Summary
Maladaptive responses to stress adversely affect human behavior, yet the signaling mechanisms underlying stress-responsive behaviors remain poorly understood. Using a conditional gene knockout approach, the α isoform of p38 Mitogen Activated Protein Kinase (MAPK) was selectively inactivated by AAV1-Cre-recombinase infection in specific brain regions or by promoter-driven excision of p38α MAPK in serotonergic neurons (by Slc6a4-Cre or ePet1-Cre) or astrocytes (by Gfap-CreERT2). Social defeat stress produced social avoidance (a model of depression-like behaviors) and reinstatement of cocaine preference (a measure of addiction risk) in wild-type mice, but not in mice having p38α MAPK selectively deleted in serotonin-producing neurons of the dorsal raphe nucleus. Stress-induced activation of p38α MAPK translocated the serotonin transporter to the plasma membrane and increased the rate of transmitter uptake at serotonergic nerve terminals. These findings suggest that stress initiates a cascade of molecular and cellular events in which p38α MAPK induces a hypo-serotonergic state underlying depression-like and drug-seeking behaviors.
Keywords: p38 MAPK (Mapk14), Plasma Membrane Serotonin Transporter (Slc6a4), membrane protein translocation mechanisms, biophysics of transmitter reuptake, regulation of neurotransmission
Introduction
Stress has significant effects on mood and can act as a motivational force for decisive action, seeking food or reward, and coping with novel environmental conditions. However, sustained stress exposure can lead to maladaptive responses including clinical depression, anxiety, and increased risk for drug addiction (Bale and Vale, 2001; Krishnan and Nestler, 2008; Bruchas et al., 2010; Koob, 2008). Recent studies have proposed that the dysphoric components of stress are coded in brain by corticotropin releasing factor (CRF) and subsequent release of the endogenous dynorphin opioid peptides in brain (Land et al., 2008, Bruchas et al., 2010, Koob 2008). Systemic blockade of these neural pathways prevents the aversive and pro-addictive effects of stress, but how these systems orchestrate affective responses at the molecular and cellular levels remain unresolved.
One group of signaling pathways involved in the cellular stress response includes the family of mitogen-activated protein kinases (MAPK). Using pharmacological approaches, p38 MAPK (also called SAPK, for stress-activated protein kinase) activity has been identified as a critical mediator of stroke-induced apoptosis, osmotic shock response, and in the regulation of transcriptional pathways responsible for cell death and differentiation (Raman et al., 2007; Coulthard et al., 2009). Recently however, inhibition of p38 MAPK was also found to block stress-induced behavioral responses including aversion (Land et al., 2009, Bruchas et al., 2007) and to prevent reflex-conditioned responses (Zhen et al., 2001). Although the cellular and molecular bases for these behavioral actions are not known, one possible site of action is the serotonergic nuclei because this transmitter has an established role in the regulation of mood (Roche et al., 2003; Paul et al., 2011; Richardson-Jones et al., 2010). The dorsal raphe nucleus (DRN) is the primary neuronal source of serotonin, and DRN neurons send diffuse projections to multiple forebrain and hindbrain structures that are critical for regulating affective state (Land et al, 2009, Hensler 2006; Zhao et al., 2007). The DRN is modulated by several afferent systems (Wylie et al., 2010; Land et al., 2009; Scott et al., 2005, Kirby et al., 2008), but how these inputs regulate serotonin neurotransmission remains unclear, and little is known about the essential signal transduction kinase cascades in the DRN that regulate serotonergic output to ultimately control behavior.
In the DRN, we found that p38α MAPK expression was widely distributed in tryptophan hydroxylase 2 (TPH) expressing cells, non-TPH cells, and astrocytes (Land et al., 2009). Several reports have demonstrated that there is a high degree of co-expression between the serotonin transporter (Slc6a4, SERT) and TPH positive neurons (MacGillivray et al., 2010; Lowry et al., 2008). Recent studies have also determined that expression of the transcription factor Pet1 is largely restricted to serotonergic (TPH-immunoreactive, ir) neurons (Scott et al., 2005; Liu et al., 2010). Thus, SERT and Pet1 represent potentially useful markers for the discrimination of serotonergic neurons within the brain. Here we used a combination of conditional p38α MAPK-null alleles generated in serotonergic neurons or astrocytes to determine the effects of p38α MAPK deletion in models of depression behaviors including place aversion and social avoidance and of drug addiction behaviors modeled by reinstatement of extinguished cocaine place preference.
RESULTS
p38α MAPK in DRN is required for behavioral responses to stress
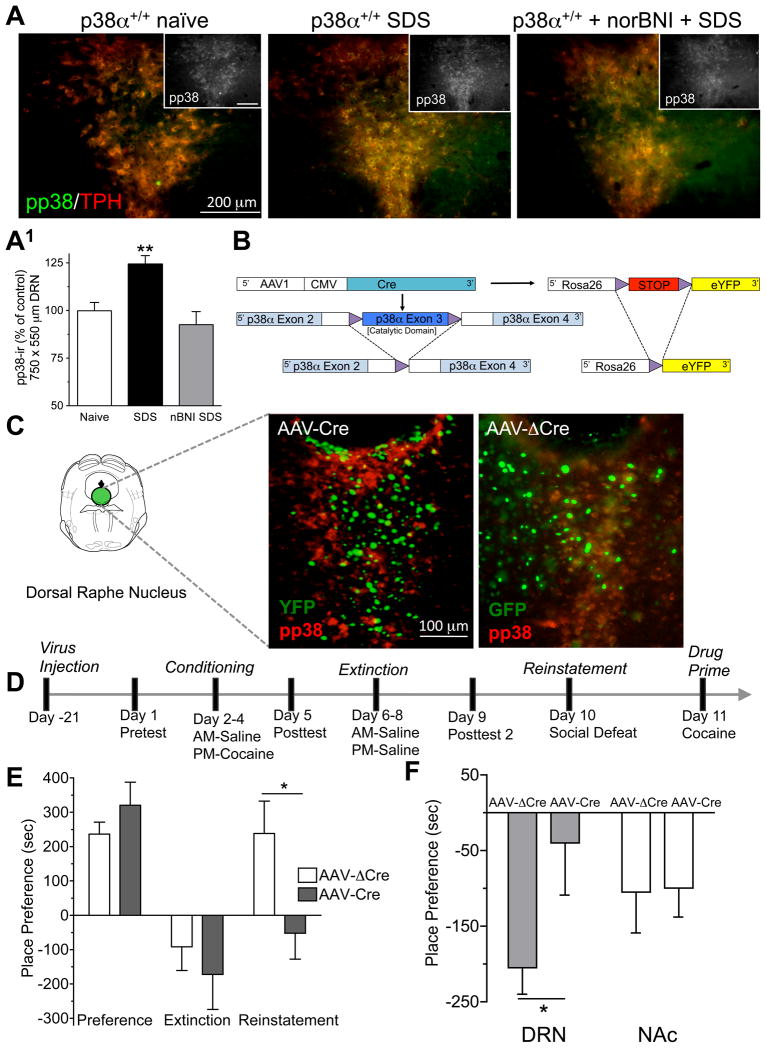
Since prior reports suggested that p38 MAPK is activated during the stress response, we first determined if social defeat stress (SDS) induces phosphorylation of p38 MAPK in the DRN. Following a single, 20-min session of SDS, mice showed an increase in phospho-p38 immunoreactivity (pp38-ir) in the DRN (Figure 1A, A1). G-protein coupled receptor activation can lead to p38 MAPK phosphorylation via recruitment of arrestin-dependent pathways (Tan et al., 2009; Gong et al., 2008), and activation of the dynorphin/kappa opioid receptor (KOR) system was shown to increase pp38-ir by this mechanism (Bruchas et al. 2006; Bruchas et al., 2007). Consistent with this concept, the increase in pp38-ir caused by SDS was prevented by blocking endogenous dynorphin activation of KOR with the selective antagonist norbinaltorphimine (norBNI) (Figure 1A, A1).
Figure 1. p38α expression in the dorsal raphe nucleus is required for stress behavior.
(A) Representative low power immunofluorescence images of social defeat stress induced pp38-ir (green) in TPH-ir cells (red) of the DRN. (A1) Quantification ±SEM of pp38-ir in DRN from unstressed (naïve), social defeat stress (SDS) and social defeated stress exposed norBNI (10 mg/kg, i.p.) injected mice (**p < 0.01, SDS vs. Naïve). Inset, representative black and white low power immunofluorescence images of social defeat stress induced pp38-ir, scale bar = 200μm (B) Schematic of AAV1 induced cre-recombination of the floxed p38α MAPK allele and STOP sequence controlling Rosa26YFP gene expression. (C) Representative images of pp38-ir (red) and YFP (green) fluorescence following AAV1-Cre-GFP or AAV1-ΔCre-GFP injection into the DRN. Mice were pretreated with KOR agonist (U50,488, 20 mg/kg, i.p, 20 min prior to perfusion). Images show that AAV-cre expressing cells lack pp38-ir, confirming effective localized DRN p38α deletion in cells where Cre-activity also promoted YFP expression by the Rosa reporter. (D) Conditioning procedure for SDS induced reinstatement of cocaine seeking. (E) Cocaine place preference scores, calculated as post-test minus pre-test on the cocaine-paired side, and SDS-induced reinstatement scores of extinguished place preference in DRN-injected animals (n = 5–8; *, P < 0.05 t-test compared to AAV1-ΔCre). Bars represent means± SEM. (F) Preference scores (mean ± SEM) for conditioned place aversion to kappa opioid agonist U50,488 (2.5 mg/kg, i.p) from mice injected with either AAV1cre-GFP or AAV1Δcre-GFP into their DRN or Nucleus Accumbens (NAc) (*p < 0.05, AAV1cre-GFP vs. AAV1Δcre-GFP; n = 8). See also Figure S1.
