Abstract
The aim of this study was to characterize the expression of paraoxonase 2 (PON2) in mouse brain and to assess its antioxidant properties. PON2 levels were highest in lung, intestine, heart and liver, and lower in brain; in all tissues, PON2 expression was higher in female than in male mice. PON2 knockout [PON2-/-] mice did not express any PON2, as expected. In brain, the highest levels of PON2 were found in the substantia nigra, the nucleus accumbens and the striatum, with lower levels in the cerebral cortex, hippocampus, cerebellum and brainstem. A similar regional distribution of PON2 activity (measured by dihydrocoumarin hydrolysis) was also found. PON3 was not detected in any brain area, while PON1 was expressed at very low levels, and did not show any regional difference. PON2 levels were higher in astrocytes than in neurons isolated from all brain regions, and were highest in cells from the striatum. PON2 activity and mRNA levels followed a similar pattern. Brain PON2 levels were highest around birth, and gradually declined. Subcellular distribution experiments indicated that PON2 is primarily expressed in microsomes and in mitochondria. The toxicity in neurons and astrocytes of agents known to cause oxidative stress (DMNQ and H2O2) was higher in cells from PON2-/- mice than in the same cells from wild-type mice, despite similar glutathione levels. These results indicate that PON2 is expressed in brain, and that higher levels are found in dopaminergic regions such as the striatum, suggesting that this enzyme may provide protection against oxidative stress-mediated neurotoxicity.
Keywords: Paraoxonase 2, PON2, striatum, antioxidant
Introduction
The paraoxonase (PON) multigene family consists of three members (PON1, PON2 and PON3), whose genes share a high degree of identity, and are located adjacent to each other on chromosome 7q21-22 in humans and on chromosome 6 in mouse (Primo-Parmo et al.,1996). Phylogenetic analysis suggests that PON2 is the oldest PON family member, from which PON1 and PON3 have evolved (Draganov and La Du, 2004). The name of these enzymes derives from paraoxon, the active metabolite of the organophosphorus insecticide parathion, which is hydrolyzed by PON1 in vitro, though not efficiently in vivo (Li et al., 2000), and has been extended to the other two PONs, which do not have esterase activity. In contrast, all three PONs are lactonases, displaying overlapping but distinct substrate specificities for lactone hydrolysis (Draganov et al., 2005). All three PONs can hydrolyze a number of acyl-homoserine lactones (acyl-HCL), molecules which mediate bacterial quorum-sensing signals, important in regulating expression of virulence factors and in inducing a host inflammatory response; PON2 has the highest acyl-HCL hydrolytic activity of the three PON isozymes (Draganov et al., 2005; Stoltz et al., 2007; Teiber et al., 2008; Horke et al., 2010). Two common polymorphisms have been found in human PON2, an Ala/Gly substitution at position 147, and a Ser/Cys substitution at position 311 (Primo-Parmo et al., 1996; Mochizuki et al., 1998). The PON2 Ser311Cys polymorphism has been shown to affect lactonase activity, with PON2 Cys311 displaying lower activity (Stoltz et al., 2009).
PON1 and PON3 are expressed primarily in liver, and their protein products are associated with high-density lipoproteins in plasma, though more recent data suggest a wider expression pattern (Marsillach et al., 2008). In contrast, PON2 is a ubiquitously expressed intracellular enzyme, but is not present in plasma (Mochizuki et al., 1998; Ng et al., 2001; Marsillach et al., 2008). PON2 mRNA and/or protein have been detected in several tissues including liver, lung, kidney, heart, pancreas, small intestine, muscle, testis, endothelial cells, tracheal epithelial cells, and macrophages (Mochizuki et al., 1998; Ng et al., 2001; Rosenblatt et al., 2003; Levy et al., 2007; Stoltz et al., 2007; Marsillach et al., 2008; Precourt et al., 2009). More limited information exists on PON2 expression in the nervous system. PON2 mRNA has been found in mouse and human brain (Primo-Parmo et al., 1996; Mochizuki et al., 1998; Ng et al., 2006), and PON2 protein has been detected in mouse brain (Ng et al., 2006; Marsillach et al., 2008).
