Abstract
Eukaryotic tail-anchored (TA) membrane proteins are inserted into the endoplasmic reticulum by a post-translational TRC40 pathway, but no comparable pathway is known in other domains of life. The crystal structure of an archaebacterial TRC40 sequence homolog bound to ADP•AlF4− reveals characteristic features of eukaryotic TRC40 including a zinc-mediated dimer and a large hydrophobic groove. Moreover, archaeal TRC40 interacts with the transmembrane domain of TA substrates and directs their membrane insertion. Thus, the TRC40 pathway is more broadly conserved than previously recognized.
Keywords: archaea, TRC40, Get3, tail-anchored, membrane proteins, post-translational, targeting, insertion
Eukaryotic TA proteins are found in all intracellular membranes where they perform wide-ranging functions (1–4). Those destined for compartments of the secretory pathway are inserted into the endoplasmic reticulum (ER) membrane by a recently discovered post-translational targeting pathway (5–9). Newly synthesized TA proteins are first captured by the mammalian Bag6-TRC35-Ubl4A ‘pre-targeting’ complex or its yeast ortholog, Sgt2-Get4-Get5(10–13). Next, the TA substrate is transferred to a cytosolic ATPase, called TRC40 (Get3 in yeast) (5, 7), which targets to its ER-localized membrane receptor, WRB (Get1/2 in yeast) (8, 14). Subsequently, the TA substrate is inserted into the membrane and TRC40/Get3 is recycled to the cytosol. The multiple components of this conserved eukaryotic targeting pathway ensure selectivity for the ER membrane and minimize TA protein aggregation in the cytosol(8, 15, 16).
Archaeal and bacterial genomes also encode TA proteins(17), but the mechanism underlying their insertion into the prokaryotic plasma membrane is not known. Indeed, given their simple membrane system, the relatively small number of predicted TA proteins (17), and the feasibility of ‘unassisted’ insertion (18), it is not known whether archaea or bacteria even require a specialized post-translational TA membrane protein targeting system.
The identification of archaeal sequence homologs of eukaryotic TRC40/Get3 led to the proposal that archaea possess a TA protein targeting pathway similar to that in eukaryotes(17). To test this, we first determined the X-ray crystal structure of ADP•AlF4−-bound TRC40 from the methanogen M. thermautotrophicus at 2.1 Å resolution (Table S1, Figure S1 and Methods). The archaeal enzyme adopts the same ‘closed’ conformation observed in the ADP•AlF4−-bound form of S. cerevisiae Get3(19) (Fig. 1a,b) (~36% sequence identity; r.m.s.d. Cα of 0.78 Å over 194 residues). This closed conformation results in an extensive dimer interface that spans the ATPase- and α-helical subdomains and buries two active-site nucleotides in a head-to-head conformation at the interface. The two monomers are linked by a zinc ion that is coordinated by four cysteine residues. These cysteines, two from each monomer, are part of the conserved ‘CXXC’ motif found in all known eukaryotic TRC40/Get3 orthologs, but not in functionally unrelated bacterial ArsA sequence homologs (Fig. 2).
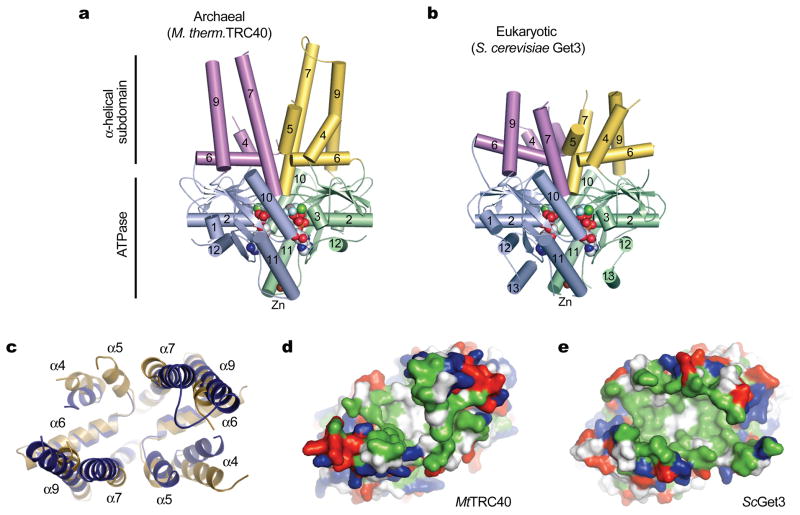
Figure 1. An evolutionarily conserved hydrophobic groove in archaeal TRC40.