There are four isoforms of p38 MAPK: α, β,δ, and γ. p38α and p38β are both expressed in neurons and glial cells, whereas p38δ and p38γ are exclusively expressed in immune cell types (Zhang et al., 2007; Zarubin and Han, 2005). Since the p38 isoforms share consensus phosphorylation sites and there are no known isoform-selective phospho-antibodies, we used non-phospho-selective, but isoform-selective antibodies in immunoprecipitation approaches to determine the phosphorylation state of each isoform. Agonist stimulation of KOR resulted in significant (p<0.05, t-test) phosphorylation of the p38α, but not p38β isoform (Supplemental Figure 1A) in HEK-293 cells expressing KOR-GFP and either FLAG-tagged p38α or p38β isoforms. No difference in immunoprecipitation efficiency or isoform expression was observed (Supplemental Figure 1B) as evidenced by equal FLAG staining. Finally, using nucleus accumbens cell lysates, we found that in vivo treatment with KOR agonist increased pp38α-ir (Supplemental Figure 1C). Together these data suggest that KOR activation during stress exposure selectively increased the phosphorylation of the α isoform of p38 MAPK.
To determine if p38α activation in DRN was required for stress-induced behavioral responses, we used a genetic approach to selectively inactivate p38α MAPK in DRN cells. Using mice with a floxed gene (Mapk14lox/lox) encoding p38α MAPK (Nishida et al., 2004), local inactivation of p38α MAPK in the DRN was achieved by stereotaxic injection of Adeno-Associated-Virus serotype 1 vector encoding Cre recombinase (AAV1-CreGFP) (Ahmed et al., 2004). These mice were also bred to carry a Gt(ROSA)26Sor-YFP (R26-YFP) reporter cassette in which Cre-mediated recombination of a transcriptional STOP promotes YFP expression as a marker of Cre activity (Figure 1B). p38α-ir was absent in AAV1-CreGFP transduced cells that co-expressed the YFP reporter (Figure 1C). In contrast, injection of AAV1-CreΔ GFP vector expressing an inactive, mutated form of the Cre-recombinase (CreΔ) did not affect p38α MAPK expression in DRN (Figure 1C).
Prior reports established that stress causes relapse to drug seeking (Nestler and Hyman 2010; Krishnan et al., 2007), and in particular, social defeat stress (SDS) represents an ethologically relevant stressor for evoking dysphoria-like behavioral states (Miczek et al., 2008). The Mapk14lox/lox mice were injected in the DRN with AAV1-CreGFP to determine whether p38α MAPK was required for SDS induced reinstatement. We followed this injection with a conditioning paradigm for cocaine place preference (Figure 1D). Both AAV1-CreGFP and AAV1-CreΔGFP injected mice developed normal place preference to cocaine (Figure 1E), suggesting that deletion of p38α in DRN cells does not disrupt associative learning components required for initial acquisition of cocaine place preference. We then extinguished the conditioned preference by substituting saline for cocaine in the drug-paired chamber (Figure 1D). After mice met extinction criteria (≤ 15% of their initial preference score, Figure 1E), mice were exposed to social defeat stress (20 min session) and then place preference was reassessed. Importantly, AAV1-CreGFP-induced deletion of p38α in the DRN completely blocked SDS-induced reinstatement of cocaine CPP, whereas floxed p38α mice injected with the virus expressing the inactive form of Cre recombinase still showed robust SDS-induced reinstatement of cocaine CPP (Figure 1E). These data suggest that expression of p38α in the DRN is required for stress-induced reinstatement of reward seeking behavior.
To expand on this concept and to parallel other studies showing that stress negatively modulates reward to initiate the drive for reward seeking (Koob, 2008), we injected Mapk14lox/lox (floxed p38α) mice with either AAV1-CreGFP or AAV1-CreΔ GFP in either the DRN or Nucleus Accumbens (NAc), and then assessed conditioned avoidance of a context paired with an aversive stimulus. Since KOR activation results from stress and is known to produce aversive behavioral responses in stress-paired contexts (Land et al., 2008; Bruchas et al., 2010; Carlezon et al., 1998; Pfeiffer et al., 1986), we conditioned mice with the KOR agonist U50,488 (2.5 mg/kg, i.p.) over 2 days and then assessed their avoidance of the drug-paired context. AAV1-CreGFP injection in the DRN of Mapk14lox/lox mice, but not the NAc, blocked conditioned place aversion (Figure 1F). This result suggests that p38α MAPK in the DRN is also required for stress-induced dysphoria-like avoidance behavior.
Selective disruption of p38α in 5HT neurons
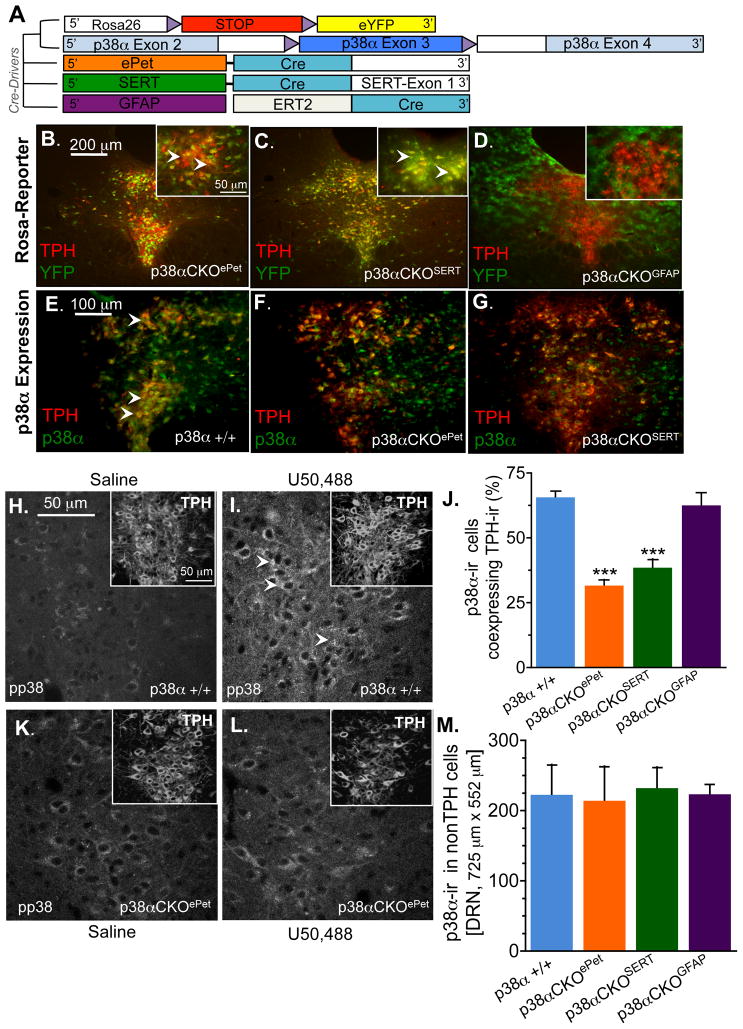
p38α MAPK is ubiquitously expressed in cells of DRN including serotonergic and non-serotonergic neurons, as well as astrocytes (Supplemental Figure 2A). Since AAV1-CreGFP transduction provides anatomical specificity but is not cell- type specific, we crossed the Mapk14lox/lox mice with mice expressing Cre-recombinase under control of either the 5HT transporter gene Slc6a4Cre (SERT-Cre) (Zhuang et al., 2005), the enhancer region of 5HT-cell-type specific transcription factor Pet-1 (ePet1-Cre) (Scott et al., 2005), or the estrogen receptor-inducible Cre variant under control of the astrocyte selective glial fibrillary acidic protein gene (GFAP-Cre-ERT2) (Hirrlinger et al., 2006) inducible Cre mouse line (Figure 2A). Due to the potential for transient and variable expression of promoter driven Cre in germ cells, males carrying the Cre recombinase alleles had an inactive Mapk14 gene (Mapk14Δ/+), and they were crossed with females carrying Mapklox/lox (See Supplemental Figure 2B for breeding scheme and Table 1 for abbreviations of each genotype used in this study). In addition, to confirm that Cre-mediated recombination by Slc6a4-Cre, ePet1-Cre, or Gfap-Cre-ERT2 were cell-type specific, we also crossed these mice with the R26-YFP reporter mice (Srinivas et al., 2001). We then used double immunofluorescence staining to detect yellow fluorescent protein (YFP) and tryptophan hydroxylase 2 (TPH), the rate-limiting enzyme for serotonin synthesis in brain and a marker for serotonergic neurons (Nakamura and Hasegawa, 2007). We observed a high level of TPH-ir and YFP co-expression in the DRN, but not in the cortex or hippocampus of p38α CKOePet (Mapk14D/lox: ePet1-Cre) mice (Figure 2B, Supplemental Figure 3A–H). Further, as would be predicted from the wide expression profile of SERT during neurodevelopment (Murphy and Lesch, 2008), we visualized a high level of TPH-ir and YFP co-expression in the DRN (Figure 2C), but YFP expression was also observed in cells of the cortex and hippocampus and thalamus of p38αCKOSERT (Mapk14Δ/lox: Slc6a4-Cre) mice (Supplemental Figure 3A). Finally, p38αCKOGFAP (Mapk14Δ/lox: GFAP-CreERT2) mice showed no YFP co-localization with TPH-ir neurons in the DRN, but showed extensive YFP signal in cells of astrocytic morphology throughout the brain including the DRN, thus establishing consistent cell-type selective Cre-recombinase activity (Figure 2C).