In several tissues, PON2 has been shown to exert an antioxidant effect (Ng et al., 2001). PON2 antagonizes oxidative stress induced by various sources in the intestine of humans and rats (Levy et al., 2007), in human vascular endothelial cells (Horke et al., 2007), in lung epithelial carcinoma cells (Horke et al., 2010), in Caco-2/15 cells (Precourt et al., 2009), and in mouse macrophages (Rosenblat et al., 2003). Given that neurotoxicity and neurodegenerative disorders are often associated with increased oxidative stress (Reynolds et al., 2007; Sayre et al., 2008), a better understanding of the expression and function of PON2 in brain tissue seemed warranted.
Materials and Methods
Materials
Neurobasal-A medium, DMEM medium, fetal bovine serum (FBS), Hank's balanced salt solution (HBSS), GlutaMAX, gentamycin, and SuperScript® III First-Strand Synthesis System were from Invitrogen (Carlsbad, CA). TaqMan Gene Expression Master Mix was from Applied Biosystems Inc. (Foster City, CA). Anti PON2, PON1, PON3 antibodies were from Abcam (Cambridge, MA, USA). Dimethylsulfoxide (DMSO), hydrogen peroxide (H2O2), 2,3-dimethoxy-1,4-naphthoquinone (DMNQ), mouse anti-β-actin antibody, reduced glutathione, 5-sulfosalicylic acid, naphthalene dicarboxaldehyde, dihydrocoumarin (3,4-dihydro-2H,1-benzopyran-2-one), and 3-(4,5-dimethyltiazol-2-yl)-2,5 diphenyltetrazolium bromide (MTT) were from Sigma-Aldrich (St. Louis, MO).
Animals
PON2 wild-type (PON2+/+) and knockout (PON2-/-) mice, kindly provided by Drs. A.J. Lusis, D.M. Shih and S. Reddy (UCLA) were used in this study. PON2-/- mice were generated using the embryonic stem cell line XE661 (strain 129/Ola) and a gene-trap vector, as described in detail elsewhere (Ng et al., 2006). After germ line transmission, backcrossing was performed with C57BL/6J mice for six generations. Control mice were wild-type littermates. Genotyping of mice was done as described by Ng et al., (2006). For developmental studies, mice were sacrificed at gestational day (GD) 20, and at postnatal days (PND) 1, 7, 14, 21, 30 and 60. Mice were housed in a specific pathogen-free facility with ad libitum access to food and water and a 12-h light cycle. All procedures for animal use were in accordance with the National Institute of Health Guide for the Use and Care of Laboratory Animals, and were approved by the University of Washington Institutional Animal Care and Use Committee.
Primary cell cultures
Primary astrocytes from all brain regions were obtained from PND 0.5 mice, except for cerebellar astrocytes, which were obtained from PND 7 mice, as previously described (Giordano et al., 2008). Briefly, brain regions were dissected, mechanically dissociated and incubated with trypsin, followed by trituration, repeated washing, and filtering. After counting, cells were plated at a density of 107 cells per flask in 75 cm2 tissue culture flasks pre-coated with poly-D-lysine and grown in DMEM containing 10% (v/v) FBS, 100 U/ml penicillin and 100 μg/ml streptomycin at 37°C in 5% CO2 / 95% (v/v) air. After 10 days in culture, cells were plated in 24-well plates for the experiments at a density of 5×104 astrocytes/well. Cultures of cerebellar granule neurons (CGN) were prepared from 7 day-old mice, as described by Giordano et al., (2006; 2008). Neurons were grown for 10-12 days before treatments. Neurons from other brain regions were prepared from PND 0.5 mice, as described by Giordano et al., (2008). Briefly, brain regions were collected in HBSS medium containing 0.02% (w/v) bovine serum albumin (BSA) and 10 mM HEPES. Tissues were digested for 25 min in HBSS containing papain (1 mg/ml) and DNAse 40 μg/ml) and centrifuged at 300 × g (max g-force is always indicated) for 5 min at room temperature. The supernatant (containing papain) was removed and the pellet was gently triturated in Neurobasal A Medium supplemented with B27, using a Pasteur pipette to dissociate larger aggregates. Cells were centrifuged at 200 × g at 4°C for 10 min and the cell pellet was gently resuspended. Neurons were then counted, seeded on poly-D-lysine coated 48-well plates at a density of 5 × 104 / cm2, and cultured in neurobasal medium supplemented with B27 (minus AO). Neurons were cultured 8 days before experiments. Microglia were isolated from the cortical astrocyte cultures by shaking the flasks, collecting the medium and spinning at 300 × g at 4°C for seven minutes. Cells were resuspended in a medium containing: DMEM-F12 (Invitrogen), 1% (v/v) G5 supplement (Invitrogen), and 0.1% (w/v) BSA, and plated on PDL-coated 24-well plates. After 3 days incubation, cells were collected and used for analysis. Cultures were determined to be at least 95% pure microglia by determining the number of β-III tubulin-positive neurons and GFAP-positive astrocytes relative to Iba-1-positive microglia (Klintworth et al., 2009).