Comparison of (a) M. thermautotrophicus TRC40 and (b) S. cerevisiae Get3 Mg2+ADP•AlF4− complexes (19). The ATPase domains are colored blue and green; α-helical subdomains are colored magenta and yellow; nucleotides and metals are shown as spheres. (c) Overlay of the composite hydrophobic groove in MtTRC40 (dark blue) and ScGet3 (gold). Surface representations of the closed dimer grooves from (d) MtTRC40 and (e) ScGet3, oriented as in (c). Hydrophobic residues are colored green; positively and negatively charged residues are colored blue and red respectively.
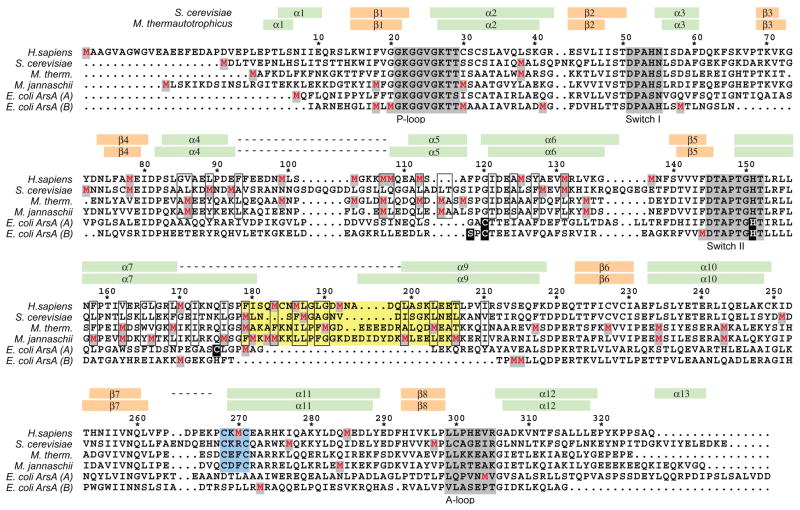
Figure 2. Sequence alignment of eukaryotic and archaeal TRC40/Get3 orthologs with the functionally distinct bacterial ArsA.
Numbering is according to M. thermautotrophicus TRC40. Secondary structure elements (green, orange) and disordered regions (dashed lines) are indicated for MtTRC40 and ScGet3. The four conserved ATPase sequence motifs are highlighted in grey. The ‘TRC40-insert’ (yellow) and the zinc-binding ‘CXXC motif’ (blue) are indicated. Hydrophobic residues lining the MtTRC40 composite groove are boxed. Methionine residues are in red; E. coli ArsA residues involved in antimony binding are in white.
The most striking structural feature of ADP•AlF4−-bound archaeal TRC40 is a large hydrophobic groove located at the dimer interface (Fig. 1a,c,d). A similar feature, observed in the ADP•AlF4−-bound structure of yeast Get3 (Fig. 1b,e), has been implicated as the site of TMD binding by a combination of structural, biochemical and genetic analysis(19–23). In archaea, as in yeast, the composite groove is constructed from a series of flexible, amphipathic helices. Notably, helix α8 (part of the ‘TRC40-insert’ sequence motif), which is observed in ‘open’ conformations of yeast Get3(19, 21, 23), is absent from the MtTRC40 structure. Instead, helices α7 and α9 form an extended hairpin structure connected by a ~12-residue loop (Fig. 1a). In the crystal, the hydrophobic surface of this loop interacts with the corresponding loop from a symmetry-related molecule. As is true for eukaryotic TRC40/Get3 orthologs, the non-polar and methionine-rich character of the groove is conserved across archaeal TRC40s (Fig. 2) (17, 19).