Figure 2.
(A) Schematic of cell type specific p38α deletion. Floxed p38α and ROSAYFP reporter mice were crossed to mice expressing Cre-recombinase under the control of Pet1, serotonin transporter, or the tamoxifen inducible glial fibrillary acidic protein (GFAP) CreERT2 transgene. Representative images showing TPH-ir and YFP in p38αCKOePet mice (B), p38αCKOSERT (C), and p38αCKOGFAP (D) mice. Insets show higher power images with arrows directed towards yellow cells indicating overlap of TPH/YFP expression. Representative images showing TPH and p38α-ir in wild type (E), p38αCKOePet (F), and p38αCKOSERT. (G) Representative images from wild type mice showing the absence of phosphorylated p38 MAPK (pp38-ir) following saline treatment (H) and increased pp38-ir following treatment with U50,488 20 mg/kg, i.p., 20 min prior) (I). Insets show intact TPH labeling in the same fields. (J) Quantitation of p38α-ir in TPH positive cells in the dorsal raphe nucleus. Data show a significant reduction in p38α expression in both p38α CKOePET and p38α CKOSERT mice (***p < 0.001, ANOVA, Bonferroni). (K). Representative images from in p38α CKOePET mice showing the absence of pp38-ir following saline treatment (K) and following treatment with U50,488 (L). Insets show intact TPH staining. (M) Quantitation of p38α-ir expressed in TPH-negative cells in the DRN. Data are representative of 4–8 animals per group. See also Figure S3.
Table 1.
Mouse Cell Lines Generated
| Official Strain Name | Reference | Shorthand Name | Genotype |
|---|---|---|---|
| B6.129-Mapk14tm1.2Otsu | Nishida et al., 2004 | floxed p38α | p38αlox/lox |
| p38α +/+ | p38α+/+ | ||
| B6.129-Tg(Slc6a4-cre)1Xz | Zhuang et al., 2005 | p38α CKOSERT | p38αΔ/lox; SERTCre/+ |
| p38α Δ/lox | p38αΔ/lox; SERT+/+ | ||
| p38α lox/+ | p38αlox/+; SERT+/+ | ||
| SERT-Cre only | p38α+/+; SERTCre/+ | ||
| B6.129-Tg(ePET-cre)1Esd | Scott et al., 2005 | p38α CKOePet | p38αΔ/lox; ePetCre/+ |
| p38α Δ/lox | p38αΔ/lox; ePet+/+ | ||
| p38α lox/+ | p38αlox/+; ePet+/+ | ||
| ePet-Cre only | p38α+/+; ePetCre/+ | ||
| B6.129-Tg(GFAP-creERT2) 1Fki | Hirrlinger et al., 2006 | p38αCKOGFAP | p38αlox/lox; GFAPCre-ERT2/+ |
| floxed p38α | p38αlox/lox; GFAP+/+ | ||
| B6.129X1-Gt(ROSA)26Sortm1(EYFP)Cos/J | Srinivas et al., 2001 | ROSA-YFP | Cre reporter (heterozygous) in genotypes above |
| B6.129S4-Mox2tm1(cre)Sor/J | Tallquist & Soriano, 2000 | Mox2-Cre | As heterozygote to produce null p38αΔ allele |
| B6.129(Cg)-Slc6a4tm1Kpl/J | Bengel et al., 1998 | Conventional SERT KO | SERT−/− |
The degree of p38α MAPK expression was also examined in the DRN of conditional knockout (CKO) mice using antibodies directed at p38α or phospho-p38 MAPK. p38αCKOePet mice displayed significantly reduced p38α MAPK expression in TPH-ir cells (ANOVA, Bonferroni post-hoc, p < 0.001) (Figure 2F, 2J) in contrast to p38α expression in wild-type mice (Figure 2E). In p38α CKOSERT mice, p38α-ir in the DRN was also significantly reduced in TPH-ir cells compared to the wild-type mice (ANOVA, Bonferroni post-hoc, p < 0.001) (Figure 2G, 2J). Importantly, expression of TPH-ir was not altered in any of the knockout mouse lines (Figure 2H,I,K,L), nor was p38α MAPK expression significantly altered in non-TPH expressing cells of CKO mice (Figure 2M). Finally, we did not observe compensatory changes in p38β MAPK expression in DRN cells in any of the mouse lines (Supplemental Figure 3I). To determine if the active isoform of p38 MAPK was selectively disrupted in TPH expressing cells, we injected mice with the KOR agonist U50,488 and then stained for pp38-ir. In wild-type mice, agonist stimulation of KOR increased pp38-ir in DRN, however p38αCKOePet mice showed no increase in pp38-ir in DRN following KOR stimulation (Figure 2K,L).
Serotonergic p38α is required for stress-induced avoidance behavior
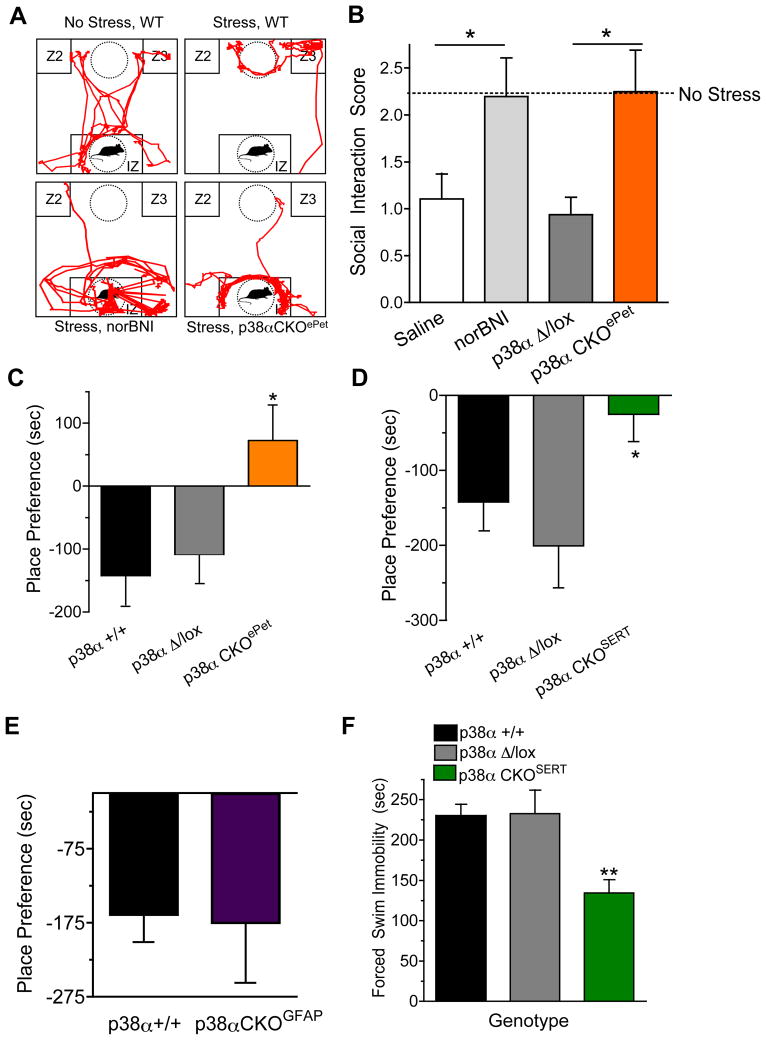
Previous reports have demonstrated that mice subjected to defeat by an aggressor mouse show subsequent decreases in motivation for social interaction that can be prevented by clinically effective antidepressants (Nestler and Hyman 2010; Cao et al., 2010; Berton et al., 2006; Avgustinovich et al., 2005; Siegfried, 1985). Using this approach, we assessed the role of p38α MAPK in stress-induced social avoidance. Previously unstressed mice readily explore and interact with a novel male mouse in the social interaction chamber (Figure 3A, B). However, socially defeated mice showed a significant social avoidance (ANOVA, F(2,29)= 2.51, p < 0.05, Bonferroni) (Figure 3A). Pretreatment with the KOR antagonist norBNI (24 hr prior to SDS, 10 mg/kg, i.p) significantly blocked the SDS-induced avoidance behavior (ANOVA, F(3,30) = 2.843, p < 0.05, Bonferroni). As expected, littermate control mice (Mapk14Δ/+: ePet1-Cre) showed avoidance behavior following SDS, whereas p38α CKOePet mice were resilient to the effects of social defeat and showed significant reduction in the SDS-induced interaction deficit (t-test, p < 0.05) (Figure 3B). Because social avoidance behavior may also be considered to be an anxiety-like response, we determined if behavior in the elevated plus maze was also affected by disruption of p38α MAPK in serotonergic neurons (Supplemental Figure 4B). Unexpectedly, there were no significant differences in the time spent in the open arms of the maze by the p38αCKOePet, p38αCKOSERT, and littermate control groups (Supplemental Figure 4B), suggesting that the blockade of SDS-induced social avoidance caused by serotonergic p38α MAPK deletion was not a consequence of a generalized decrease in anxiety-like responses.
Figure 3. Negative affective behavior requires expression of p38α in serotonergic neurons.
