus regulates the normal development of visual corticocortical projections in the cat. Exp Neurol. 1999;156:353–362. doi: 10.1006/exnr.1999.7031. [DOI] [PubMed] [Google Scholar]
- 7.Ferrer JM, Kato N, Price DJ. Organization of association projections from area 17 to areas 18 and 19 and to suprasylvian areas in the cat’s visual cortex. J Comp Neurol. 1992;316:261–278. doi: 10.1002/cne.903160302. [DOI] [PubMed] [Google Scholar]
- 8.Ferrer JM, Price DJ, Blakemore C. The organization of corticocortical projections from area 17 to area 18 of the cat’s visual cortex. Proc R Soc Lond B Biol Sci. 1988;233:77–98. doi: 10.1098/rspb.1988.0013. [DOI] [PubMed] [Google Scholar]
- 9.Fish SE, Rhoades RW, Bennett-Clarke CA, Figley B, Mooney RD. Organization, Development and Enucleation-induced Alterations in the Visual Callosal Projection of the Hamster: Single Axon Tracing with Phaseolus vulgaris leucoagglutinin and Di-I. Eur J Neurosci. 1991;3:1255–1270. doi: 10.1111/j.1460-9568.1991.tb00059.x. [DOI] [PubMed] [Google Scholar]
- 10.Gilbert CD, Wiesel TN. Columnar specificity of intrinsic horizontal and corticocortical connections in cat visual cortex. J Neurosci. 1989;9:2432–2442. doi: 10.1523/JNEUROSCI.09-07-02432.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kennedy H, Salin P, Bullier J, Horsburgh G. Topography of developing thalamic and cortical pathways in the visual system of the cat. J Comp Neurol. 1994;348:298–319. doi: 10.1002/cne.903480211. [DOI] [PubMed] [Google Scholar]
- 12.Lewis JW, Olavarria JF. Two rules for callosal connectivity in striate cortex of the rat. J Comp Neurol. 1995;361:119–137. doi: 10.1002/cne.903610110. [DOI] [PubMed] [Google Scholar]
- 13.Mori S, Zhang J. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron. 2006;51:527–539. doi: 10.1016/j.neuron.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 14.Olavarria J, Malach R, Van Sluyters RC. Development of visual callosal connections in neonatally enucleated rats. J Comp Neurol. 1987;260:321–348. doi: 10.1002/cne.902600302. [DOI] [PubMed] [Google Scholar]
- 15.Olavarria J, Montero VM. Relation of callosal and striate-extrastriate cortical connections in the rat: morphological definition of extrastriate visual areas. Exp Brain Res. 1984;54:240–252. doi: 10.1007/BF00236223. [DOI] [PubMed] [Google Scholar]
- 16.Olavarria JF, Hiroi R. Retinal influences specify cortico-cortical maps by postnatal day six in rats and mice. J Comp Neurol. 2003;459:156–172. doi: 10.1002/cne.10615. [DOI] [PubMed] [Google Scholar]
- 17.Olavarria JF, Li CP. Effects of neonatal enucleation on the organization of callosal linkages in striate cortex of the rat. J Comp Neurol. 1995;361:138–151. doi: 10.1002/cne.903610111. [DOI] [PubMed] [Google Scholar]
- 18.Olavarria JF, Safaeian P. Development of callosal topography in visual cortex of normal and enucleated rats. J Comp Neurol. 2006;496:495–512. doi: 10.1002/cne.20938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olavarría JF, Laing R, Hiroi R, Lasiene J. Topography and axon arbor architecture in the visual callosal pathway: effects of deafferentation and blockade of N-methyl-D-aspartate receptors. Biol Res. 2008;41:413–424. [PubMed] [Google Scholar]
- 20.Perkel DJ, Bullier J, Kennedy H. Topography of the afferent connectivity of area 17 in the macaque monkey: a double-labelling study. J Comp Neurol. 1986;253:374–402. doi: 10.1002/cne.902530307. [DOI] [PubMed] [Google Scholar]
- 21.Price DJ, Ferrer JM, Blakemore C, Kato N. Postnatal development and plasticity of corticocortical projections from area 17 to area 18 in the cat’s visual cortex. J Neurosci. 1994;14:2747–2762. doi: 10.1523/JNEUROSCI.14-05-02747.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richter CP, Warner CL. Comparison of Weigert stained sections with unfixed, unstained sections for study of myelin sheaths. Proc Natl Acad Sci U S A. 1974;71:598–601. doi: 10.1073/pnas.71.3.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruthazer ES, Bachleda AR, Olavarria JF. Role of interstitial branching in the development of visual corticocortical connections: A time-lapse and fixed-tissue analysis. J Comp Neurol. 2010;518:4963–4979. doi: 10.1002/cne.22502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salin PA, Bullier J, Kennedy H. Convergence and divergence in the afferent projections to cat area 17. J Comp Neurol. 1989;283:486–512. doi: 10.1002/cne.902830405. [DOI] [PubMed] [Google Scholar]
- 25.Shimony JS, Burton H, Epstein AA, McLaren DG, Sun SW, Snyder AZ. Diffusion tensor imaging reveals white matter reorganization in early blind humans. Cereb Cortex. 2006;16:1653–1661. doi: 10.1093/cercor/bhj102. [DOI] [PMC free article] [PubMed] [Google Scholar]