The conservation of key structural features suggested that the archaeal and eukaryotic TRC40 homologs share a common function. To address this directly we asked whether MtTRC40 interacts with TA substrates in vitro. Crosslinking analysis in a rabbit reticulocyte lysate (RRL) translation extract supplemented with purified MtTRC40 revealed a strong interaction with full-length human Sec61β (a model TA protein) (Fig. 3a). In contrast, an insertion-deficient ‘triple arginine’ Sec61β mutant and a construct lacking a TMD fail to interact appreciably with MtTRC40 (Fig. 3a). Thus, as is observed with eukaryotic TRC40(11), TMD hydrophobicity is an important determinant of TA protein binding to MtTRC40.
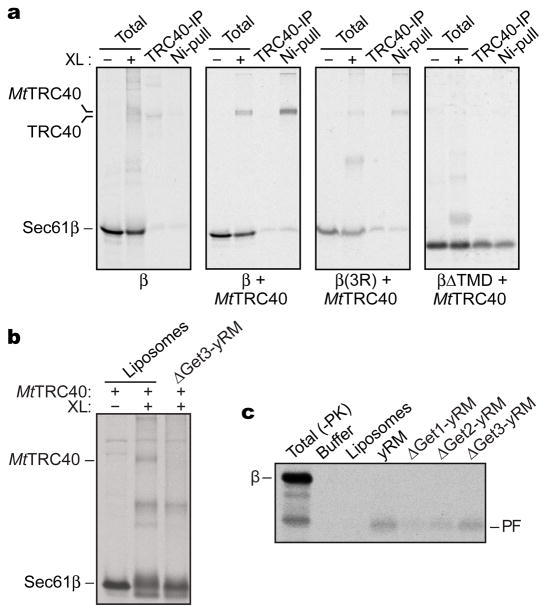
Figure 3. TA substrate binding and insertion by MtTRC40.
(a) Full-length human Sec61β, an insertion-deficient triple arginine mutant (3R) or a construct lacking its TMD (ΔTMD) were synthesized in RRL in the presence or absence of 100 ng/μl recombinant 6xHis-tagged MtTRC40. Fractions from a sucrose gradient were treated with an amine-specific crosslinker and pulled-down using anti-TRC40 antibodies (‘TRC40-IP’) or Ni-NTA sepharose beads (‘Ni-pull’). The positions of non-crosslinked Sec61β and the major crosslinked partner (endogenous mammalian TRC40 or recombinant MtTRC40) are indicated. (b) Sec61β was synthesized in PD-RRL supplemented with 5 ng/μl MtTRC40, incubated with liposomes or yeast rough microsomes prepared from a ΔGet3 strain (ΔGet3-yRM), and then subjected to chemical crosslinking. (c) Sec61β was synthesized in PD-RRL supplemented with 5 ng/μl MtTRC40. After incubation with with the indicated vesicles, insertion was monitored using a protease protection assay described previously (7, 18). The protected fragment (PF) diagnostic of proper insertion of the TA substrate is indicated.
We also examined whether archaeal TRC40 could functionally replace the endogenous mammalian TRC40 at physiological concentrations. When added to an RRL translation extract depleted of TRC40 and pre-targeting factors, MtTRC40 could be crosslinked efficiently to Sec61β (Fig. 3b). This crosslink was lost upon incubation with yeast rough microsomes (yRM) (prepared from a ΔGet3 strain) but not liposomes (Fig. 3b), suggesting that the TA protein had inserted into the yRM membrane.
To test this, we used a protease-protection assay to measure insertion of these MtTRC40-Sec61β complexes. Strikingly, Sec61β inserted efficiently into yRMs, but not into the protein-free liposomes (Fig. 3c). Moreover, microsomes from ΔGet1 or ΔGet2 yeast strains displayed reduced levels of insertion, while ΔGet3 microsomes were similar to wild-type yRMs (Fig. 3c).