(A) Representative traces of mouse locomotion (red lines) in unstressed and social defeat stressed wild type or mice lacking p38α in serotonergic neurons (p38α CKOePet). Data show that SDS caused mice to retreat to Zone 2 or 3 (Z2,3, far corners). Mice pretreated with norBNI (10 mg/kg, i.p., 24 hr prior) or with serotonergic p38α deletion (p38αCKOePet) show normal exploration of the interaction zone (IZ). (B). Quantification of social interaction scores in mice following SDS. Dashed line represents the social interaction scores for unstressed mice (n=8, * p < 0.05 vs. control saline or p38αΔ/lox, t-test). (C) Place Preference scores following conditioning with U50,488 (2.5 mg/kg) in wild type, p38αΔ/lox and p38αCKOePet mice (n = 8–10, ANOVA, p < 0.05 vs. control). (D) Place Preference scores (means± SEM following conditioning with U50,488 (2.5 mg/kg) in p38α wild type vs. p38α Δ/lox and p38α CKOSERT mice (n = 8–10, ANOVA, p < 0.05 vs. control). (E) Place Preference scores ± SEM following conditioning with U50,488 (2.5mg/kg) in wild type or p38αCKOGFAP mice (n = 6–8). (F) Swim-stress induced immobility scores for wild type mice, p38αΔ/lox, or p38αCKOSERT (Data are means ± SEM; ANOVA, * p < 0.01, n = 6–8). See also Figure S3.
Avoidance behavior is a complex response known to be regulated by serotonergic systems as well as other hormones and neuropeptides (Bari et al., 2011; Eriksson et al., 2011; Cao et al, 2010; Bromberg et al., 2010; Pamplona et al., 2011). To determine if context-dependent avoidance requires serotonergic p38α MAPK expression, we assayed conditioned place aversion (CPA) to U50,488, a KOR agonist that acts as a pharmacological stressor. KOR activation causes aversion behavior in rodents in Pavlovian conditioning paradigms (Pfeiffer et al., 1986; Land et al., 2008). We conditioned mice with U50,488 (2.5 mg/kg, i.p.) over 2 days and then assessed their preference for the drug-paired context. As expected, wild type and Mapk14 Δ/lox mice showed significant CPA to the drug-paired context (Figure 3C,D). In contrast, mice lacking p38α MAPK in either their ePet-1 or SERT-expressing cells (p38αCKOePet or p38αCKOSERT, respectively) failed to show significant place aversion (for p38αCKOePet, ANOVA, F(2,19) = 5.626, p < 0.05 Bonferroni; for p38αCKOSERT, ANOVA, F(2,32) = 4.193, p < 0.05 Bonferroni) (Figure 3C,D). Since previous studies have shown SERT is also expressed in astrocytes (Hirst et al., 1998; Bal et al., 1997; Pickel and Chan, 1999) and to further confirm 5HT neuronal selectivity of the behavioral effects, we induced Cre activity by tamoxifen in p38αCKOGFAP (Mapk14 Δ/lox:Gfap-CreERT2) then assayed their behavioral responses to KOR agonist. Although Cre activity was confirmed in astrocytes of tamoxifen-treated p38αCKOGFAP mice (Figure 2D), they still developed significant CPA (Figure 3E), suggesting that aversion does not require p38α MAPK expression in astrocytes. Furthermore, since place conditioning requires locomotor activity for normal exploratory behavior and aversive compounds such as KOR agonists can reduce locomotion, we also measured locomotor activity in p38α CKOs and controls. We did not observe any effect of genotype on basal or U50,488-induced locomotor scores before or during conditioning (Supplemental Figure 4C), suggesting that the lack of context dependent place aversion to a pharmacological stressor is not attributable to a deficit in locomotor activity or lack of pharmacological activation of KOR.
Serotonergic systems have been widely studied in models of depression and many groups use forced swim stress (FSS) as an animal model of stress-induced affect and measuring behavioral efficacy of anti-depressant-like compounds (Porsolt, 1977). To determine if p38α MAPK deletion in SERT expressing cells prevents swim stress-immobility, we exposed mice to FSS and then measured their immobility during the first trial and again 24 hr later. p38αCKOSERT mice showed significantly less immobility compared to control groups (Figure 3F; ANOVA, F (2,15) = 8.924, p < 0.01 Bonferroni). Furthermore, since previous reports have suggested that stress causes dynorphin-dependent analgesia (McLaughlin et al., 2003), we determined if deletion of p38α MAPK altered stress-induced analgesic responses. Following swim-stress, all control groups and p38αCKOSERT mice showed equivalent and significant stress-induced analgesia (Supplemental Figure 4), suggesting that p38α MAPK deletion does not alter stress-induced dynorphin release or KOR activation. Taken together, these data indicate that p38α MAPK in serotonergic neurons play a critical role in the modulation of affective behavioral responses including avoidance and stress-induced immobility.
p38α MAPK deletion blocks social defeat stress-induced reinstatement
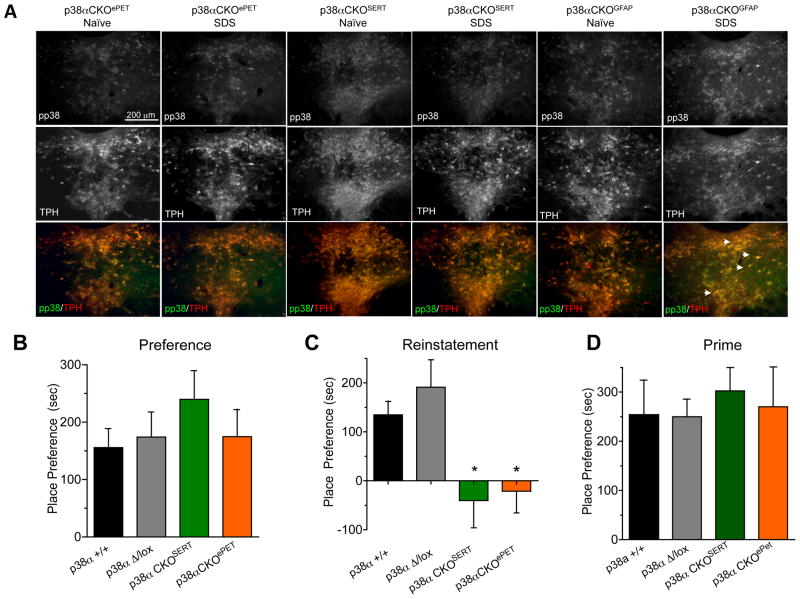
Because negative affect and drug seeking responses share common neural and molecular pathways, we next determined if p38α MAPK deletion in serotonergic neurons prevents stress-induced reinstatement of drug seeking. First, we used immunohistochemistry to determine if SDS-induced increases in pp38-ir were prevented in the CKO mice. Consistent with previous results in this study, SDS did not cause an increase in pp38-ir in TPH-ir cells in p38αCKOSERT or p38αCKOePet mice (Figure 4A, Supplemental Figure 3J). In contrast, SDS increased pp38-ir in TPH-ir cells of p38αCKOGFAP mice, further supporting selective isolation of stress-induced p38α to serotonergic neurons (Supplemental figure 3J). Next we used a similar conditioning procedure as in Figure 1 to determine if serotonergic p38α MAPK deletion altered cocaine place preference. All groups showed similar levels of place preference for cocaine (Figure 4B), suggesting that deletion of serotonergic p38α does not alter either the associative learning required for place preference or the rewarding properties of cocaine. We then extinguished place preference over 3 days, and mice that met extinction criteria were socially defeated, then tested in the place preference apparatus. We found that SDS caused reinstatement of cocaine place preference in both wild type and control Mapk14 Δ/+ mice, but stress-induced reinstatement was not evident in p38αCKOSERT or p38αCKOePet mice (t-test, p < 0.05 vs. matched control) (Figure 4C). Finally, since cocaine injection (i.e. priming) is known to initiate reinstatement to drug seeking by distinct mechanisms (Thomas et al, 2008; Shaham and Hope, 2005), on the following day mice that did not reinstate to stress were injected with 15 mg/kg of cocaine and retested for place preference. All four groups of mice reinstated following a cocaine priming injection (Figure 4D), suggesting that serotonergic p38α MAPK deletion selectively alters only stress-induced modulation of drug-seeking. In conclusion, these results implicate serotonergic p38α MAPK in the regulation of affective state and show that selective deletion of p38α MAPK in serotonergic cells protects mice from stress-induced relapse of cocaine-seeking behaviors.
Figure 4. Disruption of p38α in serotonergic neurons protects against SDS-induced reinstatement of drug seeking.
(A) Representative images of SDS induced phospho-p38-ir in each mouse line. Data show an absence of SDS-induced pp38-ir in TPH-ir cells in both p38αCKOSERT and p38αCKOePet mice, but show an intact increase in pp38-ir in TPH-ir cells of p38α CKOGFAP mice. (Pixel intensities of pp38-ir were quantified from these and replicate images and shown in Supplemental Figure 3J. SDS did not significantly increase pp38-ir in DRN of p38αCKOePet or p38αCKOSERT; whereas pp38-ir was significantly increased in DRN of p38αCKOGFAP mice. (B) Mouse place preference scores (± SEM) following cocaine (15 mg/kg, s.c.) conditioning (C) Mouse place preference scores after extinction (± SEM) and following social defeat (p < 0.05, ANOVA, Bonferroni post hoc). (D) Mouse place preference scores following extinction then cocaine priming (15 mg/kg, s.c.) (n = 8–20). See also Figure S4 for additional behavioral characterization.
p38α MAPK and KOR modulate SERT activity
To define the mechanism for the effects of p38α MAPK, we looked to studies in heterologous gene expression systems that previously suggested the plasma membrane serotonin transporter could be a p38 MAPK substrate (Zhu et al., 2005; Samuvel et al., 2005). Building on in vitro data showing that p38 MAPK increases SERT activity, we first asked whether the serotonergic p38α-dependent CPA response was sensitive to the selective SERT reuptake inhibitor citalopram (Ravna et al., 2003). Mice were conditioned as previously described with a KOR agonist and then assayed for preference to the stressor-paired context. Control mice showed normal place aversion to the U50,488-paired compartment, whereas citalopram pretreated mice (15 mg/kg, i.p 30 min prior to KOR agonist) showed significantly less U50,488 place aversion (Figure 5A) (ANOVA, F(2,15) = 4.082, Bonferroni, p < 0.05 vs. saline). These behavioral data strongly implicate the regulation of extracellular serotonin as a plausible mechanism for p38α-dependent effects.
