Abstract
Secretins form mega-Dalton bacterial membrane channels in at least four sophisticated multi-protein systems that are crucial for translocation of proteins and assembled fibers across the outer membrane of many species of bacteria. Secretin subunits contain multiple domains, which interact with numerous other proteins, including pilotins, secretion system partner proteins and exoproteins. Our understanding of the structure of secretins is rapidly progressing, and we now recognize that features common to all secretins include a cylindrical arrangement of 12–15 subunits, a large periplasmic vestibule with a wide opening on one end and a periplasmic gate at the other end. Secretins might also play a key role in the biogenesis of their cognate secretion systems.
Secretins: functions and characteristics
Life as we know it requires busy traffic across cellular membranes. This includes transport of large and small molecules in either direction across bacterial envelopes. In Gram-negative bacteria, multiple systems are involved in the secretion of proteins to the extracellular space and in assembly of fiber structures on the cell surface [1]. Three of these systems feature large, multimeric, outer membrane channels formed by membrane proteins called secretins: the type II secretion system (T2SS), the type IV pili system (T4PS) and the type III secretion system (T3SS) (Figure 1). Secretins also participate in the assembly and extrusion of filamentous bacteriophages. In plant, animal and human bacterial pathogens, many proteins secreted by these systems are important virulence factors.
Figure 1. Secretins in Gram-negative bacteria.
Schematic view of the type II and type III secretion systems, type IV pili system and bacteriophage assembly system. The secretin is the major outer membrane component of all these systems. The insertion of secretins into the outer membrane is often assisted by specific lipoproteins called pilotins. The T2SS secretes exoproteins from the periplasm to the extracellular space in the folded form. The T2SS pseudopilus is formed by multiple pseudopilin subunits; the pseudopilus is thought to act as a piston and/or plug during the secretion process (Box 1). The T4PS is related to the T2SS in several architectural and functional aspects, but a key difference is that the pilus extends outside the bacterial surface. The T3SSs transport effectors directly to the eukaryotic cytoplasm or membrane via a hollow needle. The inner membrane complexes of the T2SS, T4PS and the T3SS are composed of multiple proteins that include at least one ATPase involved in providing energy for secretion or pilus extension/retraction processes. The filamentous phage assembly system is composed of a secretin and two inner membrane proteins. E, extracellular space; OM, outer membrane; P, periplasm; IM, inner membrane; C, cytoplasm.
The T2SS is responsible for secreting toxins and hydrolytic enzymes from the periplasm to the extracellular milieu in many Gram-negative bacteria. In Vibrio cholerae and enterotoxicogenic Escherichia coli (ETEC), it secretes cholera toxin and heat-labile enterotoxin, the hallmark virulence factors of cholera and children’s diarrhea, respectively. The T2SS consists of multiple copies of 12–14 different proteins distributed over three subassemblies: the outer membrane complex, a filamentous pseudopilus which remains in the periplasm, and the inner membrane platform [2]. The T4PS assembles and disassembles long extracellular polymeric fibers on the surfaces of many pathogenic and environmental bacteria, including Neisseria gonorrhoeae, Pseudomonas aeruginosa and V. cholerae [3]. The individual pilus is composed of multiple type 4 pilin (T4P) subunits from two subclasses: the T4aP and T4bP. The T4P systems are responsible for a wide variety of functions as diverse as host cell attachment, twitching motility, biofilm formation and DNA uptake; some T4PSs are also capable of secreting specific exoproteins [4–6]. The T4PS is an assembly of more than 12 different proteins [7] and shares many functional and structural features with the T2SS [8]. The T3SS, also called the injectisome, is a protein transport pathway that delivers virulence factors from the bacterial cytoplasm directly into the membrane or cytosol of the target animal or plant cell [9]. Many human pathogens, including enteropathogenic E. coli (EPEC), Salmonella typhimurium, Shigella flexneri and Yersinia enterocolitica, use the T3SS to transport multiple proteins called effectors, which modulate a variety of crucial biochemical functions in the host cell, such as inflammatory responses, cytoskeleton remodeling, apoptosis and phagocytosis. The injectisome consists of a multi-protein basal body spanning both membranes with an attached hollow needle, or pilus [10]. Filamentous bacteriophages use a relatively simple system composed of a secretin and two additional proteins to assemble into particles and exit bacteria without causing lysis [11].
The widespread emergence of antibiotic resistance in pathogenic bacteria requires the exploration of novel classes of antibacterial compounds. Because the T2SS, T3SS and T4PS are important for virulence of many human, animal and plant pathogens, targeting bacterial virulence functions — and secretin-containing systems in particular — has been suggested as an attractive strategy for the development of new drugs [12].
In these four families of secretion and assembly systems, secretins provide the 50–80 Å wide outer membrane pores for translocation of folded proteins, assembly of oligomeric fibers or DNA uptake. During secretin function, the pore opening and closing must be carefully regulated to maintain the integrity of the outer membrane and the periplasmic content. All secretins are large multi-domain proteins with a canonical secretin domain at or near the C-terminus [13, 14] (Figure 2a). Secretin assembly is a multi-step process which is often carried out with the help of small lipoproteins, called pilotins. Here, we review recent studies of secretins and pilotins, with an emphasis on their overall architecture and domain structure (Table 1). Rapid progress is being made in unraveling their symmetries and in understanding the functions of their different domains. In particular, crystal structures of domains now can be placed into electron microscopy maps of increasingly high resolution. The assembly process remains a mystery in many ways, but the role of pilotins in this process are being unraveled for some cases. Exciting times are ahead based on (i) the first views of the conformational changes secretins must undergo to perform their functions; (ii) the first discernable images of an exoprotein in the periplasmic vestibule of a secretin; and (iii) the preliminary steps undertaken to inhibit these sophisticated outer membrane proteins.
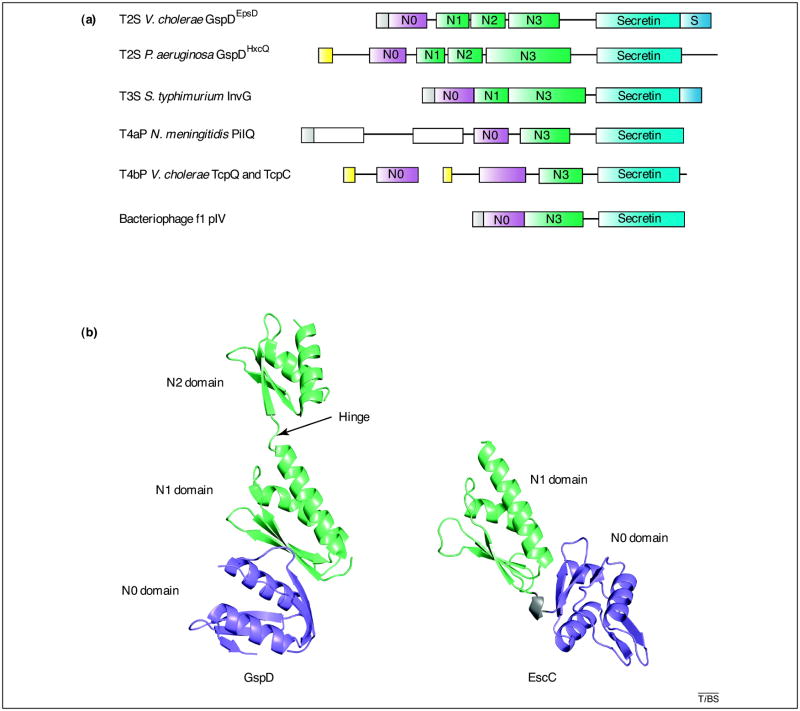
Figure 2. Secretin domains – modular organization and structures.