The discovery of a structurally and biochemically conserved TA protein targeting factor in archaea indicates that the post-translational TRC40 pathway is more broadly conserved than previously appreciated. The absence of recognizable sequence homologs for any of the upstream eukaryotic components (e.g., Sgt2, Bag6, TRC35 or Ubl4A) suggests that the archaeal pathway represents a simplified and ancient predecessor. That MtTRC40 directs insertion into yRMs in a Get1 and Get2 stimulated manner is consistent with a functionally orthologous integral membrane receptor in archaea. If this were the case, the primary sequence identity is apparently limited since no clear homolog to eukaryotic WRB, Get1 or Get2 is seen in archaeal genomes. Thus, whether a membrane receptor for MtTRC40 exists or is required for efficient insertion remains to be determined.
Alternatively, the single membrane architecture of archaea may obviate the need for a dedicated TRC40 receptor, with TA protein insertion occurring instead by an ‘unassisted’ mechanism (18) facilitated by the lipid composition of the archaeal plasma membrane. In this view, TRC40 is serving solely as a chaperone, with its targeting function co-evolving with diversification of intracellular membranes. Defining the mechanistic basis of TA protein capture and insertion in this minimal archaeal system is an important goal for future studies.
Methods
Protein cloning, expression and purification
The gene encoding full-length M. thermautotrophicus TRC40 was amplified by PCR from genomic DNA and subcloned into pCDF-1b (Novagen). This construct was co-transformed with C-terminally 6xHis-tagged M. jannaschii Sec61β in pET21c (Novagen) into E. coli BL21(DE3)/pRIL (Novagen). Expression was carried out at room temperature for ~12 h by induction with 0.1 mM IPTG after the cells reached an A600 of ~0.6. Cells were disrupted in lysis buffer (50 mM Tris, pH 7.5, 500 mM NaCl, 5 mM β-mercaptoethanol, 10 mM imidazole, 5% glycerol, 20 μg/mL Dnase1 and 1 mM PMSF) using a high-pressure microfluidizer (Avestin). After clearing by centrifugation, the supernatant was batch-purified by nickel affinity chromatography (Ni-NTA His Bind Resin, Novagen) followed by gel filtration (Superdex 200 10/300 GL, GE Healthcare). Uncomplexed MtTRC40 dimer fractions were pooled, re-purified by gel filtration, concentrated to ~5–10 mg/ml in 10 mM Tris, pH 7.5, 150 mM NaCl and 2 mM DTT, and stored at −80°C. For biochemical analysis, the gene encoding MtTRC40 was subcloned into a pET28 derivative (Novagen) modified to incorporate a tobacco etch virus (TEV) protease cleavage site between an N-terminal 6xHis tag and the polylinker; 6xHis-tagged protein was purified by nickel affinity and size exclusion chromatography essentially as described above.
Crystallization, data collection and refinement
Crystals of MtTRC40 complexed with ADP•AlF4− were grown at room temperature using hanging drop vapor diffusion by mixing equal volumes of a ~6 mg/mL protein solution containing 2 mM ADP, 2 mM MgCl2, 2 mM AlCl3 and 8 mM NaF with a reservoir solution containing 34% pentaerythritol propoxylate (5/4 PO/OH), 50 mM HEPES, pH 7.0 and 25 mM KCl. Crystals were flash frozen directly in liquid nitrogen. Data were collected at APS beamline 21-IDF (λ=0.97856) and processed using HKL2000 (HKL Research).
The structure was determined by molecular replacement with PHASER (19) using the closed-dimer form of S. cerevisiae Get3 (2WOJ) with the α-helical subdomain removed as the search model. A data set to 2.1 Å was used for model building and refinement with COOT (20) and PHENIX (21). The final model contains two MtTRC40 homodimers, four Mg2+ADP•AlF4− complexes, two zinc atoms and 433 water molecules, and was refined to an R-factor (Rfree) of 17.7% (22.5%). No electron density was observed for residues: 1, 94–114 and 323–324 in chain A; 1, 93–105, 179–198 and 323–324 in chain B; 1, 92–115, 180–191 and 322–324 in chain C; and 1, 94–106 and 322–324 in chain D.