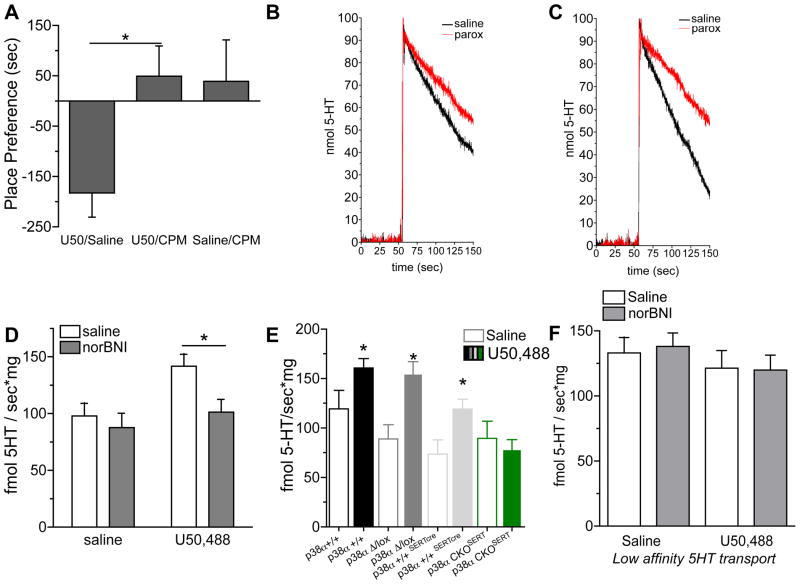
Figure 5. Investigation of 5HT uptake by SERT.
(A) Place preference scores (± SEM) following conditioning of wild type mice treated either with U50,488 (2.5 mg/kg) (U50/Saline), with the selective SERT reuptake inhibitor citalopram (CPM) (15 mg/kg, i.p., 30 min prior to U50,488) (U50/CPM), or with citalopram alone (Saline/CPM). Citalopram prior to KOR agonist significantly blocked U50,488 CPA (ANOVA, p < 0.05, n =8–10) (B,C). Representative RDEV traces of 5-HT uptake from paroxetine (red traces) and non-paroxetine (black traces) treated synaptosomes isolated from control (B) or U50,488 (2.5 mg/kg, i.p. x2) treated animals (C). Note the larger difference in slope for U50,488 treated than control animals. (D) Administration of U50,488 (2.5 mg/kg, i.p. x2 24hr apart) to mice, 30 min prior to synaptosomal isolation, increased 5-HT uptake by SERT compared to saline treated controls (n = 10–16, * P < 0.01). This effect of U50,488 was blocked by pretreatment of the mice with norBNI (10 mg/kg). (E) Administration of U50,488 (2.5 mg/kg, i.p., x2), increased serotonin uptake by SERT in synaptosomes generated from p38α+/+, p38αΔ/lox, and p38α+/+,SERTcre mice, but not from p38αCKOSERT mice (n =10–16, * p < 0.05). (F) Administration of U50,488 (2.5 mg/kg, i.p. x2) 30 min prior to preparation of synaptosomes, did not significantly increase serotonin uptake by the low-affinity transporters (n=10–16).
To determine if p38α MAPK activation actually modulates SERT function in vivo, we used rotating disk electrovoltammetry (RDEV), a validated measure of monoamine transport kinetics (McElvain 1992; Burnette et al. 1996; Earles and Schenk, 1998; Hagan et al., 2010), to measure 5HT uptake rates in synaptosomes isolated from stressed or unstressed mice. To isolate G-protein coupled receptor mediated p38α MAPK activation and to mimic the conditioned aversion paradigm described above, mice received either saline or U50,488 (2.5 mg/kg, i.p.) 24 hr prior to and again 30 min prior to preparation of whole-brain synaptosomes. Synaptosomes isolated from mice injected with KOR agonist (Figure 5C) showed a marked increase rate of SERT specific 5HT clearance compared with synaptosomes from control, saline injected mice (Figure 5B,D). This increase in uptake rate was blocked by in vivo pretreatment with norBNI (2×2 ANOVA, significant effect of pretreatment, p < 0.05) (Figure 5D). We then determined whether deletion of p38α in serotonergic cells blocked the KOR induced increase in SERT uptake. Both wild type (p38α+/+) (t-test vs. saline control, p < 0.05) and control Mapk14 Δ/lox mice (t-test vs. saline control, p < 0.001) showed a significant U50,488-mediated increases in SERT uptake as compared to saline treated animals of the same genotype (Figure 5E). In contrast, KOR stimulation did not significantly increase 5HT uptake in p38αCKOSERT (Mapk14 Δ/lox: Slc6a4-Cre) mice (t-test vs. control, p < 0.01) (Figure 5E), suggesting that p38α MAPK deletion prevented modulation of SERT activity. Because 5HT can also be taken up by a low-affinity, high-capacity transporter (Daws, 2009), we also examined the rate of 5HT uptake in the combined presence of selective NET, SERT, DAT inhibitors. The low affinity transport was not significantly changed by treatment with KOR agonist in vivo (Figure 5F). Taken together these results strongly suggest that SERT activity in nerve terminals of serotonergic neurons is positively modulated in a p38α MAPK-dependent manner.
p38α MAPK regulates SERT cell surface trafficking
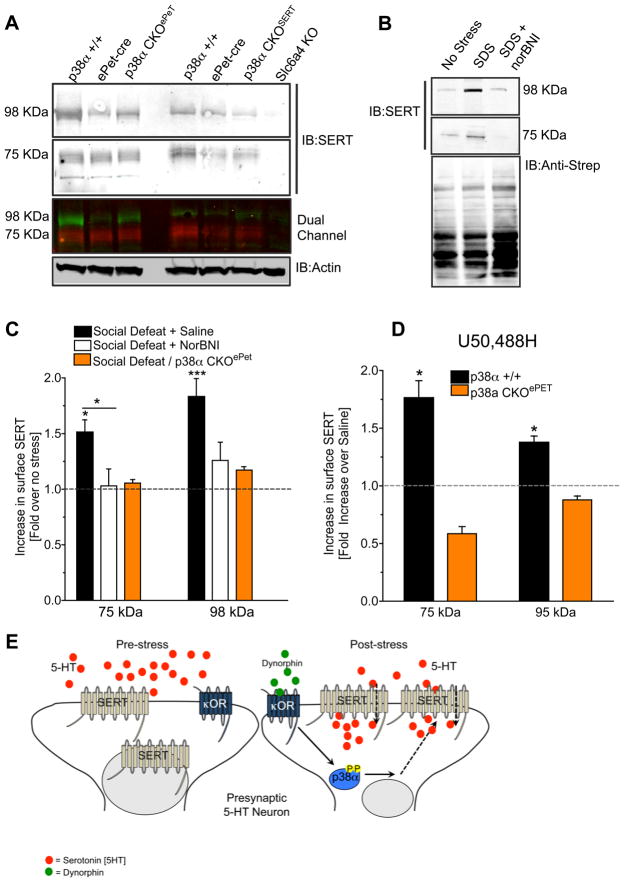
To determine if the increase in uptake rate was caused by increased SERT expression, we isolated synaptosomes and immunoblotted for SERT in each mouse genotype. Consistent with previous reports (Samuvel et al., 2005, Zhu et al., 2005), we found that SERT-ir migrates at both 75 and 98 KDa (Figure 6A). We confirmed the selectivity of the two different SERT antibodies by showing an absence of staining in synaptosomes isolated from SERT knockout mice (Figure 6A) and absence of SERT-ir in untransfected HEK293 cells, but presence in cells transfected with cDNA encoding SERT (Supplemental Figure 5). Total SERT expression in p38αCKOSERT or p38αCKOePet mice was not significantly different from wild-type mice (Figure 6A, Supplemental Figure 5).
Figure 6. p38α MAPK is required for social defeat stress induced cell surface SERT trafficking.
(A) Representative immunoblot of total SERT levels in the different mouse lines used in this study. Data show both species of SERT (75 and 98 kDa) are present in these strains and the absence of SERT-ir in the Slc6a4 knockout (SERT-KO) mouse. Actin-ir was used to control for protein loading. (B). Representative immunoblot of surface SERT expression in biotinylated synaptosomes isolated from unstressed mice (no stress), from mice after SDS, and from mice pretreated with norBNI (10 mg/kg) 24 hr prior to SDS. (Anti-streptavidin-ir confirms equal protein loading after biotinylation and pull down. (C) Quantification of SERT-ir surface expression following SDS of saline-treated wild type, norBNI-treated wild type, and p38αCKOePet mice. (* p < 0.05, ** p< 0,01, *** P< 0.001, ANOVA, Bonferroni post-hoc) (D). Quantification of SERT-ir surface expression following U50,488 treatment of wild type and p38α CKOePet mice. (*p < 0.05, t-test). n= 8–10 in replicate, and each was taken from a separate animal (E) Cartoon model depicting p38α MAPK dependent SERT translocation and decreased extracellular 5HT. See also Figure S5.