(a) Domain composition of secretins. Secretins are synthesized as precursors with N-terminal signal sequences recognized and cleaved off by a signal peptidase (grey) or a prolipoprotein signal peptidase (yellow). In the latter case, secretins are lipoproteins with acylated N-terminal Cys residues. The C-terminal secretin core homology domain (light blue; Pfam family PF00263 [75]) contains putative amphipathic transmembrane β-strands. The N-terminal N0 domain (purple; Pfam PF07660) is followed by one or several homologous repeat domains (light green; Pfam PF03958) termed N1–N3 depending on the number of repeats; some N3 domains have long loop insertions. The T4bPS secretins require small periplasmic proteins for stability and multimerization [65, 66] that also have putative N0 domains. Some secretins require specific lipoproteins, known as pilotins, for correct outer membrane targeting and contain C-terminal pilotin-interaction domains (dark blue), called S domains in the T2SS secretins. The pilotin-interaction domains are not related in sequence, which reflects the diversity of cognate pilotins. Some secretins contain domains of unknown topology (white).
(b) Crystal structures of secretin domains. The N0-N1-N2 domains structure of ETEC GspD [15] (PDB 3EZJ) and the N0-N1 domains structure of EPEC EscC [16] (PDB 3GR5) are superimposed and shown in the same orientation relative to the N1 domains. The core of the N0 domain (purple) has a βαββαββ fold that is structurally related to the signaling domain of the TonB-dependent outer membrane receptors [18], a lipoprotein DotD from the Legionella pneumophila type IVb secretion system [20], a domain of protein VgrG from the E. coli type VI secretion system [19], and a domain of protein gp27 from T4-related bacteriophages [17]. As expected from sequence homology, the repeat N1 and N2 domains (light green) have similar βαββα folds; the first helix in the N1 domain is a tandem of 310 and α helices. The fold of N1 domain is different from N0, but structurally related to the eukaryotic type I KH (hnRNP K homology) domain [76], which is also found in several ring-forming inner membrane T3SS proteins: EPEC EscJ [21], S. typhimurium PrgH [16] and InvA [22, 23]. Although the structures of individual N0 and N1 domains of GspD and EscC superimpose well, the relative orientation of these domains and the N0–N1 contact interface is different in the T2SS and T3SS secretins. In the ETEC GspD structure, the N2 domain connects to the N0–N1 lobe via a potentially flexible linker. The relative orientation of the N2 domain with respect to the N0-N1 lobe is stabilized by crystal contacts and interactions with a nanobody (not shown) and is presumed to differ from the orientation in the secretin multimer [15].
Table 1.
Structural studies of secretins
| X-ray crystallography | ||||
|---|---|---|---|---|
| Protein name | System | Domains | Reference | PDB |
| ETEC GspD | T2SS | N0-N1-N2 | [15] | 3EZJ |
| EPEC EscC | T3SS | N0-N1 | [16] | 3GR5 |
| Electron microscopy | ||||
| Protein name | System and sample state | Symmetry and | Reference | EMDBa |
| K. oxytoca GspDPulD | T2SS secretinb | C12 | [48] | |
| K. oxytoca GspDPulD | T2SS secretin | [84] | ||
| K. oxytoca GspDPulD | T2SS secretinc | C12, closed | [24] | |
| V. cholerae GspDEpsD | T2SS secretin | C12, closed | [25] | 1763 |
| Y. enterocolitica YscC | T3SS secretin | C13 | [85] | |
| S. typhimurium InvG | T3SS complexd | C20 | [30] | 1100, 1224 |
| S. typhimurium InvG | T3SS complexe | C20 | [86] | 1214, 1215 |
| S. typhimurium InvG | T3SS complex | C15 | [32] | 1871, 1874, 1875 |
| S. flexneri MxiD | T3SS complex | [87] | 1422 | |
| S. flexneri MxiD | T3SS complex | C12 | [31] | 1617 |
| N. meningitidis PilQ | T4PS secretin | C12 | [88] | |
| N. meningitidis PilQ | T4PS secretin | C12 | [26] | |
| N. meningitidis PilQ | T4PS secretin | C4 (quasi C12) | [27] | |
| N. meningitidis PilQ | T4PS secretin-pilus complex | C4 (quasi C12) | [89] | |
| N. meningitidis PilQ | T4PS complexf | C14 | [28] | |
| N. gonnorrhoea PilQ | T4PS complexf | C14 | [28] | |
| T. thermophilus PilQ | T4PS secretin | [29] | ||
| bacteriophage f1 pIV | phage assembly system secretin | C14 (D14), closed | [34] | |
Available deposition numbers are indicated.
Secretin–pilotin complex
Secretin–pilotin complex and trypsinized complex reconstructions.
Needle and base complexes.
T3SS complexes from ΔinvJ and ΔprgJ mutants.
Complexes in isolated membranes
Crystallographic studies of secretin domains
The crystal structure of the first three N-terminal domains N0-N1-N2 of the T2SS secretin GspD from ETEC was solved with the assistance of a nanobody, a camelid antibody fragment (Figure 2b) [15]. The structure shows that the three periplasmic GspD domains are arranged into two lobes: a compact N-terminal lobe containing the N0 and N1 domains; and the second lobe containing the N2 domain. The GspD domains N1 and N2 share the same fold which is different from that of the N0 domain.
A study on periplasmic domains from the EscC secretin from the EPEC T3SS shows two N-terminal domains that are connected by a linker (Figure 2b) [16]; these two N-terminal domains adopt folds similar to those observed in the N0 and N1 domains of the ETEC GspD T2SS secretin. The mutual orientation of these domains with respect to each other in the T2SS and T3SS secretins is, however, surprisingly different. When the N1 domains of T2SS and T3SS are superimposed, the N0 domains are rotated by not less than 143 degrees (Figure 2b). Further studies are needed to establish whether these structural differences (i) simply indicate that the first two domains are differently organized in these two secretins; or, (ii) represent different conformations of a flexible unit that can be adopted by both secretins during secretion or assembly. Interestingly, both N0 and N1 folds also occur in several other, non-secretin, bacterial membrane proteins [16–23].
Electron microscopy studies of secretins
A number of electron microscopy studies on secretins have been published, including several three-dimensional cryo-electron microscopy (cryo-EM) reconstructions (Table 1). Cryo-EM studies on the T2SS secretin GspDPulD (in this review we use the standard T2SS nomenclature followed by a species-specific name in superscript) from Klebsiella oxytoca show a cylindrical dodecameric arrangement of secretin subunits with the N-terminal domains located in the periplasm [24]. An open ring is present in the outer leaflet of the outer membrane with weak connections to a second ring in the periplasm and the inner leaflet of the outer membrane. The periplasmic entrance of the cylinder is wide open, but a continuous density closes off the periplasmic part of the structure from the outer membrane region. The most recent T2SS cryo-EM structure is that of the secretin GspDEpsD from V. cholerae [25]. The 12-fold symmetric multimeric channel has the shape of an inverted cup of 200 Å in length and an outer diameter of 155 Å (Figure 3a). The bottom periplasmic part of the channel appears highly convoluted. Above, the smooth outer membrane part of the channel is followed by a narrower extracellular gate with a 10 Å opening. Furthermore, the cross-section of the map reveals a 125 Å-long cylindrical periplasmic vestibule that is closed off at one end by the periplasmic gate. This periplasmic vestibule has an opening to the periplasm with an inner diameter of 75 Å (Figure 3a). The internal diameter of the periplasmic vestibule narrows to a 55 Å-wide constriction located 70 Å from the opening. The periplasmic gate appears as a continuous density and closes off the periplasmic vestibule from an extracellular chamber that is 100 Å in diameter.
Figure 3. Electron microscopy structures of secretins.