Crosslinking assay
Full-length 35S-labelled human Sec61β or its mutants were synthesized in a rabbit reticulocyte lysate (RRL) translation extract in the presence or absence of 100 ng/μl 6xHis-tagged MtTRC40. After translation for 30 min. at 32 °C, the mixture was subjected to 5%–25% (w/v) sucrose gradient in physiological salt buffer (50 mM HEPES pH 7.4, 100 mM potassium acetate, 7 mM magnesium acetate) for 5 hr at 55,000 in a TLS-55 rotor at 4°C. Ten fractions were collected manually from the sucrose gradient. Fractions 4 to 6 were pooled and DSS crosslinker was added to a final concentration of 250 μM. After incubating for 30 min at RT the reaction was terminated by adding 100-fold molar excess of Tris-HCl pH 7.5. Samples were boiled with 1% SDS and diluted with 10-fold excess of immunoprecipitation buffer (IP: 50 mM Tris pH 7.5, 150 mM NaCl, 1% Triton X-100) at 4°C. Anti-TRC40 antibodies (against human TRC40) or Ni-NTA Sepharose beads were added and incubated with mixing for 90 min at 4°C. To recover the immunoglobulins, Protein A agarose beads were added and mixed for 90 min at 4°C. The beads were washed three times with 1 ml of IP buffer, eluted with SDS-PAGE sample buffer and analyzed by 12% Tris-Tricine gel and autoradiography. Crosslinking was also performed in the absence of endogenous mammalian TRC40 using a phenyl- and DEAE-sepharose-depleted RRL (PD-RRL) translation extract (see below). Following synthesis of Sec61β in the presence or absence of 5 ng/μl 6xHis-tagged MtTRC40, ribosomes were removed by centrifugation and crosslinking was carried out on the supernatant as described above.
Insertion assay
Full-length 35S-labelled human Sec61β containing a double strep-tag at the N-terminus and a 3F4 epitope tag at the C-terminus was synthesized in PD-RRL supplemented with 5 ng/μl of 6xHis-tagged MtTRC40. After removing ribosomes by centrifugation, the supernatant was tested for insertion into liposomes or yeast rough microsomes (prepared from wild-type or Get deletion strains from Open Biosystems) in the presence of an energy regenerating system (2 mM ATP, 10 mM creatine phosphate, and 40 μg/ml creatine kinase). After incubating at 32°C for 30 min, the samples were treated with proteinase K (0.5 mg/ml) for 60 min on ice. The protease protected fragment (PF) was immunoprecipitated with an antibody against the 3F4 tag at the C-terminus of Sec61β.
Miscellaneous
Liposomes containing egg phosphatidylcholine and di-palmotyl phosphatidylethanolamine in a 4:1 (w/w) ratio were prepared by extrusion through 100 nm polycarbonate filter. Depleted RRL translation extract was prepared by removing ribosomes by centrifugation and passing the supernatant over a phenyl-sepharose column. The flow-through was bound to and eluted from a DEAE column, and the depleted translation extract (PD-RRL) was reconstituted from the DEAE elution and ribosomes. This lysate is devoid of TRC pathway chaperones, and is deficient for TA substrate insertion unless supplemented with physiological concentrations of TRC40/Get3 (Fig. S2).
Supplementary Material
Table S1. Data collection and refinement statistics
Figure S1. Cross-eyed stereo view of electron density for the Mg2+ADP•AlF4− complex of M. thermautotrophicus TRC40. The refined model is superimposed onto a σA-weighted 2Fo-Fc map calculated at 2.1 Å resolution and contoured at 2σ.
Figure S2. Characterization of the depleted translation extract (PD-RRL). Sec61β was translated in PD-RRL in the presence or absence of recombinant yeast Get3. An aliquot was subjected to chemical crosslinking with DSS to evaluate substrate interactions with 5 ng/μl Get3 (top panel). The remainder was incubated with yRMs and analyzed for insertion by the protease protection assay (bottom panel). PD-RRL lacks all known chaperones that interact with ER-directed TA proteins, and is deficient for insertion; activity was restored by supplementing with physiological levels of TRC40/Get3.