Western blots
Immunoblots were carried out as described by Giordano et al., (2006). Twentyfive μg of protein were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis and immunoblotted using antibodies against PON1, PON2, PON3 and β-actin. After electrophoresis, proteins were transferred to polyvinylidene difluoride membranes that were incubated with antibodies using the following dilutions: 1:250 (for PON1, PON2, and PON3), and 1:1500 (for β-actin). After the transfer the blots were rinsed in Tris-buffered saline (pH=7.5) and incubated with horseradish peroxidase-conjugated anti-rabbit secondary antibody at the appropriate dilution of 1:750 (for PON2 and PON3), or incubated with horseradish peroxidase-conjugated anti-mouse secondary antibody at the dilutions of 1:2500 for β-actin, or 1:750 (for PON1). Band intensity was measured by densitometry, and the intensity of the bands was normalized to β-actin content.
Deglycosylation of PON2 with Peptide-N-glycosidase F
Peptide-N-glycosidase F (PNGase F) (Biolabs) digestion was performed as described by Marsillach et al. (2010) and Stoltz et al. (2009), and according to the manufacturer's directions. This enzyme releases asparagine-linked oligosaccharides from glycoproteins by hydrolyzing the amide group of the asparagine side chain. Briefly, cell lysates were collected in lysis buffer (50 mM Tris HCl, pH 7.5 (at 18°C), 138 mM NaCl, 5 mM EDTA, 1 mM EGTA, 1 mM NaF, 1 mM phenylmethylsulfonyl fluoride, 10 ug/ml leupeptin and aprotinin), and incubated with PNGase F (5 units) for 1 or 4 h at 37 °C. The reaction was stopped by adding SDS buffer and incubating at 100 °C for 5 min. Deglycosylation was then assessed with SDS-PAGE followed by immunoblot.
RT-PCR
Reverse transcription was performed according to the manufacturer's established protocol using total RNA and the SuperScript® III First-Strand Synthesis System. For gene expression measurements, 4 μL of cDNA were included in a PCR reaction (25 μL final volume) that also consisted of the appropriate forward (FP) and reverse (RP) primers at 360 nM each, 80 nM TaqMan probe, and TaqMan Gene Expression Master Mix. The PCR primers and the dual-labeled probes [6-carboxyfluorescein (FAM) and 6-carboxy-tetramethyl-rhodamine (TAMRA)] for all genes were designed using ABI Primer Express v.1.5 software (Applied Biosystems Inc., Foster City, CA). Amplification and detection of PCR amplicons were performed with the ABI PRISM 7900 system (Applied Biosystems Inc., Foster City, CA) with the following PCR reaction profile: 1 cycle of 95°C for 10 min, 40 cycles of 95°C for 30 sec and 62°C for 1 min. β-actin amplification plots derived from serial dilutions of an established reference sample were used to create a linear regression formula in order to calculate expression levels, and β-actin gene expression levels were utilized as an internal control to normalize the data.
PON2 activity assay
PON2 activity was measured as described by Rosenblatt et al., (2003). Briefly, tissues and primary cells were washed and resuspended in Tris buffer containing 25 mM Tris/HCL, pH 7.6 at 18°C / 1 mM CaCl2. The homogenates were sonicated twice for 10 seconds on ice. An aliquot was saved to measure protein concentration. Enzyme activities were measured using 200 to 300 μg of protein per mL assay mixture. The lactonase activity was measured kinetically using dihydrocoumarin (DHC) as substrate, by monitoring the absorbance at 270 nm in a SpectraMax 190 microplate reader (Molecular Devices, Sunnyvale, CA).