(a) Cryo-EM reconstruction of V. cholerae T2SS secretin GspDEpsD [25] (EMDB 1763). In side view, three domains are identified from top to bottom: the extracellular cap, the outer membrane domain and the periplasmic domain. In a cross-section, the channel reveals an extracellular gate, an extracellular chamber, a periplasmic gate and a periplasmic vestibule with a constriction (yellow).
(b) The secretin architecture is conserved in different secretion systems. (i): fitting of 12-fold symmetrical ring models of the N-terminal periplasmic domains (N0 domain in purple, N1–N3 domains in light green) into the GspDEpsD density map [25]. The N0 and N1 domains ring is anchored at the bottom of the map. The N2 domain ring fits into the central periplasmic domain density and the N3 domain ring into the periplasmic constriction. (ii): EM reconstruction of S. typhimurium T3SS base complex in the closed state [30] (EMDB 1224). OR – outer ring, IR – inner ring. (iii): EM reconstruction of the S. flexneri T3SS needle complex in the open state [31] (EMDB 1617). OMR – outer membrane ring, IMR – inner membrane ring. The secretin (blue) occupies the top part of the T3SS reconstructions above the inner membrane complex – (ii) and (iii). V. cholerae GspDEpsD secretin is in a closed state; compare (i) and (ii). The modeled locations of N0 (purple solid ovals) and N1 (light green solid ovals) domain rings in the T3SS secretins are shown [33, 41], as well as putative locations of the N3 (light green open ovals) domain ring in the constriction site (upper neck or OMR3) based on the corresponding N3 domain ring fit in the T2SS secretin.
(c) Cryo-EM reconstruction of S. typhimurium T3SS secretin InvG as part of the needle complex [32]. (i): fitting of the periplasmic domains of InvG (N0 domain in purple, N1 domain in light green) into the density map (EMDB 1875). Inner membrane part of the needle complex is omitted for clarity. (ii)–(iii): tilted and top views of the C15 map (EMDB 1871) corresponding to the neck region of InvG secretin with fitting of the periplasmic domains (PDB 2Y9K).
(d) Experimental visualization of the secretin in the T3SS complex. Class averages of the S. typhimurium T3SS needle complexes purified from wild type (i) and ΔinvG secretin mutant (ii) strains. (iii) The density difference between averaged images of wild type and ΔinvG complexes [(i) − (ii) = (iii)] indicates the position of the secretin in the complex. Reproduced from [33] under the terms of the Creative Commons Attribution License. Copyright: © 2010 Schraidt et al.
(e) Electron microscopy analysis of the T. thermophilus T4PS secretin PilQ. A class average of purified PilQ shows a 150 Å wide and 340 Å long particle with features similar to the T2SS and T3SS secretins (compare with the side view (a) and (b, ii)) [29]. The upper (outer membrane) part of the PilQ particle reveals a bisecting density corresponding to the periplasmic gate. The conical lower (periplasmic) part of PilQ consists of six concentric rings that likely correspond to the N-terminal secretin domains; sequence and fold-recognition analysis of PilQ by the Pcons server [63] indicates the presence of an N0 domain and five putative N1-like domains. Reproduced with permission from [29]. Copyright: © 2011 the American Society for Biochemistry and Molecular Biology.
The structure of the T4PS secretin PilQ from Neisseria meningitidis was initially described as a doughnut-like channel with one open end and C12 symmetry [26]. Subsequent cryo-EM and two-dimensional crystal analyses led to a C4 (quasi-C12) symmetrical model that is closed on both ends but lacks the periplasmic gate found in all other secretin reconstructions [27]. The T4PS secretin PilQ from N. meningitidis, as well as its N. gonorrhoeae homolog, has also been studied in isolated membranes [28]. The axial views show a double-ring structure with 14–15 fold symmetry for the central ring which is assumed to be the secretin. Furthermore, a recent study on the T4PS PilQ from Thermus thermophilus [29] shows in class-averaged side-views a particle with a width of 150 Å and a length of 340 Å. The reconstruction reveals six rings in the periplasm, thought to be formed by the N-terminal 500 residues, underneath a cup structure in the membrane (Figure 3e). The views along the cylinder axis indicate an assembly of approximately 12 to 14 subunits.
The T3SS secretins have been studied by electron microscopy as part of the needle complex that spans both outer and inner membranes, and includes inner membrane, needle and shaft proteins (Figure 3b). Initial reconstructions of the S. typhimurium needle and base complexes were obtained using 20-fold averaging [30]. However, the reconstruction of the S. flexneri needle complex indicates a 12-fold symmetry for the outer membrane, secretin-containing region and 24-fold symmetry for the inner membrane region [31]. Surprisingly, a very recent higher resolution reconstruction of the S. typhimurium needle complex revealed a 15-fold symmetry for the secretin and a 24-fold symmetry for the inner membrane ring, giving a 3-fold symmetry for the overall complex [32]. By comparing 3D reconstructions of the T3SS complexes with available secretin structures from other systems, it has been suggested that the secretin occupies most of the outer membrane rings [16, 31]. The actual location of the secretin in the complex has been experimentally shown by comparing basal complexes isolated from wild-type and secretin-deficient strains [33] (Figure 3d). Thus, the available data suggest that the secretin occupies the top 3 ring structures in the needle and also in the base complexes of T3SS (Figure 3b). Importantly, in the base structure without needle components, several major T3SS secretin features strongly resemble those of the T2SS GspD secretin. Indeed, one can identify a periplasmic vestibule, a periplasmic gate (or septum) and an extracellular chamber in both the T3SS and the T2SS secretin (Figure 3b).
The pIV secretin of the phage assembly system that has been studied by cryo-EM reveals a barrel-like structure composed of two multimeric secretin channels with D14 symmetry [34]. A single multimer channel has a diameter of 135 Å and length of 120 Å. A large pore that has a variable diameter from 60 to 88 Å is blocked by a feature similar to the periplasmic gate seen in the GspDEpsD secretin structure [25]. Interestingly, the pore diameter in the pIV structure is smaller than the diameter of a phage particle, pointing to a similar mechanism of gating as in the T2SS that might involve a constriction site that initiates considerable conformational changes (Box 1).
Box 1. Possible mode of action of the secretin from the T2SS.
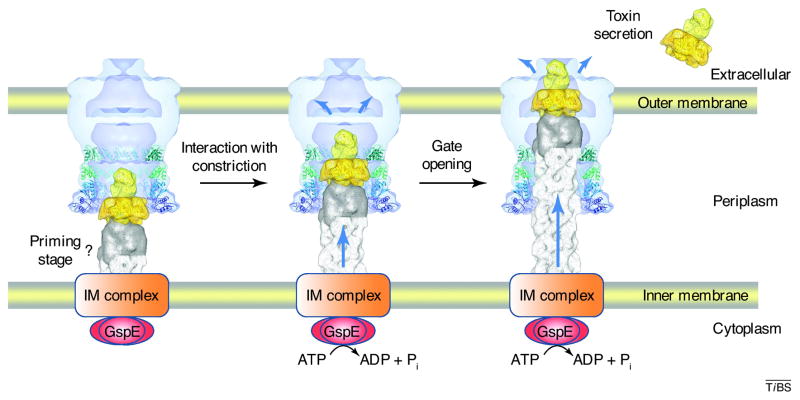
A model for the mechanism of action of the T2SS has been proposed [25], continuing on the ideas of a pseudopilus piston mechanism (Figure I) [46, 77, 78]. The model is based on a wealth of data [2, 79], of which here only recent results can be mentioned. Surface plasmon resonance (SPR) studies showed that the N0-N1-N2 domains of ETEC GspD interact with an exoprotein, the B-pentamer of heat-labile enterotoxin [25]. More recently, electron microscopy studies indicated a binding site of the B-pentamer of cholera toxin in the entrance to the periplasmic vestibule of V. cholerae GspDEpsD [39]. The fact that the N-terminal part of the secretin is (by sequence) the most divergent domain of this protein, is consistent with the notion that its N-terminus is involved in the recognition of exoproteins. Other studies report that the HR domain of the inner membrane protein GspC interacts with the N-terminal domains of GspD [71, 72]. In addition, it has been shown that the periplasmic domains of the T2SS secretin from ETEC interacts with the tip of the T2SS pseudopilus formed by the pseudopilins GspK, GspI and GspJ [25].