Acknowledgments
Data were collected at beamline 21-IDF at the Advanced Photon Source (APS), Argonne National Laboratory, and we thank the beamline staff for support. Use of the Advanced Photon Source, an Office of Science User Facility operated for the U.S. Department of Energy (DOE) Office of Science by Argonne National Laboratory, was supported by the U.S. DOE under Contract No. DE-AC02-06CH11357. We thank Maureen Downing for cloning the M. therm. TRC40 homolog, and Agnieszka Mateja and Malgorzata Dobosz for help with data collection. This work was supported by the Intramural Research Program of the NIH (to R.S.H.), NIH grant T32 GM008720 (to J.S.), an Edward Mallinckrodt, Jr. Foundation Grant (to R.J.K.), and NIH Grant R01 GM086487 (to R.J.K.).
Footnotes
Author Contributions
J.S. and P.D. carried out cloning, protein purification and crystallization. J.S., P.D. and R.J.K. performed data collection and J.S. carried out the structure determination and model building. M.M. and R.S.H. performed the substrate-binding and membrane insertion assays. R.J.K. conceived the project, guided experiments and wrote the paper. All authors discussed the results and commented on the manuscript.
Author Information
The x-ray structure of Mg2+ADP•AlF4− bound M. therm. TRC40 is deposited in the Protein Data Bank under ID code 3zq6.
References
- 1.Kutay U, Hartmann E, Rapoport TA. A class of membrane proteins with a C-terminal anchor. Trends Cell Biol. 1993;3(3):72–75. doi: 10.1016/0962-8924(93)90066-a. [DOI] [PubMed] [Google Scholar]
- 2.Beilharz T, Egan B, Silver PA, Hofmann K, Lithgow T. Bipartite signals mediate subcellular targeting of tail-anchored membrane proteins in Saccharomyces cerevisiae. J Biol Chem. 2003;278(10):8219–8223. doi: 10.1074/jbc.M212725200. [DOI] [PubMed] [Google Scholar]
- 3.Kalbfleisch T, Cambon A, Wattenberg BW. A bioinformatics approach to identifying tail-anchored proteins in the human genome. Traffic. 2007;8(12):1687–1694. doi: 10.1111/j.1600-0854.2007.00661.x. [DOI] [PubMed] [Google Scholar]
- 4.Kriechbaumer V, Shaw R, Mukherjee J, Bowsher CG, Harrison AM, Abell BM. Subcellular distribution of tail-anchored proteins in Arabidopsis. Traffic. 2009;10(12):1753–1764. doi: 10.1111/j.1600-0854.2009.00991.x. [DOI] [PubMed] [Google Scholar]
- 5.Favaloro V, Spasic M, Schwappach B, Dobberstein B. Distinct targeting pathways for the membrane insertion of tail-anchored (TA) proteins. J Cell Sci. 2008;121(Pt 11):1832–1840. doi: 10.1242/jcs.020321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Favaloro V, Vilardi F, Schlecht R, Mayer MP, Dobberstein B. Asna1/TRC40-mediated membrane insertion of tail-anchored proteins. Journal of Cell Science. 2010 doi: 10.1242/jcs.055970. [DOI] [PubMed] [Google Scholar]
- 7.Stefanovic S, Hegde RS. Identification of a targeting factor for posttranslational membrane protein insertion into the ER. Cell. 2007;128(6):1147–1159. doi: 10.1016/j.cell.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 8.Schuldiner M, Metz J, Schmid V, Denic V, Rakwalska M, Schmitt HD, Schwappach B, Weissman JS. The GET complex mediates insertion of tail-anchored proteins into the ER membrane. Cell. 2008;134(4):634–645. doi: 10.1016/j.cell.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borgese N, Fasana E. Targeting pathways of C-tail-anchored proteins. Biochim Biophys Acta. 2010 doi: 10.1016/j.bbamem.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 10.Leznicki P, Clancy A, Schwappach B, High S. Bat3 promotes the membrane integration of tail-anchored proteins. J Cell Sci. 2010;123(Pt 13):2170–2178. doi: 10.1242/jcs.066738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mariappan M, Li X, Stefanovic S, Sharma A, Mateja A, Keenan RJ, Hegde RS. A ribosome-associating factor chaperones tail-anchored membrane proteins. Nature. 2010;466(7310):1120–1124. doi: 10.1038/nature09296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang F, Brown EC, Mak G, Zhuang J, Denic V. A chaperone cascade sorts proteins for posttranslational membrane insertion into the endoplasmic reticulum. Mol Cell. 2010;40(1):159–171. doi: 10.1016/j.molcel.2010.