Cytotoxicity assay
Cell viability was quantified colorimetrically using the metabolic dye MTT, as previously described (Giordano et al., 2006; Huang et al., 2010). Cells in 24-well plates were treated with either H2O2 (1-10 μM) or DMNQ (1-50 μM), dissolved in distilled water and DMSO, respectively, for 24 h at 37°C. At the end of exposure, medium was removed and cells were incubated with 500 μl/well of Locke's buffer solution containing 4 μg/ml MTT for 30 min. MTT was removed and the reaction product was dissolved in 0.25 ml DMSO/well. The absorbance was read at 570 nm in a SpectraMax 190 microplate reader, and results expressed as percentage of viable cells relative to controls. Values of IC50 were determined from concentration-response curves using 4-5 concentrations of each compound.
Subcellular fractionation
Subcellular fractionation of cerebellar granule neurons and of cerebellar astrocytes was performed as described by Cox and Emili, (2006), with some modifications (Huang et al., 2010), to isolate the following fractions: cytosol, nucleus, mitochondria, microsomes (representing endoplasmic reticulum, Golgi, intracellular vesicles and plasma membrane), and cytoskeleton. Briefly, cells were collected and homogenized as described by Darte and Beaufay, (1983) in ice-cold lysis buffer containing 250 mM sucrose, 50 mM Tris–HCl (pH 7.4, 18°C), 5 mM MgCl2, 1 mM DDT and 1 mM PMSF, using a tight fitting Teflon pestle, and then disrupted by ten slow passages through a syringe needle (16G × 0.5 cm). After centrifugation at 800 × g at 4°C for 15 min, the supernatant was removed and saved, and the pellet was resuspended for another series of five passages through the needle. The whole process was repeated until a total of 30 passages were reached. The supernatants (crude cytoplasmic fraction) were pooled, and served as source for cytosol, mitochondria and microsomes. The pellet, which contained the nuclei and cytoskeletal fraction, was re-homogenized in buffer containing 1% (v/v) Nonidet P-40, as described by van den Bos et al., (1996) and Ramsby and Makowski, (1999). The insoluble pellet was denoted as “cytoskeletal fraction”, resuspended in buffer containing 1% (v/v) SDS, and stored at -80°C until further analysis. The supernatant was saved as the nuclear fraction. Mitochondria were isolated from the crude cytoplasmic fraction by bench-top centrifugation at 6000 × g at 4°C for 15 min. The mitochondrial pellet was washed and pelleted twice in lysis buffer. The cytosolic fraction was obtained after removal of the microsomal fraction by ultracentrifugation at 100,000 × g at 4°C in for 1 h.
Assay of reactive oxygen species (ROS) formation
ROS formation was determined by fluorescence using 2′,7′-dichlorofluorescein diacetate (DCFH2-DA), as previously described (Giordano et al., 2006; Huang et al., 2010). DCFH2-DA is readily taken up by cells and is subsequently de-esterified to DCFH2, which can be oxidized to dichlorofluorescein (DCF) by hydrogen peroxide, peroxynitrite, and other ROS or reactive nitrogen species. Cells were first washed with Locke's solution and then preincubated for 30 min at 37°C with DCFH2-DA (50 nmol/mg cell protein) in Locke's solution. DCFH2 was added from a stock solution prepared in DMSO which was also added to the blank. Cells were then washed with Locke's solution to remove extracellular DCFH2-DA before treatments with H2O2 or DMNQ. After treatments (at 37°C), cells were washed twice with Locke's buffer and lysed with 0.1 M KH2PO4 and 0.5% (v/v) Triton X-100, pH 7.2 for 5 min. Cell lysates were then scraped from the dishes, and the supernatant was collected. The fluorescence (λEX = 488 nm and λEM = 525 nm) was read immediately, using a SpectraMax 190 spectrophotometric plate reader. ROS formation was expressed as the amount of fluorescence per mg of protein measured using the bicinchoninic acid protein assay kit.