Comparing the diameter of the constriction site in V. cholerae GspDEpsD with the dimensions of secreted proteins and the tip of the pseudopilus [80] led to the hypothesis that interactions with the constriction site of the secretin might be important for opening and closing of the periplasmic gate[25]. A possible sequence of events is the following: Once the inner membrane protein GspC has contacted N0, and sensed the arrival of an exoprotein in the periplasmic vestibule of GspD, it transmits a signal to the secretion ATPase, whereupon the latter starts expending energy to elongate the pseudopilus. The inner membrane protein GspLEpsL interacts with both the secretion ATPase GspEEpsE [81, 82] and the major pseudopilin GspGEpsG [83], and very likely plays a key role in adding subunits to the pseudopilus. The growing pseudopilus pushes the exoprotein from the secretin vestibule further upwards until the constriction site is reached. This contact induces large conformational changes in the T2SS secretin which results in opening of the periplasmic gate and eventually to release of the exoprotein into extracellular space. The nature and extent of the conformational changes which the T2SS secretin undergo still have to be determined, but a comparison of the large structural differences in the T3SS secretin with and without needle (Figure 3b) indicates that secretins can adopt very different conformations.
The range of 12–15 subunits represented by secretins from different systems and species is intriguing, yet, in other cases, homologous proteins have also been observed to form assemblies with dramatically different symmetries [35, 36]. Indeed, ATPases have been reported to change symmetry in response to nucleotide binding [37]. Moreover, detergents vs. lipid environments can alter the symmetry of membrane proteins [38]. Hence, a diversity of symmetries is certainly also a possibility in the case of the secretins.
Combining Crystallographic and Electron Microscopy Structural Data
With high-resolution domain crystal structures and electron microscopy reconstructions of secretins in hand, several groups have reported on combining the results of these studies. The structures of the N0-N1-N2 domains of GspD have been used to generate 12-fold symmetrical rings [15]. Although the crystal structure of the N3 domain has not been solved yet, it is predicted to have a structure similar to the N1 and N2 domains based on high sequence homology. Dodecameric ring models composed of N0-N1, of N2 and of N3 domains have been placed in the density map of V. cholerae GspDEpsD [25]. The N0-N1 ring fits into the widest area at the bottom of the periplasmic part of the secretin, and the N2 and N3 rings can be positioned directly above the N0-N1 ring in the map (Figure 3b). In this model, the N3 domain ring fits at the constriction site of the cryo-EM map, suggesting an important function for this domain (Box 1). Interestingly, a recent study shows that the B-pentamer of cholera toxin binds in the lower part of the periplasmic vestibule of the V. cholerae GspDEpsD secretin [39].
For the T3SS, the structures of the N0 and N1 domains of EPEC EscC have been used to generate ring-models with C12 and C14 symmetry of secretin domains by a novel procedure for modeling symmetrical protein assemblies [40]. These ring models, with symmetries ranging from 12- to 14-fold, have been fitted into cryo-EM reconstructions of S. typhimurium and S. flexneri T3SS complexes [16, 41]. The 14-fold symmetrical models fit the density best when placed in the lower part of the secretin or neck region (Figure 3b). The N0 domain is located below the N1 domain in this model — close to the inner membrane rings — consistent with biochemical data which show interactions between the N0 domain of the secretin InvG and the C-terminal domain of inner membrane protein PrgH [33, 41]. In another study, the secretin InvG was reported to occupy the outer rings and ‘neck’ regions of the S. typhimurium needle complex but the symmetry of the secretin has not been determined [33]. Most recently, a homology model of the N0 and N1 domains of the InvG secretin fits in the neck region of the recent S. typhimurium needle complex cryo-EM map with 15-fold symmetry for the secretin[32] (Figure 3c), consistent with previous cross-linking and modeling data [33, 41].
It appears that the general placements of ring models of N0 and N1 domains of the T3SS secretin are presently in agreement with each other, and also with the models for the T2SS secretin. The N3 domain has not been formally placed in the density maps of T3SS; however, based on the high degree of homology between T3SS and T2SS secretins, we can tentatively place the N3 ring in the narrower part of the connector region of the injectisome complex corresponding to the constriction site of the T2SS secretin in the periplasmic vestibule (Figure 3b).
Another striking common feature of both the T2SS and T3SS secretin cryo-EM reconstructions is the periplasmic gate (Figure 3b). The importance of this feature for the mechanism of action of these two secretins might be quite different, however, given that the inner wall of the T3SS secretin dodecamer becomes, to a large degree, covered internally by rings of other proteins (Figure 3b), in contrast with the T2SS and T4PS secretins. A recent study on the bacteriophage secretin pIV identified two regions, called GATE1 and GATE2, within the C-terminal secretin domain in which amino acid substitutions result in a ‘leaky’ channel phenotype, indicating destabilization of the closed state [42]. Interestingly, several mutations resulting in a similar phenotype have also been identified in the N3 domain, in agreement with a possible triggering role of this domain in secretin gate opening (Box 1).
Pilotins assist in the assembly of secretins
In many cases, the assembly of secretins in the outer membrane requires a specific small outer membrane lipoprotein — called a pilotin [43]. These proteins might assist its cognate secretin in targeting to the outer membrane and in formation or stabilization of the secretin multimer [44]. The C-terminal region in the T2SS and T3SS secretins interacts with their corresponding T2SS and T3SS pilotins (Figure 2a) [45–47]. A pilotin may or may not form a stable complex with the secretin [48]; moreover, ionic detergents typically required for secretin solubilization could affect the complex stability during in vitro studies. Intriguingly, pilotins from different systems appear to be entirely unrelated in sequence and structure, yet, they play similar functional roles in the biogenesis of their secretins.
The lipoprotein MxiM from the S. flexneri T3SS is required for the stability, outer membrane targeting, and multimerization of the secretin MxiD [49]. The crystal structure of the pilotin MxiM reveals a conical shape β-barrel structure interrupted by an α-helix (Figure 4a) [50]. The hydrophobic cleft at the top of the β-barrel serves as a binding site for either lipids or the C-terminal domain of MxiD [50, 51]. The ability of MxiM to bind lipids and secretin at the same site prompted a model for pilotin-mediated targeting of secretins to the outer membrane [51]. It has been suggested that as a first step MxiM binds its own N-terminal lipid. Subsequent binding of the C-terminal region of MxiD substitutes the lipid moiety and makes it available to engage the Lol sorting pathway [52]. As a result, the MixM–MxiD complex is targeted to the outer membrane where formation of the secretin multimer can occur. Interestingly, the characterized T3SS pilotins from S. typhimurium InvH [47, 53] and Yersinia enterocolitica YscW [54] are not homologous in sequence to S. flexneri MxiM, or to each other.