Using a membrane impermeant biotinylation procedure to label cell-surface proteins (Samuvel et al., 2005), we next assessed changes in SERT-ir expression on the synaptosomal surface. SDS (20 min exposure) of wild-type mice significantly increased (ANOVA, F(2,24) = 4.7122, p < 0.05) synaptosomal surface SERT expression (Fig 6), and this increase was blocked by pretreatment with norBNI (10 mg/kg, i.p.) 1 hr prior to SDS (Figure 6B,C). Furthermore, socially defeated (20 min exposure) or KOR agonist treated (2.5 mg/kg, 2x 24 hr, i.p.) p38α CKOePet mice did not show stress-induced increases in surface SERT expression, defining a critical role for p38α MAPK in SERT surface trafficking following stress and KOR activation (Figure 6C,D). The proposed mechanism of p38α MAPK-SERT interaction is illustrated in Figure 6E.
DISCUSSION
In this study we present evidence that p38α MAPK is an essential mediator of stress-induced adverse behavioral responses through regulation of serotonergic neuronal functioning. Our data demonstrate that p38α expression in 5HT neural circuits is required for local regulatory control of serotonin transport that ultimately controls behavioral responses including social avoidance, relapse of drug seeking, and the dysphoria-like responses underlying aversion. These results are important because they implicate a critical requirement for p38α MAPK signaling in 5HT neuronal function during stress, and demonstrate that p38α MAPK, in spite of its ubiquitous expression profile, has the ability to specifically regulate selected downstream targets to shape behavioral output. The evidence presented here strongly links molecular events, physiological responses and behavioral output through p38α MAPK signaling actions in serotonergic neurons.
The dorsal raphe nucleus (DRN) contains a major cluster of serotonergic neurons that project broadly throughout the brain (Wylie et al., 2010). Its circuits have impact on mood regulation and nociception (Scott et al., 2005; Zhao et al., 2007). However, the DRN is not homogeneous and contains a diversity of cell types whose local circuit interactions and projections are not completely defined (Wylie et al., 2010). Expression of the transcription factor Pet1 during development is highly correlated with the production of TPH, the rate-limiting enzyme in 5HT synthesis (Liu et al., 2010; Scott et al., 2005). GABA and glutamatergic inputs are known to regulate tonic DRN neuronal activity (Lemos et al., 2011; Tao and Auerbach 2000), although how these different systems are integrated remains an active area of study. All serotonergic cell bodies express SERT peri-synaptically at their terminal regions to clear extracellular 5HT following transmitter release (Murphy and Lesch, 2008). Using the selective expression of Cre driven by SERT and Pet1 promoters, we found that the genetic inactivation of p38α MAPK in Pet1- and SERT-expressing cells caused a loss of p38α and pp38 staining selectively in TPH ir-positive cells of DRN. We were not surprised to find that expression of Cre driven by the SERT promoter was widespread (Supplemental Figure 3) because transient SERT expression during brain development had previously been noted (Gaspar et al., 2003; Narboux-Neme et al., 2008). Nevertheless, the SERT-Cre mice provide important corroborative results consistent with the effects of two other tools we used to excise p38α in serotonergic neurons. The selectivity of Cre expression and subsequent p38α excision by AAV1-CreGFP, SERT-Cre or ePet1-Cre are demonstrably different. AAV1-CreGFP acts on all DRN cells at the site of injection; SERT-Cre expression was not restricted to DRN; and ePet1-Cre is expressed in TPH-ir neurons of the median raphe as well as DRN. Nevertheless, the consistent behavioral results suggest the p38α deletion in the common TPH-ir cells of DRN mediates these effects. In addition, although p38-dependent stress responses also include activation, hypertrophy, and proliferation of astrocytes (Xu et al., 2007), we found no evidence that activation of p38α in GFAP-ir astrocytes was involved in the behavioral responses assessed. The lack of effect of p38α deletion in astrocytes was surprising since other investigators have noted that many aspects of the brain’s response to stress resemble inflammation (Wager-Smith and Markou, 2011).
The conditional deletion of p38α and lack of compensation by p38β caused profound behavioral effects in models of stress-induced depression and addiction and establishes a distinct role of the p38α isoform over p38β isoforms in dorsal raphe function. The selective role for the p38α MAPK isoform was unexpected, but is consistent with prior reports suggesting that the α and β isforms may be expressed in different subcellular compartments (Lee et al., 2000). In addition, differences in functional roles are consistent with isoform differences in other signaling kinases including the various PKC isoforms (Haubensak et al., 2010; Sajikumar and Korte, 2011).
The 5HT transmitter system in mammalian brain is known to be an essential modulator of homeostatic responses that control emotional behaviors and the interaction of animals with their environments (Holmes, 2008; Ansorge et al., 2004; Hen et al., 2001). It is widely accepted that 5HT function is necessary for the normal functioning of neural circuits required for adult emotional behaviors (Gaspar et al, 2003). However, few studies have identified the critical kinases involved in serotonergic function, and few have established how disruption of signal transduction in serotonergic neurons impacts emotional behaviors. Pharmacological blockade of p38 MAPK has been suggested to prevent conditioned place aversion and learned helplessness in animal models of depression (Bruchas et al., 2007). Furthermore, expression of mutant kappa opioid receptors that are ineffective at activating p38 MAPK prevents place aversion in behavioral assays (Land et al., 2009). However, a definitive role for p38 MAPK in behavioral regulation following stress had not previously been directly demonstrated.
Rodent models of social interaction have gained acceptance by neurobiologists as useful models of depression-like behavior since they respond to antidepressant compounds, and the DSM-IV criteria includes decreased motivation for social interaction as major component of human depression (Berton et al., 2006; Biedel et al., 2010). p38α MAPK may represent the first kinase mediator in a series of neurochemical events that underlie the chronic behavioral changes. The block of social avoidance by KOR antagonist further establishes the dynorphin system a critical part of the stress response and strengthens the concept that this system may be novel therapeutic targets to promote stress resilience (Land et al., 2009, Bruchas and Chavkin 2010).
The regulation of extracellular serotonin levels and subsequent post-synaptic effects have long been thought to be a primary component of depression and anhedonic behavioral responses in humans (Haenisch and Bonisch, 2011); however, few reports have demonstrated that interruption of the signal transduction that controls SERT protects against the depressive-like effects of stress. Although regulation of SERT by p38 had been implicated based on in vitro studies (Zhu et al., 2005; Samuvel et al., 2005), the demonstration that stress-induced p38α MAPK causes translocation of SERT to the plasma membrane in brain provides a clear molecular explanation for stress-induced dysphoria. The data presented here show that in serotonin neurons, p38α MAPK acts to directly influence SERT trafficking and ultimately to increase the rate of serotonin reuptake. In conclusion, understanding the molecular and cellular mechanisms that control stress-induced behaviors delineates the neurobiological mechanisms involved in depression and addiction-like behaviors, while also providing insight to potential therapeutic targets. Although prior studies have demonstrated a role for p38α MAPK in cellular development and apoptotic mechanisms, its role in the regulation of mood disorders and addiction risk was not previously appreciated. Furthermore, although antidepressant efficacies of drugs that inhibit the plasma membrane serotonin transporter are clear, the profound effects of stress on the serotonin system function defined by this study provide key molecular insight into the underlying mechanisms of stress-vulnerability and resilience.
Experimental Procedures
For detailed experimental procedures see supplemental information.
Animals
Experimental procedures were carried out in accordance with the USPHS Guide for Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the University of Washington. Male C57BL/6 mice (20–30 gm) were group-housed, four to a cage, in ventilated mouse cages (Thoren Caging Systems, Hazelton, PA) within the Animal Core Facility at the University of Washington, given access to food pellets and water ad libitum, and maintained in specificpathogen-free housing.
Generation of serotonin-specific conditional knockout mice
Breeding and genotyping procedures were as described in the Supplemental Data.
Behavior
Conditioned Place Aversion
Mice were trained in an unbiased, balanced three-compartment conditioning apparatus as described (Land et al., 2009, Bruchas et al., 2007). Stress-induced Social Avoidance and Stress-Induced Cocaine Reinstatement were as performed as described in the Supplemental Data.
Viral Preparation and Injections Local intracranial injections were performed as previously reported (Zweifel et al., 2008, Land et al., 2009) and described more fully in the Supplemental Data.
Immunohistochemistry was performed as previously described (Land et al., 2007; Bruchas et al., 2007) and described more fully in the Supplemental Data.
Synatosomes were prepared from whole brain according to published protocols (Hagan et al., 2010; Ramamoorthy, 2007) and described more fully in the Supplemental Data.
Rotating Disk Electrovoltammetry (RDEV) was used to measure initial velocities of serotonin (5-HT) transport into mouse synaptosomal preparations as previously described (Hagan et al. 2010) and described more fully in the Supplemental Data.
Data Analysis/Statistics
Data are expressed as means ± SEM. Data were normally distributed, and differences between groups were determined using independent t-tests or one-way ANOVA, or two-way ANOVAs followed by post hoc Bonferroni comparisons if the main effect was significant at P < 0.05. Statistical analyses were conducted using GraphPad Prism (version 4.0; GraphPad) or SPSS (version 11.0; SPSS).