Measurement of glutathione levels
Total intracellular GSH levels were measured as described by Giordano et al., (2006). Briefly, cells were homogenized in Locke's buffer and an aliquot was taken to measure protein concentration by the bicinchoninic acid method. A second aliquot was diluted (1:1) in 10% (w/v) 5-sulfosalicylic acid (SSA), centrifuged at 13,000 × g for 5 min at 4°C, and the supernatant was used for GSH determination. Aliquots from the SSA fraction were added to a black flat-bottomed 96-well plate, and pH was adjusted to 7 with 0.2 M N-ethylmorpholine / 0.02 M KOH. Oxidized glutathione was reduced by adding 10 μl of 10 mM tris-(2-carboxyethyl)-phosphine hydrochloride (TCEP) for 15 min at room temperature. The pH was then adjusted to 12.5 by using 0.5 NaOH before derivatizing samples with 10 mM naphthalene dicarboxaldehyde for 30 min. Finally, samples were analyzed on a spectrofluorometric plate reader (λEX = 472 and λEM = 528 nm). After incubation, the total amount of GSH in the sample was expressed as nanomoles per mg of protein, determined from a standard curve obtained by plotting known amounts of GSH incubated in the same experimental conditions versus fluorescence.
Statistical analysis
Data are expressed as the mean ± S.D. of at least three independent experiments. Statistical analysis was performed using one-way or two-way ANOVA followed by a Bonferroni test for multiple comparisons.
Results
PON2 expression in mouse tissues
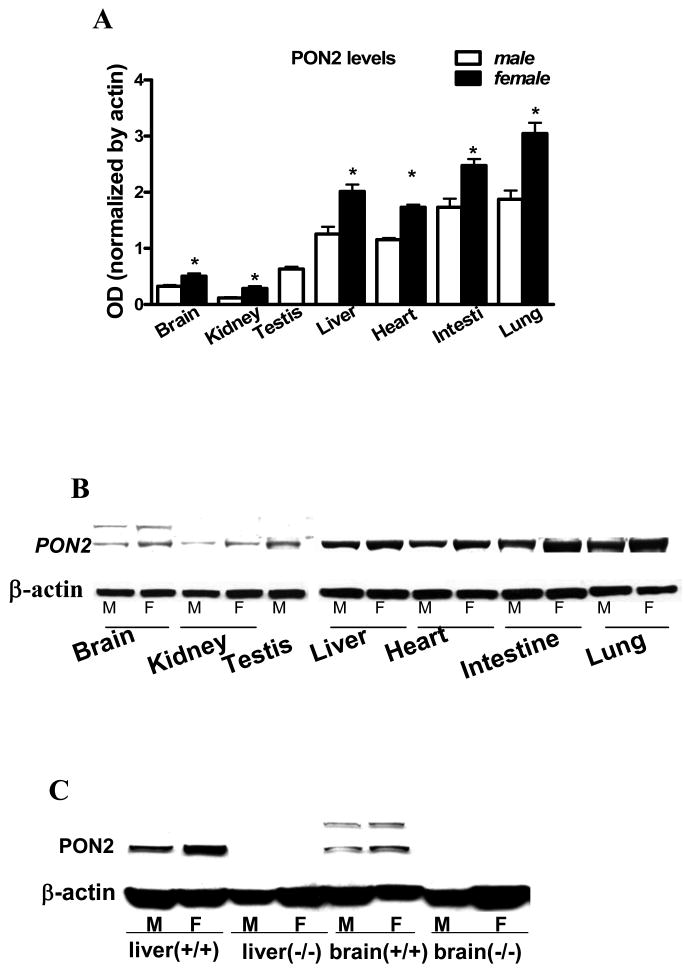
PON2 protein was identified in several mouse tissues by Western blot (Fig. 1A,B). Highest levels were found in lung and small intestine, followed by heart and liver, while lower levels were present in testis, kidney and brain. Interestingly, PON2 expression in tissues from female mice was always significantly higher than in male animals (Fig. 1A,B). In brain, and to a lesser extent in kidneys and testis, but not in other tissues, the PON2 antibody recognized two bands, the lower at MW ∼43 kDa, which corresponds to the reported MW of PON2. The upper band (MW ∼53 kDa) had been found at times by other investigators (see Discussion), and may represent a PON2 isoform. As expected, no PON2 (either of the two bands) was detected in brain or liver from PON2-deficient (PON2-/-) mice (Fig. 1C).
Fig. 1.
PON2 protein expression in tissues from male and female mice. A. Quantification of PON2 levels in tissues (mean ± SD; n = 3). *Significantly different from male, p<0.05. B. Representative Western blot of results shown in Fig. 1A. C. PON2 expression in liver and brain of PON2+/+ and PON2-/- mice.