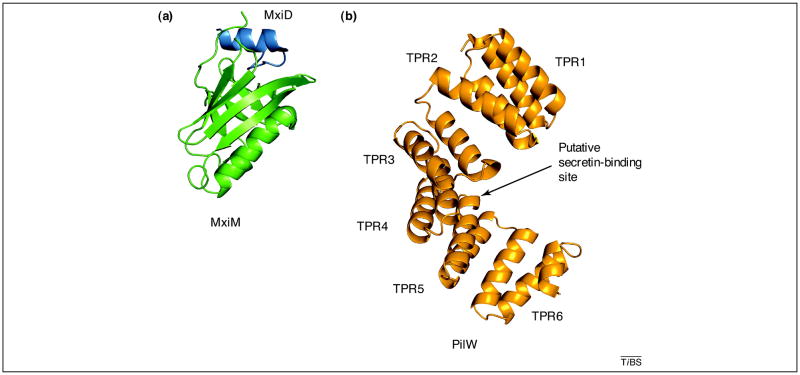
Figure 4. Structures of pilotins.
These structures are remarkably dissimilar in spite of a common function. (a) The structure of the S. flexneri T3SS pilotin MxiM (green) in complex with the C-terminal fragment of the secretin MxiD (dark blue) [51] (PDB 2JW1). The ‘cracked β barrel’ of MxiM forms a cleft at the top for binding of a short α helix of MxiD. (b) The structure of the N. meningitidis T4PS pilotin PilW (orange) [59] (PDB 2VQ2) is composed of six tetratricopeptide repeat (TPR) motifs arranged as a super-helix. Structures of a homologous P. aeruginosa pilotin PilF (26% sequence identity with N. meningitidis PilW) show a similar arrangement of TPR motifs [56, 58].
The pilotin PilF from P. aeruginosa is required for T4aPS biogenesis [55] and is involved in the outer membrane targeting and multimerization of the T4PS secretin PilQ [56]. A homologous pilotin PilW from N. meningitidis appears to be essential for secretin multimer formation, but not for targeting to the outer membrane [57]. Both P. aeruginosa PilF and N. meningitidis PilW are composed of six tetratricopeptide repeats (TPRs) arranged as a super helix (Figure 4b) [56, 58, 59]. The orientation of TPR motifs 5 and 6 is different in the structures of PilF and PilW, with the N- and C-terminal parts of PilW being closer compared to the corresponding parts of PilF [56]. It is not clear whether this structural flexibility is important for function or is a result of crystal packing. The concave surfaces on the PilF and PilW structures have been suggested as potential binding sites for the secretin. However, which part of T4aP secretin interacts with a pilotin is still unknown. Indeed, T4aP secretins lack the C-terminal domain that is responsible for pilotin binding in T2SS and T3SS secretins (Figure 2a). Notably, N. meningitidis lipoprotein PilP was initially described as a pilotin due to its role in assembly and stabilization of the T4aPS secretin PilQ [60], but subsequent studies showed that PilP is an inner membrane lipoprotein [61] and part of the inner membrane of the T4PS [62]. Therefore, PilP should not be referred to as pilotin; it is instead a functional homolog of the inner membrane T2SS protein GspC, and also a predicted structural homolog of the homology region (HR) domain of GspC [63].
Some secretins are lipoproteins and do not depend on separate pilotins for correct outer membrane targeting. A recently identified lipoprotein secretin GspDHxcQ from the second T2SS of P. aeruginosa has a long N-terminal linker that presumably extends from the outer membrane to the bottom part of the secretin multimer (Figure 2a) [64]. The lipid moiety is essential for GspDHxcQ function, given that a non-lipidated mutant secretin is incorrectly targeted to the inner membrane. The lipoprotein secretins BfpB and TcpC from the T4bP systems of EPEC and V. cholerae, respectively, require periplasmic proteins BfpG and TcpQ for multimerization and stability [65, 66]. However, fold-recognition analysis by the Pcons server [63] indicates that BfpG and TcpQ have an N0-secretin-like fold and therefore could function as integral parts of the secretin multimeric channel, not as pilotins (Figure 2a).
Role of secretins in the assembly of secretion systems
In the T2SS, T3SS and T4P systems, secretins are connected to multiprotein complexes in the inner membrane (Figure 1). Does the secretin multimer form first and the inner membrane platform assembles later, is it the other way around, or do they assemble independently and interact when needed? Several recent studies have provided some clues, but the answer is complicated. In the K. oxytoca T2SS, the fusion of the secretin GspDPulD with a monomeric red fluorescent protein, mCherry, forms distinct fluorescent foci at the cell periphery [67]. Furthermore, in the V. cholerae T2SS, the GFP-fusions of two inner membrane complex proteins, GspCEpsC and GspMEpsM, localize in punctate fluorescent foci along the full length of the bacterial membrane [68]. Those fluorescent foci, which likely represent the fully-assembled functional T2SS complexes, have not been observed in a V. cholerae strain with the secretin gene deleted. Expression of the GspDEpsD secretin from a plasmid in secretin deletion mutants restores the punctate fluorescence of GFP-GspCEpsC and GFP-GspMEpsM, as well as secretion activity. Therefore, the secretin GspDEpsD appears to be crucial for proper localization of the inner membrane proteins GspCEpsC and GspMEpsM, and also for assembly of the complete T2SS complex. A study of the Y. enterocolitica T3SS assembly using fluorescent protein fusions with secretin YscC, and also with the inner membrane proteins YscD and YscJ, reveals a similar pattern of isolated fluorescent foci on the cell surface [69]. Moreover, the formation of fluorescent foci by YscD or YscJ is dependent on the presence of the secretin YscC, whereas the assembly of YscC is independent of YscD or YscJ. It has been suggested that the assembly of the Y. enterocolitica T3SS injectisome follows an outside–in process that starts with secretin ring formation [69], analogous to the proposed order in V. cholerae T2SS assembly [68]. However, the assembly of the S. typhimurium T3SS does not appear to depend on the presence of a secretin. It has also been shown that the inner membrane proteins PrgH and PrgK, homologs of Y. enterocolitica YscD and YscJ, can form a ring-like structure in the absence of other proteins [70]. Even more remarkably, an incomplete S. typhimurium needle complex has been isolated from a mutant strain that lacks the secretin (Figure 3d) [33]. Taken together, the studies on Y. enterocolitica and S. typhimurium injectisomes indicate that T3SS biogenesis might have evolved to follow different pathways in these species. Therefore, the role of secretins in assembly of cognate secretion complexes appears to be system- and possibly even species-specific.
Concluding remarks
Secretins are key components of several complex transport systems present in many bacteria, including a number of major bacterial pathogens. The structures of isolated secretins, or as parts of larger assemblies, have been studied by electron microscopy methods. It is only recently that high resolution crystal structures of soluble secretin domains from T2SS and T3SS, which are localized in the periplasmic regions of the electron microscopy maps, have become available. Secretins assemble into 12- to 15-meric cylindrical channels with the C-terminal domain embedded into the outer membrane and the N-terminal domains arranged into rings that form a large periplasmic vestibule. This vestibule is generally wide open towards the periplasm and closed off by the periplasmic gate, except in the assembled injectisome. This gate probably undergoes conformational changes after interacting with exoproteins and/or partner proteins of the secretion system. The functions of several domains of the secretins are emerging: the N-terminal domains interact with domains from inner membrane proteins [33, 41, 71, 72], and at least in some cases, with exoproteins [25, 39, 46]; the N3 domains might contain a constriction site which could play a role in triggering conformational changes [25, 42]. The secretin domain is inserted into the outer membrane; and the S-domain of the T2SS and a functional homologous C-terminal domain of the T3SS interacts with a pilotin, an assembly assistant. A proposed mechanism for the transport of cholera toxin across the outer membrane by the T2SS of V. cholerae combines multiple functional and structural results (Box 1).
However, we still need to obtain a large amount of additional information — both structural and biochemical — to fully understand how secretins function to secrete proteins, take up DNA, or assemble fibrous filaments. Establishing the symmetry of secretins across species and systems will be an important area of future research. Future studies of the secretin family of proteins in different conformational states are needed for a better understanding of transmembrane transport in bacteria, and this understanding could assist in the development of novel drugs interfering with the translocation process.