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang Y-W, Chuang Y-C, Ho Y-C, Cheng M-Y, Sun Y-J, Hsiao C-D, Wang C. Crystal structure of Get4-Get5 complex and its interactions with Sgt2, Get3, and Ydj1. J Biol Chem. 2010;285(13):9962–9970. doi: 10.1074/jbc.M109.087098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vilardi F, Lorenz H, Dobberstein B. WRB is the receptor for TRC40/Asna1-mediated insertion of tail-anchored proteins into the ER membrane. J Cell Sci. 2011;124(Pt 8):1301–1307. doi: 10.1242/jcs.084277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jonikas MC, Collins SR, Denic V, Oh E, Quan EM, Schmid V, Weibezahn J, Schwappach B, Walter P, Weissman JS, Schuldiner M. Comprehensive characterization of genes required for protein folding in the endoplasmic reticulum. Science. 2009;323(5922):1693–1697. doi: 10.1126/science.1167983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Copic A, Dorrington M, Pagant S, Barry J, Lee MC, Singh I, Hartman JLt, Miller EA. Genomewide analysis reveals novel pathways affecting endoplasmic reticulum homeostasis, protein modification and quality control. Genetics. 2009;182(3):757–769. doi: 10.1534/genetics.109.101105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borgese N, Righi M. Remote origins of tail-anchored proteins. Traffic. 2010;11(7):877–885. doi: 10.1111/j.1600-0854.2010.01068.x. [DOI] [PubMed] [Google Scholar]
- 18.Brambillasca S, Yabal M, Soffientini P, Stefanovic S, Makarow M, Hegde RS, Borgese N. Transmembrane topogenesis of a tail-anchored protein is modulated by membrane lipid composition. EMBO J. 2005;24(14):2533–2542. doi: 10.1038/sj.emboj.7600730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mateja A, Szlachcic A, Downing ME, Dobosz M, Mariappan M, Hegde RS, Keenan RJ. The structural basis of tail-anchored membrane protein recognition by Get3. Nature. 2009;461(7262):361–366. doi: 10.1038/nature08319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bozkurt G, Stjepanovic G, Vilardi F, Amlacher S, Wild K, Bange G, Favaloro V, Rippe K, Hurt E, Dobberstein B, Sinning I. Structural insights into tail-anchored protein binding and membrane insertion by Get3. Proc Natl Acad Sci USA. 2009;106(50):21131–21136. doi: 10.1073/pnas.0910223106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu J, Li J, Qian X, Denic V, Sha B. The crystal structures of yeast Get3 suggest a mechanism for tail-anchored protein membrane insertion. PLoS ONE. 2009;4(11):e8061. doi: 10.1371/journal.pone.0008061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suloway CJ, Chartron JW, Zaslaver M, Clemons WM., Jr Model for eukaryotic tail-anchored protein binding based on the structure of Get3. Proc Natl Acad Sci U S A. 2009;106(35):14849–14854. doi: 10.1073/pnas.0907522106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamagata A, Mimura H, Sato Y, Yamashita M, Yoshikawa A, Fukai S. Structural insight into the membrane insertion of tail-anchored proteins by Get3. Genes Cells. 2010;15(1):29–41. doi: 10.1111/j.1365-2443.2009.01362.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Data collection and refinement statistics
Figure S1. Cross-eyed stereo view of electron density for the Mg2+ADP•AlF4− complex of M. thermautotrophicus TRC40. The refined model is superimposed onto a σA-weighted 2Fo-Fc map calculated at 2.1 Å resolution and contoured at 2σ.
Figure S2. Characterization of the depleted translation extract (PD-RRL). Sec61β was translated in PD-RRL in the presence or absence of recombinant yeast Get3. An aliquot was subjected to chemical crosslinking with DSS to evaluate substrate interactions with 5 ng/μl Get3 (top panel). The remainder was incubated with yRMs and analyzed for insertion by the protease protection assay (bottom panel). PD-RRL lacks all known chaperones that interact with ER-directed TA proteins, and is deficient for insertion; activity was restored by supplementing with physiological levels of TRC40/Get3.