Abstract
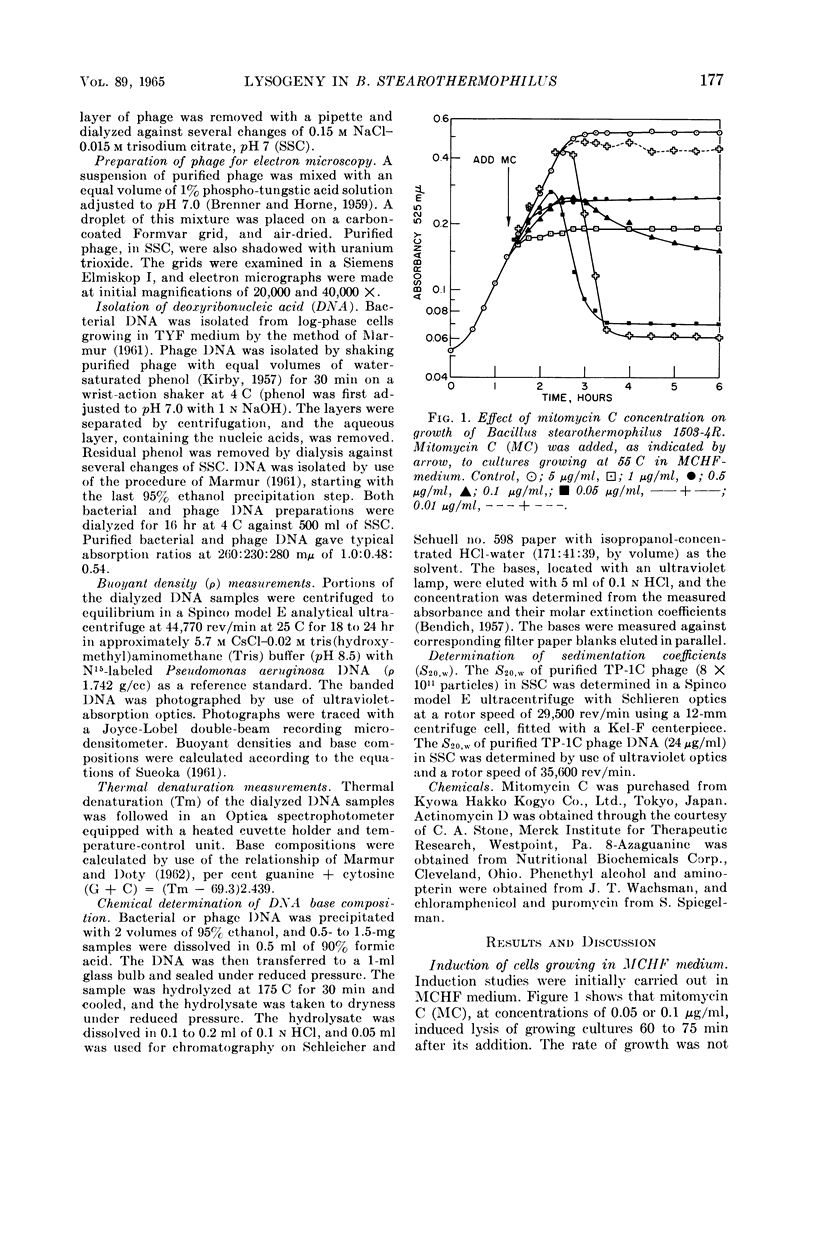
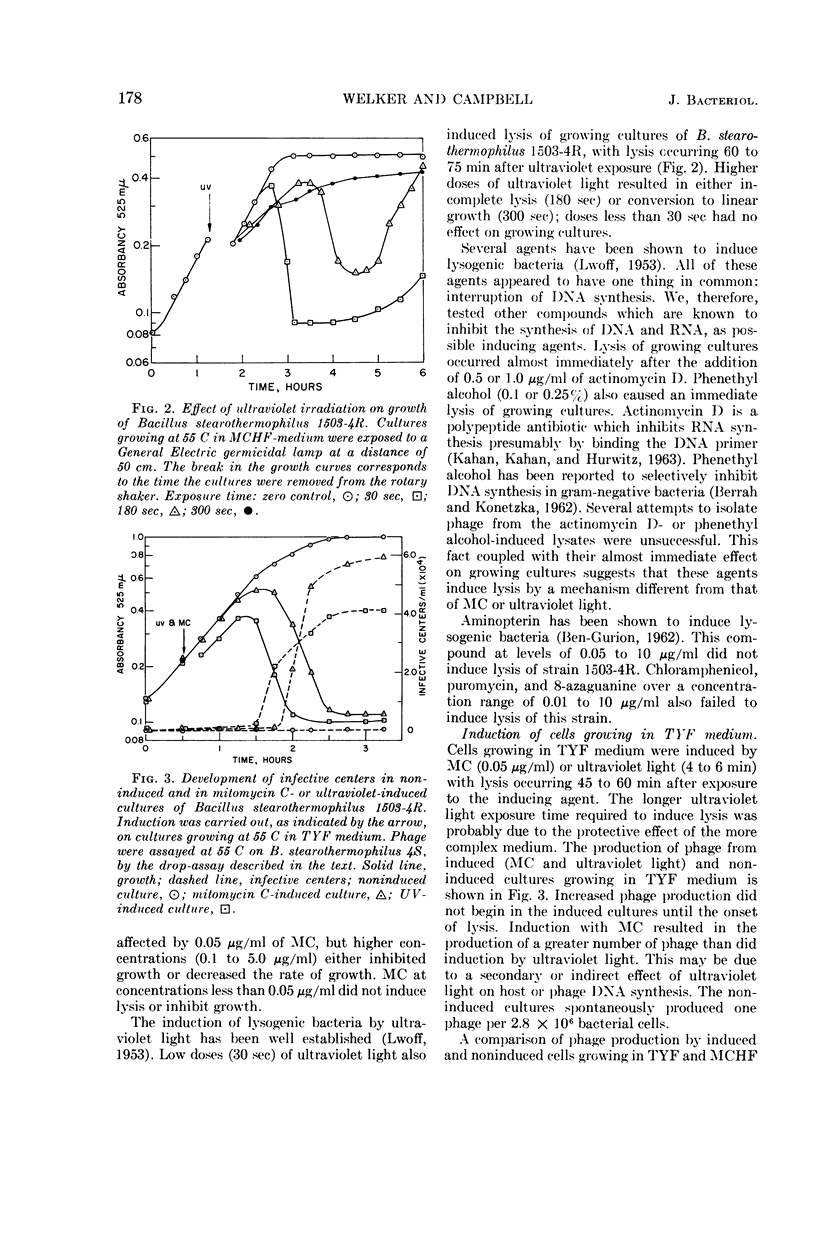
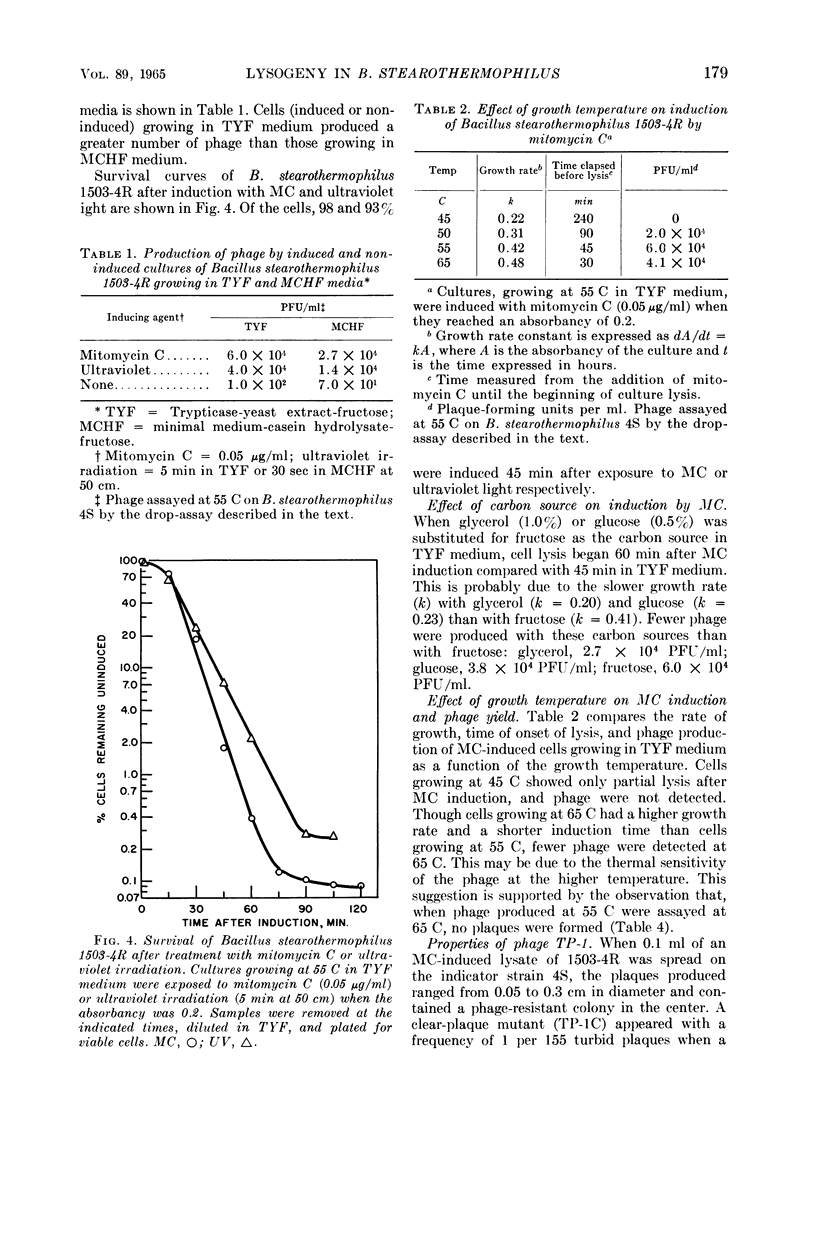
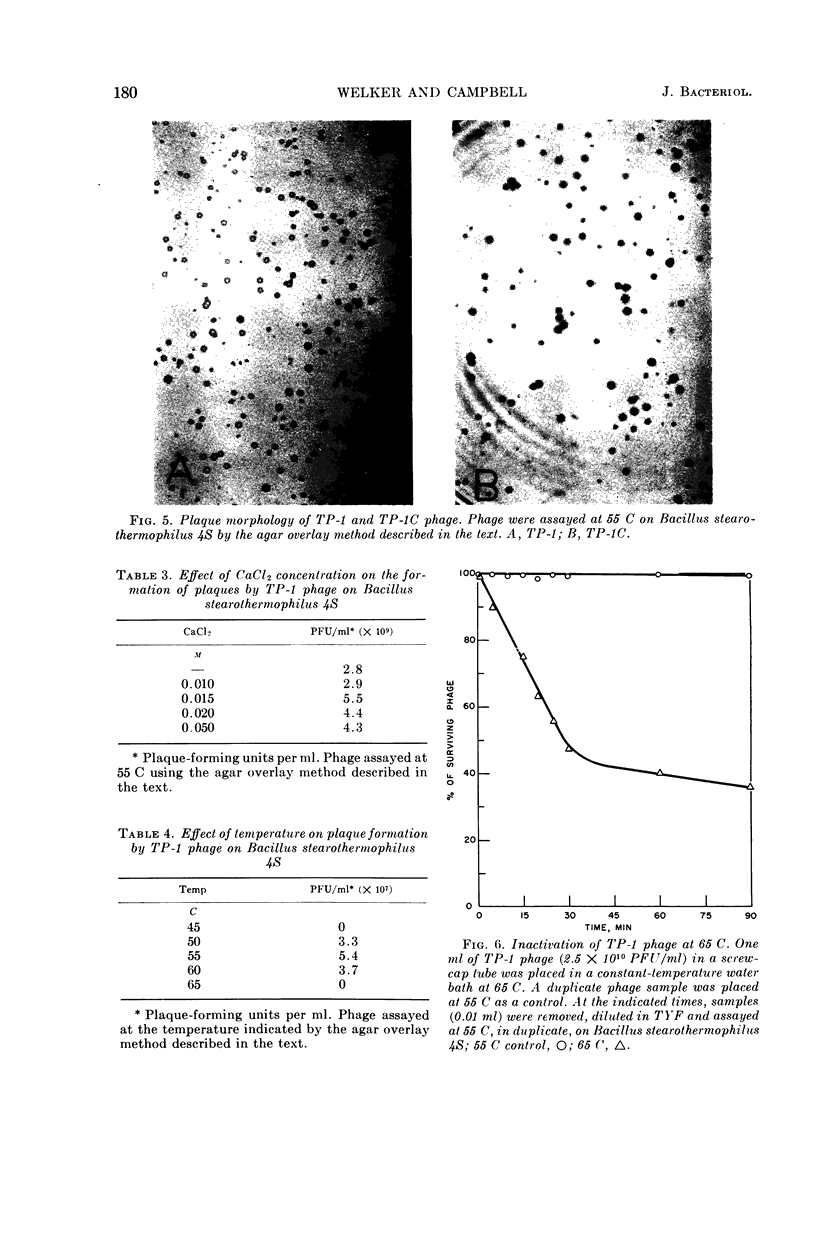
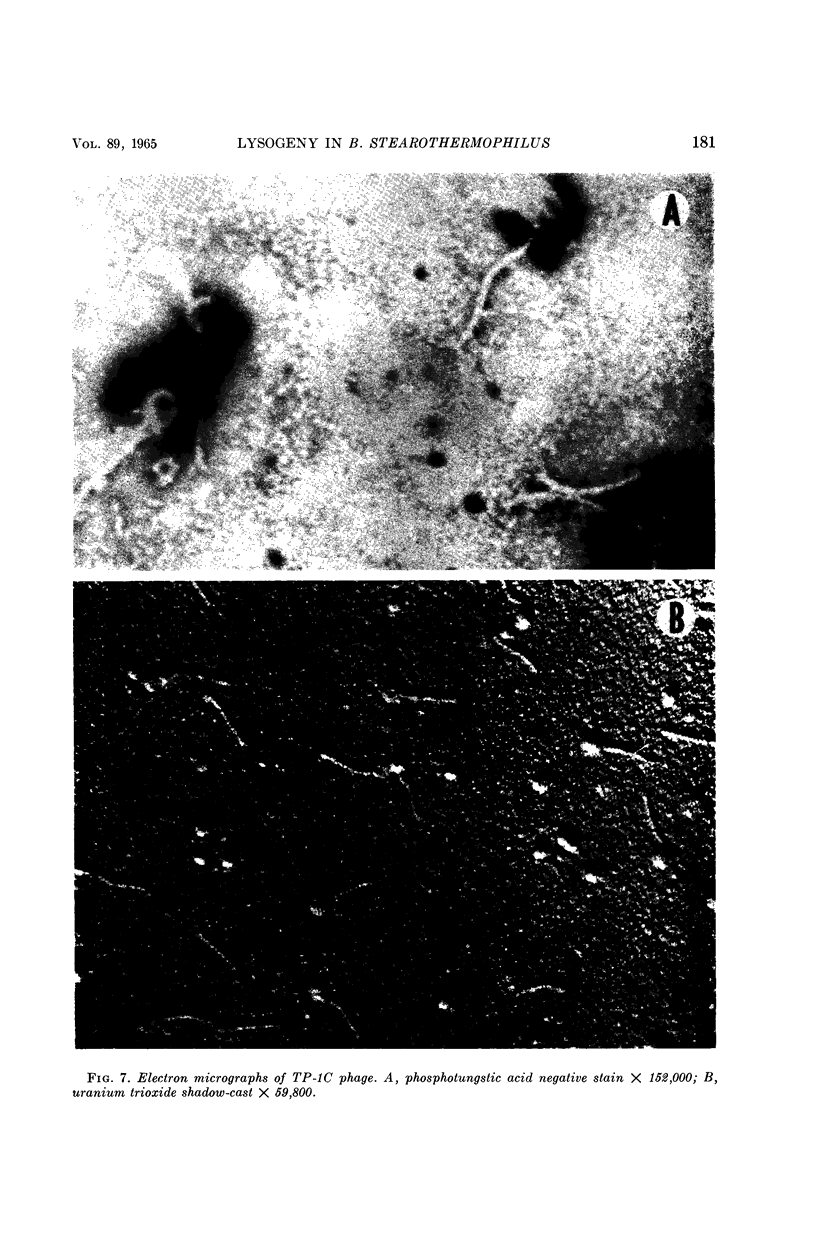
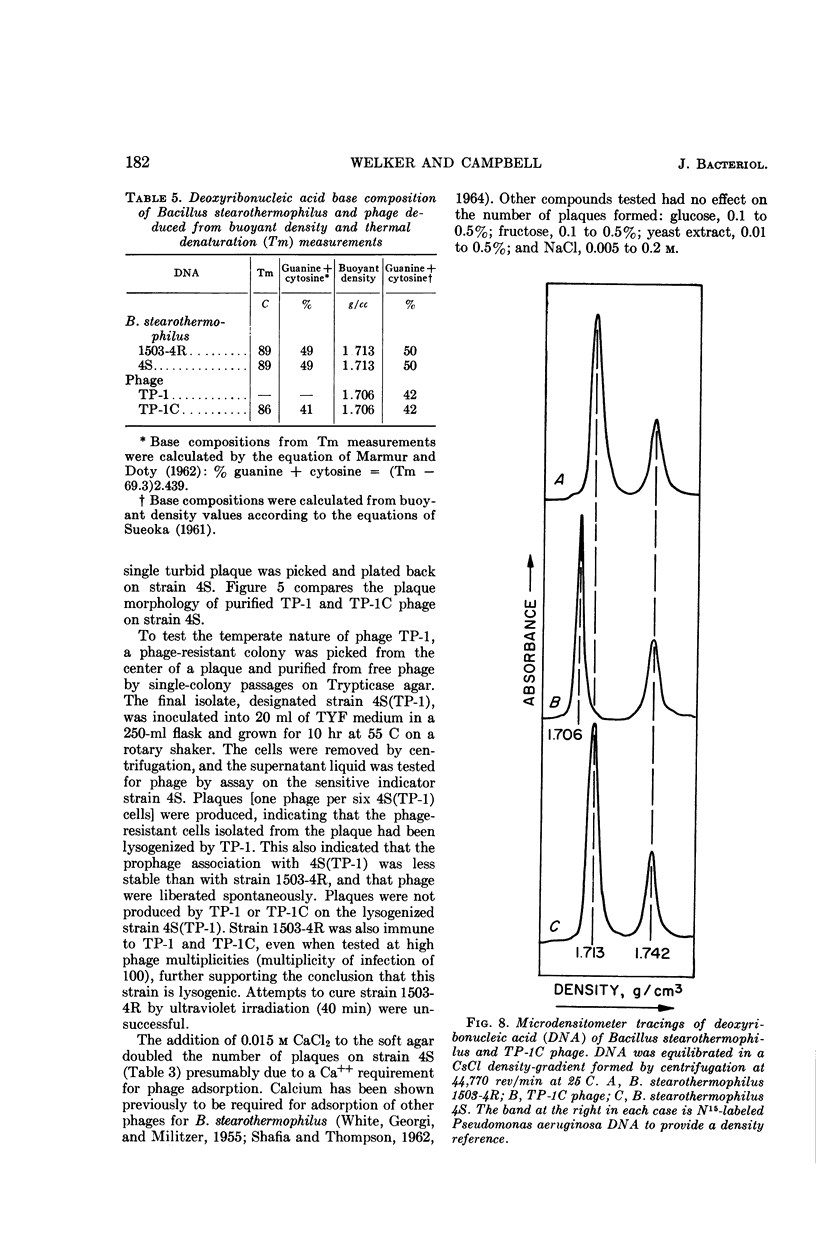
Welker, N. E. (University of Illinois, Urbana), and L. Leon Campbell. Induction and properties of a temperate bacteriophage from Bacillus stearothermophilus. J. Bacteriol. 89:175–184. 1965.—Bacillus stearothermophilus 1503-4R growing at 55 C was induced to lyse either when 0.05 μg/ml of mitomycin C was added or when it was exposed to ultraviolet light for 30 sec. Lysis of the induced cultures occurred 45 to 60 min after induction. Phage were assayed on B. stearothermophilus 4S giving turbid plaques 0.05 to 0.3 cm in diameter. Noninduced cultures of 1503-4R spontaneously produced one phage per 2.8 × 106 bacterial cells. The optimal