The recent progress in our understanding of the architecture and assembly of secretins, and the beginning of our insight into their interactions with pilotins, is especially important because secretins and pilotins represent attractive targets for the development of novel antibacterial compounds. The possibility to specifically inhibit secretion was demonstrated by engineering specific binding proteins against the K. oxytoca secretin GspDPulD [73]. Furthermore, in a functional screen for T3SS inhibitors, a compound was identified that interfered with needle complex assembly in S. typhimurium [74]. Most intriguingly, the same compound also inhibited the function of Y. enterocolitica T3SS, P. aeruginosa T2SS and P. aeruginosa T4PS. These studies might represent the very first steps in what could become a fruitful translation of our understanding of these impressive outer membrane proteins into compounds of relevance for preventing and curing several major infectious diseases.
Figure I.
Mechanism of the T2SS. Modified and reproduced with permission from [25]. Copyright: © 2010 Reichow et al.
Acknowledgments
We thank Stewart Turley, Steve Reichow, Jan Steyaert and Els Pardon for contributions to the studies on the T2SS secretin in our laboratories, Ariel Blocker for discussion of unpublished data and Maria Sandkvist for stimulating discussions of secretion mechanism. This work was supported by award RO1AI34501 (to W.G.J.H) from the National Institute of Allergy and Infectious Diseases. We thank the Murdock Charitable Trust and the Washington Research Foundation for generous support of our electron cryo-electron microscopy laboratory. T.G. is a Howard Hughes Medical Institute Early Career Scientist.
Glossary box
- Effectors
proteins secreted by the T3SS into host target cells
- Exoproteins
proteins secreted across the outer membrane
- Needle complex
a large multiprotein complex, spanning the inner and outer membrane from bacteria, involved in delivering proteins from bacteria to the membrane and the cytosol of target host cells via an extended hollow needle
- Pilotin
small lipoprotein involved in the assembly of the secretin multimer
- Secretin
large outer membrane protein, part of the T2SS, T4PS, T3SS and phage assembly systems
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wooldridge K. Bacterial secreted proteins: secretory mechanisms and role in pathogenesis. Caister Academic Press; 2009. [Google Scholar]
- 2.Johnson TL, et al. Type II secretion: from structure to function. FEMS Microbiol Lett. 2006;255:175–186. doi: 10.1111/j.1574-6968.2006.00102.x. [DOI] [PubMed] [Google Scholar]
- 3.Pelicic V. Type IV pili: e pluribus unum? Mol Microbiol. 2008;68:827–837. doi: 10.1111/j.1365-2958.2008.06197.x. [DOI] [PubMed] [Google Scholar]
- 4.Kirn TJ, et al. Secretion of a soluble colonization factor by the TCP type 4 pilus biogenesis pathway in Vibrio cholerae. Mol Microbiol. 2003;49:81–92. doi: 10.1046/j.1365-2958.2003.03546.x. [DOI] [PubMed] [Google Scholar]
- 5.Hager AJ, et al. Type IV pili-mediated secretion modulates Francisella virulence. Mol Microbiol. 2006;62:227–237. doi: 10.1111/j.1365-2958.2006.05365.x. [DOI] [PubMed] [Google Scholar]
- 6.Han X, et al. Type IV fimbrial biogenesis is required for protease secretion and natural transformation in Dichelobacter nodosus. J Bacteriol. 2007;189:5022–5033. doi: 10.1128/JB.00138-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Craig L, Li J. Type IV pili: paradoxes in form and function. Curr Opin Struct Biol. 2008;18:267–277. doi: 10.1016/j.sbi.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ayers M, et al. Architecture of the type II secretion and type IV pilus machineries. Future Microbiol. 2010;5:1203–1218. doi: 10.2217/fmb.10.76. [DOI] [PubMed] [Google Scholar]
- 9.Cornelis GR. The type III secretion injectisome, a complex nanomachine for intracellular ‘toxin’ delivery. Biol Chem. 2010;391:745–751. doi: 10.1515/BC.2010.079. [DOI] [PubMed] [Google Scholar]
- 10.Marlovits TC, Stebbins CE. Type III secretion systems shape up as they ship out. Curr Opin Microbiol. 2010;13:47–52. doi: 10.1016/j.mib.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Russel M, et al. Filamentous phage assembly: variation on a protein export theme. Gene. 1997;192:23–32. doi: 10.1016/s0378-1119(96)00801-3. [DOI] [PubMed] [Google Scholar]
- 12.Baron C. Antivirulence drugs to target bacterial secretion systems. Curr Opin Microbiol. 2010;13:100–105. doi: 10.1016/j.mib.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Martin PR, et al. Characterization of pilQ, a new gene required for the biogenesis of type 4 fimbriae in Pseudomonas aeruginosa. Mol Microbiol. 1993;9:857–868. doi: 10.1111/j.1365-2958.1993.tb01744.x. [DOI] [PubMed] [Google Scholar]
- 14.Genin S, Boucher CA. A superfamily of proteins involved in different secretion pathways in Gram-negative bacteria: modular structure and specificity of the N-terminal domain. Mol Gen Genet. 1994;243:112–118. doi: 10.1007/BF00283883. [DOI] [PubMed] [Google Scholar]
- 15.Korotkov KV, et al. Crystal structure of the N-terminal domain of the secretin GspD from ETEC determined with the assistance of a nanobody. Structure. 2009;17:255–265. doi: 10.1016/j.str.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spreter T, et al. A conserved structural motif mediates formation of the periplasmic rings in the type III secretion system. Nat Struct Mol Biol. 2009;16:468–476. doi: 10.1038/nsmb.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanamaru S, et al. Structure of the cell-puncturing device of bacteriophage T4. Nature. 2002;415:553–557. doi: 10.1038/415553a. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Herrero A, Vogel HJ. Nuclear magnetic resonance solution structure of the periplasmic signalling domain of the TonB-dependent outer membrane transporter FecA from Escherichia coli. Mol Microbiol. 2005;58:1226–1237. doi: 10.1111/j.1365-2958.2005.04889.x. [DOI] [PubMed] [Google Scholar]
- 19.Leiman PG, et al. Type VI secretion apparatus and phage tail-associated protein complexes share a common evolutionary origin. Proc Natl Acad Sci U S A. 2009;106:4154–4159. doi: 10.1073/pnas.0813360106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakano N, et al. Crystal structure of Legionella DotD: insights into the relationship between type IVB and type II/III secretion systems. PLoS Pathog. 2010;6:e1001129. doi: 10.1371/journal.ppat.1001129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yip CK, et al. Structural characterization of the molecular platform for type III secretion system assembly. Nature. 2005;435:702–707. doi: 10.1038/nature03554. [DOI] [PubMed] [Google Scholar]
- 22.Worrall LJ, et al. Crystal structure of the C-terminal domain of the Salmonella type III secretion system export apparatus protein InvA. Protein Sci. 2010;19:1091–1096. doi: 10.1002/pro.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lilic M, et al. A conserved domain in type III secretion links the cytoplasmic domain of InvA to elements of the basal body. Acta Crystallogr D Biol Crystallogr. 2010;66:709–713. doi: 10.1107/S0907444910010796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chami M, et al. Structural insights into the secretin PulD and its trypsin-resistant core. J Biol Chem. 2005;280:37732–37741. doi: 10.1074/jbc.M504463200. [DOI] [PubMed] [Google Scholar]