Supplementary Material
Acknowledgments
The authors would like to thank Drs. Larry Zweifel and Ali Guler (University of Washington) for helpful discussion. The floxed p38α (p38αlox) transgenic mice were provided by Dr. K. Otsu (Osaka University) though the RIKEN Bioresearch Center. The SERT-Cre mice were provided by Dr. Xiaoxi Zhuang (University of Chicago). The GFAP-CreERT2 mice were provided by Dr. Hans Kirchoff (University of Leipzig). Dr. Evan Deneris (Case Western Reserve University) provided the ePET1-Cre driver line. Support was provided by USPHS grants from the National Institute on Drug Abuse RO1-DA030074, R21-DA025970, RO1-DA016898, T32-DA07278, KO5-DA020570 (CC), K99-DA025182 (MRB), and the Hope for Depression Research Foundation (CC).
References
- Ahmed BY, Chakravarthy S, Eggers R, Hermens WTJMC, Zhang JY, Niclou SP, Levelt C, Sablitzky F, Anderson PN, Lieberman AR, et al. Efficient delivery of Cre-recombinase to neurons in vivo and stable transduction of neurons using adeno-associated and lentiviral vectors. BMC Neurosci. 2004;5:4. doi: 10.1186/1471-2202-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansorge MS, Zhou M, Lira A, Hen R, Gingrich JA. Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science. 2004;306:879–881. doi: 10.1126/science.1101678. [DOI] [PubMed] [Google Scholar]
- Avgustinovich DF, Kovalenko IL. Formation of behavioral pathology in female C57BL/6J mice exposed to prolonged negative psychoemotional conditions. Neurosci Behav Physiol. 2005;35:959–967. doi: 10.1007/s11055-005-0152-8. [DOI] [PubMed] [Google Scholar]
- Bal N, Figueras G, Vilaró MT, Suñol C, Artigas F. Antidepressant drugs inhibit a glial 5-hydroxytryptamine transporter in rat brain. Eur J Neurosci. 1997;9(8):1728–1738. doi: 10.1111/j.1460-9568.1997.tb01530.x. [DOI] [PubMed] [Google Scholar]
- Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Bari A, Theobald DE, Caprioli D, Mar AC, Aidoo-Micah A, Dalley JW, Robbins TW. Serotonin modulates sensitivity to reward and negative feedback in a probabilistic reversal learning task in rats. Neuropsychopharmacology. 2010;35:1290–1301. doi: 10.1038/npp.2009.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beidel DC, Rao PA, Scharfstein L, Wong N, Alfano CA. Social skills and social phobia: an investigation of DSM-IV subtypes. Behav Res Ther. 2010;48:992–1001. doi: 10.1016/j.brat.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron. 2010;68:815–834. doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Aita M, Xu M, Barot SK, Li S, Chavkin C. Stress-induced p38 mitogen-activated protein kinase activation mediates kappa-opioid-dependent dysphoria. J Neurosci. 2007;27:11614–11623. doi: 10.1523/JNEUROSCI.3769-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Chavkin C. The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res. 2010;1314:44–55. doi: 10.1016/j.brainres.2009.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette WB, Bailey MD, Kukoyi S, Blakely RD, Trowbridge CG, Justice JB. Human norepinephrine transporter kinetics using rotating disk electrode voltammetry. Anal Chem. 1996;68:2932–2938. doi: 10.1021/ac960022x. [DOI] [PubMed] [Google Scholar]
- Cao J, Covington HE, Friedman AK, Wilkinson MB, Walsh JJ, Cooper DC, Nestler EJ, Han M. Mesolimbic dopamine neurons in the brain reward circuit mediate susceptibility to social defeat and antidepressant action. J Neurosci. 2010;30:16453–16458. doi: 10.1523/JNEUROSCI.3177-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Thome J, Olson VG, Lane-Ladd SB, Brodkin ES, Hiroi N, Duman RS, Neve RL, Nestler EJ. Regulation of cocaine reward by CREB. Science. 1998;282:2272–2275. doi: 10.1126/science.282.5397.2272. [DOI] [PubMed] [Google Scholar]
- Coulthard LR, White DE, Jones DL, McDermott MF, Burchill SA. p38(MAPK): stress responses from molecular mechanisms to therapeutics. Trends Mol Med. 2009;15:369–379. doi: 10.1016/j.molmed.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daws LC. Unfaithful neurotransmitter transporters: focus on serotonin uptake and implications for antidepressant efficacy. Pharmacol Ther. 2009;121:89–99. doi: 10.1016/j.pharmthera.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earles C, Schenk JO. Rotating disk electrode voltammetric measurements of dopamine transporter activity: an analytical evaluation. Anal Biochem. 1998;264:191–198. doi: 10.1006/abio.1998.2850. [DOI] [PubMed] [Google Scholar]
- Eriksson TM, Delagrange P, Spedding M, Popoli M, Mathé AA, Ogren SO, Svenningsson P. Emotional memory impairments in a genetic rat model of depression: involvement of 5-HT/MEK/Arc signaling in restoration. Mol Psychiatry. 2011 doi: 10.1038/mp.2010.131. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar P, Cases O, Maroteaux L. The developmental role of serotonin: news from mouse molecular genetics. Nat Rev Neurosci. 2003;4:1002–1012. doi: 10.1038/nrn1256. [DOI] [PubMed] [Google Scholar]
- Gong K, Li Z, Xu M, Du J, Lv Z, Zhang Y. A novel protein kinase A-independent, beta-arrestin-1-dependent signaling pathway for p38 mitogen-activated protein kinase activation by beta2-adrenergic receptors. J Biol Chem. 2008;283:29028–29036. doi: 10.1074/jbc.M801313200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haenisch B, Bönisch H. Depression and antidepressants: Insights from knockout of dopamine, serotonin or noradrenaline re-uptake transporters. Pharmacol Ther. 2011;129:352–368. doi: 10.1016/j.pharmthera.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Hagan CE, Neumaier JF, Schenk JO. Rotating disk electrode voltammetric measurements of serotonin transporter kinetics in synaptosomes. J Neurosci Methods. 2010;193:29–38. doi: 10.1016/j.jneumeth.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haubensak W, Kunwar PS, Cai H, Ciocchi S, Wall NR, Ponnusamy R, Biag J, Dong H, Deisseroth K, Callaway EM, et al. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature. 2010;468:270–276. doi: 10.1038/nature09553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingrich JA, Hen R. Dissecting the role of the serotonin system in neuropsychiatric disorders using knockout mice. Psychopharmacology (Berl) 2001;155:1–10. doi: 10.1007/s002130000573. [DOI] [PubMed] [Google Scholar]
- Hensler JG. Serotonergic modulation of the limbic system. Neurosci Biobehav Rev. 2006;30:203–214. doi: 10.1016/j.neubiorev.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Hirrlinger PG, Scheller A, Braun C, Hirrlinger J, Kirchhoff F. Temporal control of gene recombination in astrocytes by transgenic expression of the tamoxifen-inducible DNA recombinase variant CreERT2. Glia. 2006;54:11–20. doi: 10.1002/glia.20342. [DOI] [PubMed] [Google Scholar]
- Hirst WD, Price GW, Rattray M, Wilkin GP. Serotonin transporters in adult rat brain astrocytes revealed by [3H]5-HT uptake into glial plasmalemmal vesicles. Neurochem Int. 1998;33:11–22. doi: 10.1016/s0197-0186(05)80003-8. [DOI] [PubMed] [Google Scholar]
- Holmes A. Genetic variation in cortico-amygdala serotonin function and risk for stress-related disease. Neurosci Biobehav Rev. 2008;32:1293–1314. doi: 10.1016/j.neubiorev.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby LG, Freeman-Daniels E, Lemos JC, Nunan JD, Lamy C, Akanwa A, Beck SG. Corticotropin-releasing factor increases GABA synaptic activity and induces inward current in 5-hydroxytryptamine dorsal raphe neurons. J Neurosci. 2008;28:12927–12937. doi: 10.1523/JNEUROSCI.2887-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Han M, Graham DL, Berton O, Renthal W, Russo SJ, Laplant Q, Graham A, Lutter M, Lagace DC, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Schattauer S, Giardino WJ, Aita M, Messinger D, Hnasko TS, Palmiter RD, Chavkin C. Activation of the kappa opioid receptor in the dorsal raphe nucleus mediates the aversive effects of stress and reinstates drug seeking. Proc Natl Acad Sci USA. 2009;106:19168–19173. doi: 10.1073/pnas.0910705106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Park J, Che Y, Han PL, Lee JK. Constitutive activity and differential localization of p38alpha and p38beta MAPKs in adult mouse brain. J Neurosci Res. 2000;60:623–631. doi: 10.1002/(SICI)1097-4547(20000601)60:5<623::AID-JNR7>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Lemos JC, Zhang G, Walsh T, Kirby LG, Akanwa A, Brooks-Kayal A, Beck SG. Stress-Hyperresponsive WKY Rats Demonstrate Depressed Dorsal Raphe Neuronal Excitability and Dysregulated CRF-Mediated Responses. Neuropsychopharmacology. 2011;36:721–734. doi: 10.1038/npp.2010.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Maejima T, Wyler SC, Casadesus G, Herlitze S, Deneris ES. Pet-1 is required across different stages of life to regulate serotonergic function. Nat Neurosci. 2010b;13:1190–1198. doi: 10.1038/nn.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry CA, Hale MW, Evans AK, Heerkens J, Staub DR, Gasser PJ, Shekhar A. Serotonergic systems, anxiety, and affective disorder: focus on the dorsomedial part of the dorsal raphe nucleus. Ann N Y Acad Sci. 2008;1148:86–94. doi: 10.1196/annals.1410.004. [DOI] [PubMed] [Google Scholar]