PON2 expression in brain regions
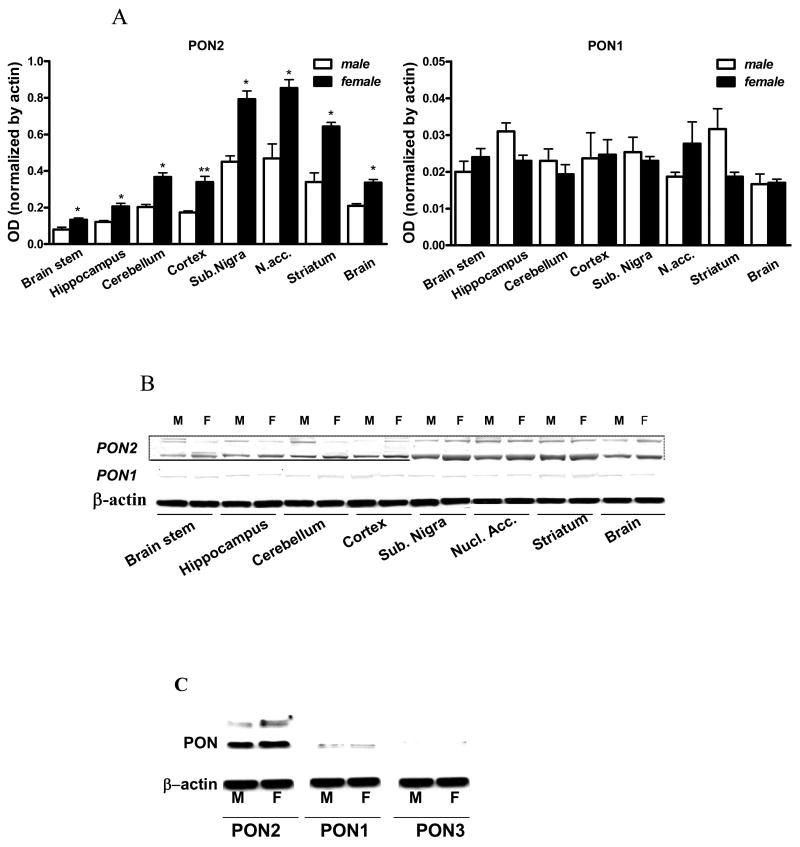
PON2 expression was then examined in several brain regions by Western blot (Fig. 2A,B). The highest levels of PON2 were found in the nucleus accumbens, the substantia nigra and the striatum, with lower levels in cerebral cortex, cerebellum, hippocampus and brainstem. In every brain region, PON2 levels were higher in female mice than in male mice (Fig. 2A,B). PON1 was detected at very low levels (20- to 40-fold less than PON2) in all brain areas, but did not show any regional or gender difference (Fig. 2A,B,C). PON3 was not detected in whole brain (Fig. 2C), or in any brain region (not shown).
Fig. 2.
Expression of PONs in mouse brain. A. PON2 (left) and PON1 (right) protein levels in brain areas of male and female mice (mean ± SD; n =3). *Significantly different from male, p<0.05. B. Representative Western blot of results shown in Fig. 2A. C. Comparison of PON1, PON2 and PON3 expression in whole female mouse brain, showing the absence of PON3.
PON2 activity in brain regions
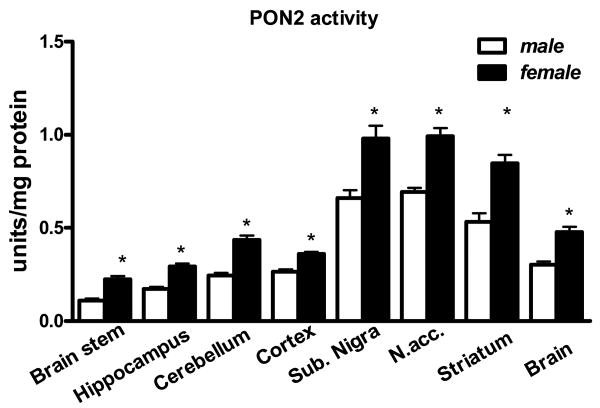
The lactonase activity of PON2 (measured by dihydrocoumarin hydrolysis) was measured in all brain regions. As shown in Fig. 3, the activity followed the same regional distribution as PON2 protein levels, with the highest activity in nucleus accumbens, substantia nigra and striatum, and lower levels in other brain areas. PON2 enzymatic activity was also higher in female than in male mice in all brain regions (Fig. 3).