- 25.Reichow SL, et al. Structure of the cholera toxin secretion channel in its closed state. Nat Struct Mol Biol. 2010;17:1226–1232. doi: 10.1038/nsmb.1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collins RF, et al. Three-dimensional structure of the Neisseria meningitidis secretin PilQ determined from negative-stain transmission electron microscopy. J Bacteriol. 2003;185:2611–2617. doi: 10.1128/JB.185.8.2611-2617.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collins RF, et al. Structure of the Neisseria meningitidis outer membrane PilQ secretin complex at 12 A resolution. J Biol Chem. 2004;279:39750–39756. doi: 10.1074/jbc.M405971200. [DOI] [PubMed] [Google Scholar]
- 28.Jain S, et al. Structural characterization of outer membrane components of the type IV pili system in pathogenic Neisseria. PLoS One. 2011;6:e16624. doi: 10.1371/journal.pone.0016624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burkhardt J, et al. Structure and function of PilQ, a secretin of the DNA transporter from the thermophilic bacterium Thermus thermophilus HB27. J Biol Chem. 2011;286:9977–9984. doi: 10.1074/jbc.M110.212688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marlovits TC, et al. Structural insights into the assembly of the type III secretion needle complex. Science. 2004;306:1040–1042. doi: 10.1126/science.1102610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hodgkinson JL, et al. Three-dimensional reconstruction of the Shigella T3SS transmembrane regions reveals 12-fold symmetry and novel features throughout. Nat Struct Mol Biol. 2009;16:477–485. doi: 10.1038/nsmb.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schraidt O, Marlovits TC. Three-dimensional model of Salmonella’s needle complex at subnanometer resolution. Science. 2011;331:1192–1195. doi: 10.1126/science.1199358. [DOI] [PubMed] [Google Scholar]
- 33.Schraidt O, et al. Topology and organization of the Salmonella typhimurium type III secretion needle complex components. PLoS Pathog. 2010;6:e1000824. doi: 10.1371/journal.ppat.1000824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Opalka N, et al. Structure of the filamentous phage pIV multimer by cryo-electron microscopy. J Mol Biol. 2003;325:461–470. doi: 10.1016/s0022-2836(02)01246-9. [DOI] [PubMed] [Google Scholar]
- 35.Smith JL, et al. Structure of trimeric haemerythrin. Nature. 1983;303:86–88. doi: 10.1038/303086a0. [DOI] [PubMed] [Google Scholar]
- 36.Izard T, et al. Principles of quasi-equivalence and Euclidean geometry govern the assembly of cubic and dodecahedral cores of pyruvate dehydrogenase complexes. Proc Natl Acad Sci U S A. 1999;96:1240–1245. doi: 10.1073/pnas.96.4.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akoev V, et al. Nucleotide-induced switch in oligomerization of the AAA+ ATPase ClpB. Protein Sci. 2004;13:567–574. doi: 10.1110/ps.03422604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reichow SL, Gonen T. Lipid-protein interactions probed by electron crystallography. Curr Opin Struct Biol. 2009;19:560–565. doi: 10.1016/j.sbi.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reichow SL, et al. The binding of cholera toxin to the periplasmic vestibule of the type II secretion channel. Channels. 2011:5. doi: 10.4161/chan.5.3.15268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andre I, et al. Prediction of the structure of symmetrical protein assemblies. Proc Natl Acad Sci U S A. 2007;104:17656–17661. doi: 10.1073/pnas.0702626104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanowar S, et al. Interactions of the transmembrane polymeric rings of the Salmonella enterica serovar Typhimurium type III secretion system. M Bio. 2010;1:e00158–00110. doi: 10.1128/mBio.00158-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spagnuolo J, et al. Identification of the gate regions in the primary structure of the secretin pIV. Mol Microbiol. 2010;76:133–150. doi: 10.1111/j.1365-2958.2010.07085.x. [DOI] [PubMed] [Google Scholar]
- 43.Hardie KR, et al. Insertion of an outer membrane protein in Escherichia coli requires a chaperone-like protein. EMBO J. 1996;15:978–988. [PMC free article] [PubMed] [Google Scholar]
- 44.Hardie KR, et al. The secretin-specific, chaperone-like protein of the general secretory pathway: separation of proteolytic protection and piloting functions. Mol Microbiol. 1996;22:967–976. doi: 10.1046/j.1365-2958.1996.01539.x. [DOI] [PubMed] [Google Scholar]
- 45.Daefler S, et al. The C-terminal domain of the secretin PulD contains the binding site for its cognate chaperone, PulS, and confers PulS dependence on pIVf1 function. Mol Microbiol. 1997;24:465–475. doi: 10.1046/j.1365-2958.1997.3531727.x. [DOI] [PubMed] [Google Scholar]
- 46.Shevchik VE, et al. Specific interaction between OutD, an Erwinia chrysanthemi outer membrane protein of the general secretory pathway, and secreted proteins. EMBO J. 1997;16:3007–3016. doi: 10.1093/emboj/16.11.3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Daefler S, Russel M. The Salmonella typhimurium InvH protein is an outer membrane lipoprotein required for the proper localization of InvG. Mol Microbiol. 1998;28:1367–1380. doi: 10.1046/j.1365-2958.1998.00908.x. [DOI] [PubMed] [Google Scholar]
- 48.Nouwen N, et al. Secretin PulD: Association with pilot PulS, structure, and ion-conducting channel formation. Proc Natl Acad Sci U S A. 1999;96:8173–8177. doi: 10.1073/pnas.96.14.8173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schuch R, Maurelli AT. MxiM and MxiJ, base elements of the Mxi-Spa type III secretion system of Shigella, interact with and stabilize the MxiD secretin in the cell envelope. J Bacteriol. 2001;183:6991–6998. doi: 10.1128/JB.183.24.6991-6998.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lario PI, et al. Structure and biochemical analysis of a secretin pilot protein. EMBO J. 2005;24:1111–1121. doi: 10.1038/sj.emboj.7600610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Okon M, et al. Structural characterization of the type-III pilot-secretin complex from Shigella flexneri. Structure. 2008;16:1544–1554. doi: 10.1016/j.str.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 52.Narita S, Tokuda H. Sorting of bacterial lipoproteins to the outer membrane by the Lol system. Methods Mol Biol. 2010;619:117–129. doi: 10.1007/978-1-60327-412-8_7. [DOI] [PubMed] [Google Scholar]
- 53.Crago AM, Koronakis V. Salmonella InvG forms a ring-like multimer that requires the InvH lipoprotein for outer membrane localization. Mol Microbiol. 1998;30:47–56. doi: 10.1046/j.1365-2958.1998.01036.x. [DOI] [PubMed] [Google Scholar]
- 54.Burghout P, et al. Role of the pilot protein YscW in the biogenesis of the YscC secretin in Yersinia enterocolitica. J Bacteriol. 2004;186:5366–5375. doi: 10.1128/JB.186.16.5366-5375.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Watson AA, et al. Identification of a gene, pilF, required for type 4 fimbrial biogenesis and twitching motility in Pseudomonas aeruginosa. Gene. 1996;180:49–56. doi: 10.1016/s0378-1119(96)00403-9. [DOI] [PubMed] [Google Scholar]
- 56.Koo J, et al. PilF is an outer membrane lipoprotein required for multimerization and localization of the Pseudomonas aeruginosa Type IV pilus secretin. J Bacteriol. 2008;190:6961–6969. doi: 10.1128/JB.00996-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carbonnelle E, et al. Type IV pilus biogenesis in Neisseria meningitidis: PilW is involved in a step occurring after pilus assembly, essential for fibre stability and function. Mol Microbiol. 2005;55:54–64. doi: 10.1111/j.1365-2958.2004.04364.x. [DOI] [PubMed] [Google Scholar]