temperature for phage production and assay was found to be 55 C. B. stearothermophilus 1503-4R was immune to the isolated temperate phage (TP-1) and to a clear-plaque mutant phage (TP-1C), even when tested at phage mutliplicities of 100. TP-1 and TP-1C phage were identical morphologically having a head 65 mμ in diameter and a tail 240 mμ long and 12 mμ wide. TP-1C phage deoxyribonucleic acid (DNA) had an S20,w value of 24.1 and a calculated molecular weight of 1.21 × 107. DNA base compositions of TP-1 and TP-1C phage were identical (42% guanine + cytosine), but differed significantly from those of the lysogenic or indicator strains of B. stearothermophilus (50% guanine + cytosine). No unusual bases were detected in either the bacterial or phage DNA.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEN-GURION R. On the induction Escherichia coli K-12 (lambda) by aminopterin. Biochem Biophys Res Commun. 1962 Aug 31;8:456–461. doi: 10.1016/0006-291x(62)90296-6. [DOI] [PubMed] [Google Scholar]
- BRENNER S., HORNE R. W. A negative staining method for high resolution electron microscopy of viruses. Biochim Biophys Acta. 1959 Jul;34:103–110. doi: 10.1016/0006-3002(59)90237-9. [DOI] [PubMed] [Google Scholar]
- BURGI E., HERSHEY A. D. Sedimentation rate as a measure of molecular weight of DNA. Biophys J. 1963 Jul;3:309–321. doi: 10.1016/s0006-3495(63)86823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWSON I. M., SMILLIE E., NORRIS J. R. The morphology of Bacillus cereus bacteriophages. J Gen Microbiol. 1962 Jul;28:517–519. doi: 10.1099/00221287-28-3-517. [DOI] [PubMed] [Google Scholar]
- HATA T., HOSHI T., KANAMORI K., MATSUMAE A., SANO Y., SHIMA T., SUGAWARA R. Mitomycin, a new antibiotic from Streptomyces. I. J Antibiot (Tokyo) 1956 Jul;9(4):141–146. [PubMed] [Google Scholar]