- MacGillivray L, Lagrou LM, Reynolds KB, Rosebush PI, Mazurek MF. Role of serotonin transporter inhibition in the regulation of tryptophan hydroxylase in brainstem raphe nuclei: time course and regional specificity. Neuroscience. 2010;171:407–420. doi: 10.1016/j.neuroscience.2010.08.055. [DOI] [PubMed] [Google Scholar]
- McElvain JS, Schenk JO. Studies of the mechanism of inhibition of the dopamine uptake carrier by cocaine in vitro using rotating disk electrode voltammetry. Ann N Y Acad Sci. 1992;654:480–482. doi: 10.1111/j.1749-6632.1992.tb26006.x. [DOI] [PubMed] [Google Scholar]
- McLaughlin JP, Marton-Popovici M, Chavkin C. Kappa opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. J Neurosci. 2003;23:5674–5683. doi: 10.1523/JNEUROSCI.23-13-05674.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA, Yap JJ, Covington HE., 3rd Social stress, therapeutics and drug abuse: preclinical models of escalated and depressed intake. Pharmacol Ther. 2008;1120:102–128. doi: 10.1016/j.pharmthera.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DL, Lesch K. Targeting the murine serotonin transporter: insights into human neurobiology. Nat Rev Neurosci. 2008;9:85–96. doi: 10.1038/nrn2284. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Hasegawa H. Developmental role of tryptophan hydroxylase in the nervous system. Mol Neurobiol. 2007;35:45–54. doi: 10.1007/BF02700623. [DOI] [PubMed] [Google Scholar]
- Narboux-Nême N, Pavone LM, Avallone L, Zhuang X, Gaspar P. Serotonin transporter transgenic (SERTcre) mouse line reveals developmental targets of serotonin specific reuptake inhibitors (SSRIs) Neuropharmacology. 2008;55:994–1005. doi: 10.1016/j.neuropharm.2008.08.020. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13:1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida K, Yamaguchi O, Hirotani S, Hikoso S, Higuchi Y, Watanabe T, Takeda T, Osuka S, Morita T, Kondoh G, et al. p38alpha mitogen-activated protein kinase plays a critical role in cardiomyocyte survival but not in cardiac hypertrophic growth in response to pressure overload. Mol Cell Biol. 2004;24:10611–10620. doi: 10.1128/MCB.24.24.10611-10620.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamplona FA, Henes K, Micale V, Mauch CP, Takahashi RN, Wotjak CT. Prolonged fear incubation leads to generalized avoidance behavior in mice. J Psychiatr Res. 2011;45:354–360. doi: 10.1016/j.jpsychires.2010.06.015. [DOI] [PubMed] [Google Scholar]
- Paul ED, Hale MW, Lukkes JL, Valentine MJ, Sarchet DM, Lowry CA. Repeated social defeat increases reactive emotional coping behavior and alters functional responses in serotonergic neurons in the rat dorsal raphe nucleus. Physiol Behav. 2011 doi: 10.1016/j.physbeh.2011.01.006. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer A, Brantl V, Herz A, Emrich HM. Psychotomimesis mediated by kappa opiate receptors. Science. 1986;233(4765):774–776. doi: 10.1126/science.3016896. [DOI] [PubMed] [Google Scholar]
- Pickel VM, Chan J. Ultrastructural localization of the serotonin transporter in limbic and motor compartments of the nucleus accumbens. J Neurosci. 1999;19:7356–7366. doi: 10.1523/JNEUROSCI.19-17-07356.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamoorthy S, Samuvel DJ, Buck ER, Rudnick G, Jayanthi LD. Phosphorylation of threonine residue 276 is required for acute regulation of serotonin transporter by cyclic GMP. J Biol Chem. 2007;282:11639–11647. doi: 10.1074/jbc.M611353200. [DOI] [PubMed] [Google Scholar]
- Raman M, Chen W, Cobb MH. Differential regulation and properties of MAPKs. Oncogene. 2007;26:3100–3112. doi: 10.1038/sj.onc.1210392. [DOI] [PubMed] [Google Scholar]
- Ravna AW, Sylte I, Dahl SG. Molecular mechanism of citalopram and cocaine interactions with neurotransmitter transporters. J Pharmacol Exp Ther. 2003;307:34–41. doi: 10.1124/jpet.103.054593. [DOI] [PubMed] [Google Scholar]
- Richardson-Jones JW, Craige CP, Guiard BP, Stephen A, Metzger KL, Kung HF, Gardier AM, Dranovsky A, David DJ, Beck SG, et al. 5-HT1A autoreceptor levels determine vulnerability to stress and response to antidepressants. Neuron. 2010;65:40–52. doi: 10.1016/j.neuron.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche M, Commons KG, Peoples A, Valentino RJ. Circuitry underlying regulation of the serotonergic system by swim stress. J Neurosci. 2003;23:970–977. doi: 10.1523/JNEUROSCI.23-03-00970.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajikumar S, Korte M. Metaplasticity governs compartmentalization of synaptic tagging and capture through brain-derived neurotrophic factor (BDNF) and protein kinase M{zeta} (PKM{zeta}) Proc Natl Acad Sci USA. 2011;108:2551–2556. doi: 10.1073/pnas.1016849108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuvel DJ, Jayanthi LD, Bhat NR, Ramamoorthy S. A role for p38 mitogen-activated protein kinase in the regulation of the serotonin transporter: evidence for distinct cellular mechanisms involved in transporter surface expression. J Neurosci. 2005;25:29–41. doi: 10.1523/JNEUROSCI.3754-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott MM, Krueger KC, Deneris ES. A differentially autoregulated Pet-1 enhancer region is a critical target of the transcriptional cascade that governs serotonin neuron development. J Neurosci. 2005;25:2628–2636. doi: 10.1523/JNEUROSCI.4979-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Hope BT. The role of neuroadaptations in relapse to drug seeking. Nat Neurosci. 2005;8:1437–1439. doi: 10.1038/nn1105-1437. [DOI] [PubMed] [Google Scholar]
- Siegfried K. Cognitive symptoms in late-life depression and their treatment. J Affect Disord Suppl. 1985;1:S33–40. doi: 10.1016/0165-0327(85)90086-2. [DOI] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallquist MD, Soriano P. Epiblast-restricted Cre expression in MORE mice: a tool to distinguish embryonic vs. extra-embryonic gene function. Genesis. 2000;26:113–115. doi: 10.1002/(sici)1526-968x(200002)26:2<113::aid-gene3>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Tan M, Walwyn WM, Evans CJ, Xie C. p38 MAPK and beta-arrestin 2 mediate functional interactions between endogenous micro-opioid and alpha2A-adrenergic receptors in neurons. J Biol Chem. 2009;284:6270–6281. doi: 10.1074/jbc.M806742200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao R, Auerbach SB. Regulation of serotonin release by GABA and excitatory amino acids. J Psychopharmacol (Oxford) 2000;14:100–113. doi: 10.1177/026988110001400201. [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Kalivas PW, Shaham Y. Neuroplasticity in the mesolimbic dopamine system and cocaine addiction. Br J Pharmacol. 2008;154:327–342. doi: 10.1038/bjp.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager-Smith K, Markou A. Depression: a repair response to stress-induced neuronal microdamage that can grade into a chronic neuroinflammatory condition? Neurosci Biobehav Rev. 2011;35:742–764. doi: 10.1016/j.neubiorev.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie CJ, Hendricks TJ, Zhang B, Wang L, Lu P, Leahy P, Fox S, Maeno H, Deneris ES. Distinct transcriptomes define rostral and caudal serotonin neurons. J Neurosci. 2010;30:670–684. doi: 10.1523/JNEUROSCI.4656-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Bruchas MR, Ippolito DL, Gendron L, Chavkin C. Sciatic nerve ligation-induced proliferation of spinal cord astrocytes is mediated by kappa opioid activation of p38 mitogen-activated protein kinase. J Neurosci. 2007;27:2570–2581. doi: 10.1523/JNEUROSCI.3728-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarubin T, Han J. Activation and signaling of the p38 MAP kinase pathway. Cell Res. 2005;15:11–18. doi: 10.1038/sj.cr.7290257. [DOI] [PubMed] [Google Scholar]
- Zhang J, Shen B, Lin A. Novel strategies for inhibition of the p38 MAPK pathway. Trends Pharmacol Sci. 2007;28:286–295. doi: 10.1016/j.tips.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Gao Y, Sun Y, Zhao C, Gereau RW, Chen Z. Central serotonergic neurons are differentially required for opioid analgesia but not for morphine tolerance or morphine reward. Proc Natl Acad Sci USA. 2007;104:14519–14524. doi: 10.1073/pnas.0705740104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen X, Du W, Romano AG, Friedman E, Harvey JA. The p38 mitogen-activated protein kinase is involved in associative learning in rabbits. J Neurosci. 2001;21:5513–5519. doi: 10.1523/JNEUROSCI.21-15-05513.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C, Carneiro AM, Dostmann WR, Hewlett WA, Blakely RD. p38 MAPK activation elevates serotonin transport activity via a trafficking-independent, protein phosphatase 2A-dependent process. J Biol Chem. 2005;280:15649–15658. doi: 10.1074/jbc.M410858200. [DOI] [PubMed] [Google Scholar]
- Zhuang X, Masson J, Gingrich JA, Rayport S, Hen R. Targeted gene expression in dopamine and serotonin neurons of the mouse brain. J Neurosci Methods. 2005;143:27–32. doi: 10.1016/j.jneumeth.2004.09.020. [DOI] [PubMed] [Google Scholar]
- Zweifel LS, Argilli E, Bonci A, Palmiter RD. Role of NMDA receptors in dopamine neurons for plasticity and addictive behaviors. Neuron. 2008;59:486–496. doi: 10.1016/j.neuron.2008.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.