- 58.Kim K, et al. Crystal structure of PilF: functional implication in the type 4 pilus biogenesis in Pseudomonas aeruginosa. Biochem Biophys Res Commun. 2006;340:1028–1038. doi: 10.1016/j.bbrc.2005.12.108. [DOI] [PubMed] [Google Scholar]
- 59.Trindade MB, et al. Structure of a widely conserved type IV pilus biogenesis factor that affects the stability of secretin multimers. J Mol Biol. 2008;378:1031–1039. doi: 10.1016/j.jmb.2008.03.028. [DOI] [PubMed] [Google Scholar]
- 60.Drake SL, et al. PilP, a pilus biogenesis lipoprotein in Neisseria gonorrhoeae, affects expression of PilQ as a high-molecular-mass multimer. Mol Microbiol. 1997;23:657–668. doi: 10.1046/j.1365-2958.1997.2511618.x. [DOI] [PubMed] [Google Scholar]
- 61.Balasingham SV, et al. Interactions between the lipoprotein PilP and the secretin PilQ in Neisseria meningitidis. J Bacteriol. 2007;189:5716–5727. doi: 10.1128/JB.00060-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ayers M, et al. PilM/N/O/P proteins form an inner membrane complex that affects the stability of the Pseudomonas aeruginosa type IV pilus secretin. J Mol Biol. 2009;394:128–142. doi: 10.1016/j.jmb.2009.09.034. [DOI] [PubMed] [Google Scholar]
- 63.Wallner B, Elofsson A. Identification of correct regions in protein models using structural, alignment, and consensus information. Protein Sci. 2006;15:900–913. doi: 10.1110/ps.051799606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Viarre V, et al. HxcQ liposecretin is self-piloted to the outer membrane by its N-terminal lipid anchor. J Biol Chem. 2009;284:33815–33823. doi: 10.1074/jbc.M109.065938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schmidt SA, et al. Structure-function analysis of BfpB, a secretin-like protein encoded by the bundle-forming-pilus operon of enteropathogenic Escherichia coli. J Bacteriol. 2001;183:4848–4859. doi: 10.1128/JB.183.16.4848-4859.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bose N, Taylor RK. Identification of a TcpC-TcpQ outer membrane complex involved in the biogenesis of the toxin-coregulated pilus of Vibrio cholerae. J Bacteriol. 2005;187:2225–2232. doi: 10.1128/JB.187.7.2225-2232.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Buddelmeijer N, et al. Type II secretion system secretin PulD localizes in clusters in the Escherichia coli outer membrane. J Bacteriol. 2009;191:161–168. doi: 10.1128/JB.01138-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lybarger SR, et al. Docking and assembly of the type II secretion complex of Vibrio cholerae. J Bacteriol. 2009;191:3149–3161. doi: 10.1128/JB.01701-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Diepold A, et al. Deciphering the assembly of the Yersinia type III secretion injectisome. EMBO J. 2010;29:1928–1940. doi: 10.1038/emboj.2010.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kimbrough TG, Miller SI. Contribution of Salmonella typhimurium type III secretion components to needle complex formation. Proc Natl Acad Sci U S A. 2000;97:11008–11013. doi: 10.1073/pnas.200209497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Korotkov KV, et al. Structural and functional studies of EpsC, a crucial component of the type 2 secretion system from Vibrio cholerae. J Mol Biol. 2006;363:311–321. doi: 10.1016/j.jmb.2006.08.037. [DOI] [PubMed] [Google Scholar]
- 72.Login FH, et al. A 20-residue peptide of the inner membrane protein OutC mediates interaction with two distinct sites of the outer membrane secretin OutD and is essential for the functional type II secretion system in Erwinia chrysanthemi. Mol Microbiol. 2010;76:944–955. doi: 10.1111/j.1365-2958.2010.07149.x. [DOI] [PubMed] [Google Scholar]
- 73.Mouratou B, et al. Remodeling a DNA-binding protein as a specific in vivo inhibitor of bacterial secretin PulD. Proc Natl Acad Sci U S A. 2007;104:17983–17988. doi: 10.1073/pnas.0702963104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Felise HB, et al. An inhibitor of Gram-negative bacterial virulence protein secretion. Cell Host Microbe. 2008;4:325–336. doi: 10.1016/j.chom.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Finn RD, et al. Pfam: clans, web tools and services. Nucleic Acids Res. 2006;34:D247–251. doi: 10.1093/nar/gkj149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Valverde R, et al. Structure and function of KH domains. FEBS J. 2008;275:2712–2726. doi: 10.1111/j.1742-4658.2008.06411.x. [DOI] [PubMed] [Google Scholar]
- 77.Filloux A, et al. GSP-dependent protein secretion in Gram-negative bacteria: the Xcp system of Pseudomonas aeruginosa. FEMS Microbiol Rev. 1998;22:177–198. doi: 10.1111/j.1574-6976.1998.tb00366.x. [DOI] [PubMed] [Google Scholar]
- 78.Sandkvist M. Biology of type II secretion. Molecular Microbiology. 2001;40:271–283. doi: 10.1046/j.1365-2958.2001.02403.x. [DOI] [PubMed] [Google Scholar]
- 79.Filloux A. The underlying mechanisms of type II protein secretion. Biochim Biophys Acta. 2004;1694:163–179. doi: 10.1016/j.bbamcr.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 80.Korotkov KV, Hol WG. Structure of the GspK-GspI-GspJ complex from the enterotoxigenic Escherichia coli type 2 secretion system. Nat Struct Mol Biol. 2008;15:462–468. doi: 10.1038/nsmb.1426. [DOI] [PubMed] [Google Scholar]
- 81.Sandkvist M, et al. Interaction between the autokinase EpsE and EpsL in the cytoplasmic membrane is required for extracellular secretion in Vibrio cholerae. EMBO J. 1995;14:1664–1673. doi: 10.1002/j.1460-2075.1995.tb07155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Abendroth J, et al. The X-ray structure of the type II secretion system complex formed by the N-terminal domain of EpsE and the cytoplasmic domain of EpsL of Vibrio cholerae. J Mol Biol. 2005;348:845–855. doi: 10.1016/j.jmb.2005.02.061. [DOI] [PubMed] [Google Scholar]
- 83.Gray MD, et al. In vivo cross-linking of EpsG to EpsL suggests a role for EpsL as an ATPase-pseudopilin coupling protein in the Type II secretion system of Vibrio cholerae. Mol Microbiol. 2011;79:786–798. doi: 10.1111/j.1365-2958.2010.07487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nouwen N, et al. Domain structure of secretin PulD revealed by limited proteolysis and electron microscop. EMBO J. 2000;19:2229–2236. doi: 10.1093/emboj/19.10.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Burghout P, et al. Structure and electrophysiological properties of the YscC secretin from the type III secretion system of Yersinia enterocolitica. J Bacteriol. 2004;186:4645–4654. doi: 10.1128/JB.186.14.4645-4654.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Marlovits TC, et al. Assembly of the inner rod determines needle length in the type III secretion injectisome. Nature. 2006;441:637–640. doi: 10.1038/nature04822. [DOI] [PubMed] [Google Scholar]
- 87.Blocker A, et al. Structure and composition of the Shigella flexneri “needle complex”, a part of its type III secreton. Mol Microbiol. 2001;39:652–663. doi: 10.1046/j.1365-2958.2001.02200.x. [DOI] [PubMed] [Google Scholar]
- 88.Collins RF, et al. Analysis of the PilQ secretin from Neisseria meningitidis by transmission electron microscopy reveals a dodecameric quaternary structure. J Bacteriol. 2001;183:3825–3832. doi: 10.1128/JB.183.13.3825-3832.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Collins RF, et al. Interaction with type IV pili induces structural changes in the bacterial outer membrane secretin PilQ. J Biol Chem. 2005;280:18923–18930. doi: 10.1074/jbc.M411603200. [DOI] [PubMed] [Google Scholar]