- KAHAN E., KAHAN F. M., HURWITZ J. The role of deoxyribonucleic acid in ribonucleic acid synthesis. VI. Specificity of action of actinomycin D. J Biol Chem. 1963 Jul;238:2491–2497. [PubMed] [Google Scholar]
- KIRBY K. S. A new method for the isolation of deoxyribonucleic acids; evidence on the nature of bonds between deoxyribonucleic acid and protein. Biochem J. 1957 Jul;66(3):495–504. doi: 10.1042/bj0660495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORN D., WEISSBACH A. Thymineless induction in Escherichia coli K12 (lambda). Biochim Biophys Acta. 1962 Nov 26;61:775–790. doi: 10.1016/0926-6550(62)90060-9. [DOI] [PubMed] [Google Scholar]
- LEVINE M. Effect of mitomycin C on interactions between temperate phages and bacteria. Virology. 1961 Apr;13:493–499. doi: 10.1016/0042-6822(61)90280-x. [DOI] [PubMed] [Google Scholar]
- LWOFF A. Lysogeny. Bacteriol Rev. 1953 Dec;17(4):269–337. doi: 10.1128/br.17.4.269-337.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its thermal denaturation temperature. J Mol Biol. 1962 Jul;5:109–118. doi: 10.1016/s0022-2836(62)80066-7. [DOI] [PubMed] [Google Scholar]
- MARMUR J. Thermal denaturation of deoxyribosenucleic acid isolated from a thermophile. Biochim Biophys Acta. 1960 Feb 26;38:342–343. doi: 10.1016/0006-3002(60)91251-8. [DOI] [PubMed] [Google Scholar]
- Meselson M., Stahl F. W., Vinograd J. EQUILIBRIUM SEDIMENTATION OF MACROMOLECULES IN DENSITY GRADIENTS. Proc Natl Acad Sci U S A. 1957 Jul 15;43(7):581–588. doi: 10.1073/pnas.43.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OTSUJI N., SEKIGUCHI M., IIJIMA T., TAKAGI Y. Induction of phage formation in the lysogenic Escherichia coli K-12 by mitomycin C. Nature. 1959 Oct 3;184(Suppl 14):1079–1080. doi: 10.1038/1841079b0. [DOI] [PubMed] [Google Scholar]
- Shafia F., Thompson T. L. Isolation and preliminary characterization of bacteriophage phi-mu-4. J Bacteriol. 1964 May;87(5):999–1002. doi: 10.1128/jb.87.5.999-1002.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WELKER N. E., CAMPBELL L. L. DE NOVO SYNTHESIS OF ALPHA-AMYLASE BY BACILLUS STEAROTHERMOPHILUS. J Bacteriol. 1963 Dec;86:1202–1210. doi: 10.1128/jb.86.6.1202-1210.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WELKER N. E., CAMPBELL L. L. EFFECT OF CARBON SOURCES ON FORMATION OF ALPHA-AMYLASE BY BACILLUS STEAROTHERMOPHILUS. J Bacteriol. 1963 Oct;86:681–686. doi: 10.1128/jb.86.4.681-686.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE R., GEORGI C. E., MILITZER W. E. Characteristics of a thermophilic bacteriophage. Proc Soc Exp Biol Med. 1955 Mar;88(3):373–377. doi: 10.3181/00379727-88-21592. [DOI] [PubMed] [Google Scholar]