Fig. 3.
PON2 lactonase activity (measured by dihydrocoumarin hydrolysis) in brain areas of male and female mice. Results are the mean (± SD) of three separate determinations. * Significantly different from male mice, p<0.05.
PON2 expression and activity in brain cells
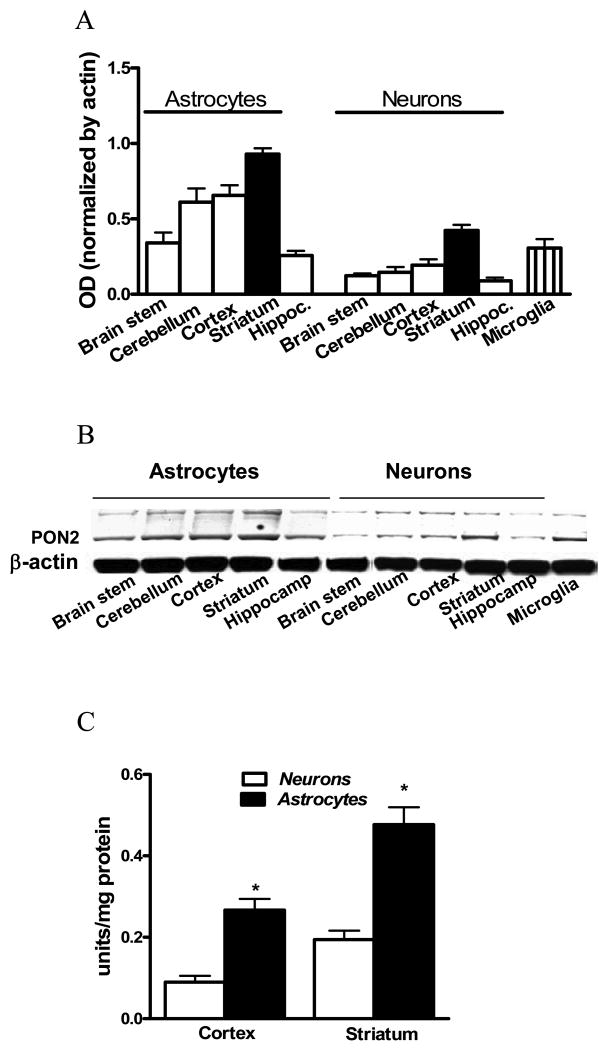
PON2 protein levels were then examined in astrocytes and in neurons isolated from several brain regions (Fig. 4). PON2 levels were significantly higher in astrocytes than in neurons in all brain regions (Fig. 4A,B). Furthermore, the highest levels of PON2 were found in cells isolated from the striatum. PON2 was also present in cortical microglia, at levels similar to those found in neurons (Fig. 4A,B). The activity of PON2 was measured in astrocytes and neurons isolated from the cerebral cortex and the striatum. As shown in Fig. 4C, the highest activity was present in astrocytes, and it was higher for both cell types in striatum than in cerebral cortex.
Fig. 4.
PON2 protein abundance and lactonase activity in astrocytes and neurons from different mouse brain areas. A. PON2 protein levels in astrocytes and neurons from different brain areas and in cortical microglia (mean ± SD; n=3). B. Representative Western blot of results shown in Fig. 4A. C. PON2 lactonase activity in neurons and astrocytes from striatum and cerebral cortex (mean ± SD; n=3). *Significantly different from neurons, p<0.05.
PON2 mRNA levels
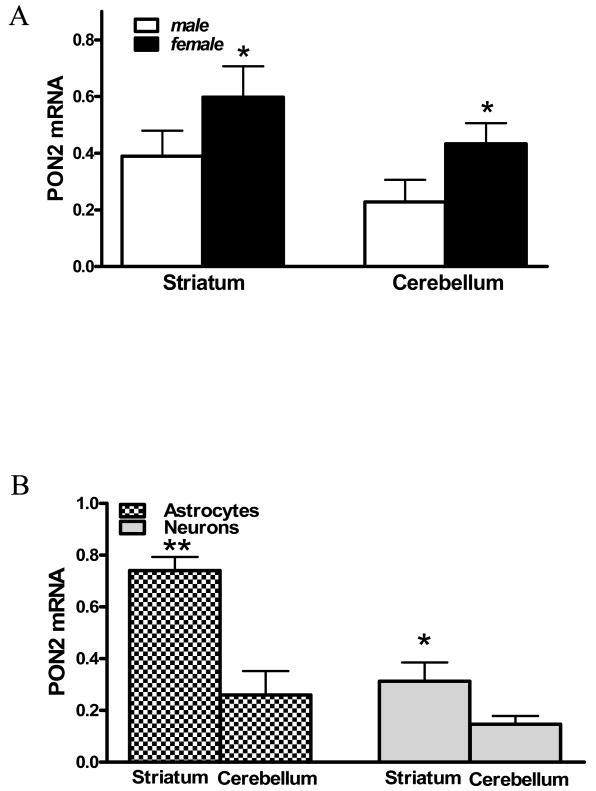
PON2 mRNA levels were measured by quantitative RT-PCR in striatum and cerebellum (Fig. 5A), as well as in astrocytes and neurons isolated from the striatum and cerebellum (Fig. 5B). As with PON2 protein (Figs. 2, 4), the levels of PON2 mRNA were higher in striatum than in cerebellum and higher in female than in male mice (Fig. 5A). As was the case with PON2 protein, astrocytes had higher levels of PON2 mRNA than neurons, and PON2 mRNA levels were higher in striatum than cerebellum in both cell types (Fig. 5B).
Fig. 5.
PON2 mRNA levels in mouse brain. A. PON2 mRNA levels in striatum and cerebellum of male and female mice (mean ± SD; n = 3). *Significantly different from male, p<0.05. B. PON2 mRNA levels in striatal and cerebellar astrocytes and in striatal neurons and cerebellar granule neurons (mean ± SD; n =3). *Significantly different from neurons, p<0.05.
Developmental expression of PON2 in mouse brain
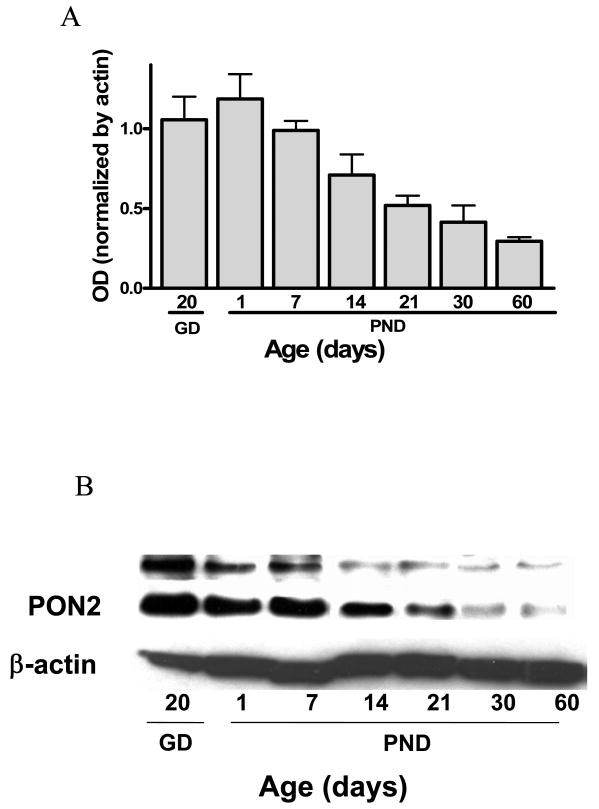
Levels of PON2 protein in the whole brain of female mice were measured by Western blot at GD 20 and at PND 1, 7, 14, 21, 30, and 60. As shown in Fig. 6, PON2 levels were highest before (GD 20), and shortly after (PND 1-7) birth, and gradually declined. In two-month old female mice, PON2 levels were only 30% of the levels on PND1 (Fig. 6).
Fig. 6.
Developmental expression of PON2 protein in female mouse brain. A. PON2 levels, measured by Western blot on GD 20 and PND 1, 7, 14, 21, 30 and 60 (mean ± SD; n =3). B. Representative blots of results shown in Fig. 6A.
Subcellular distribution of PON2 in neurons and in astrocytes
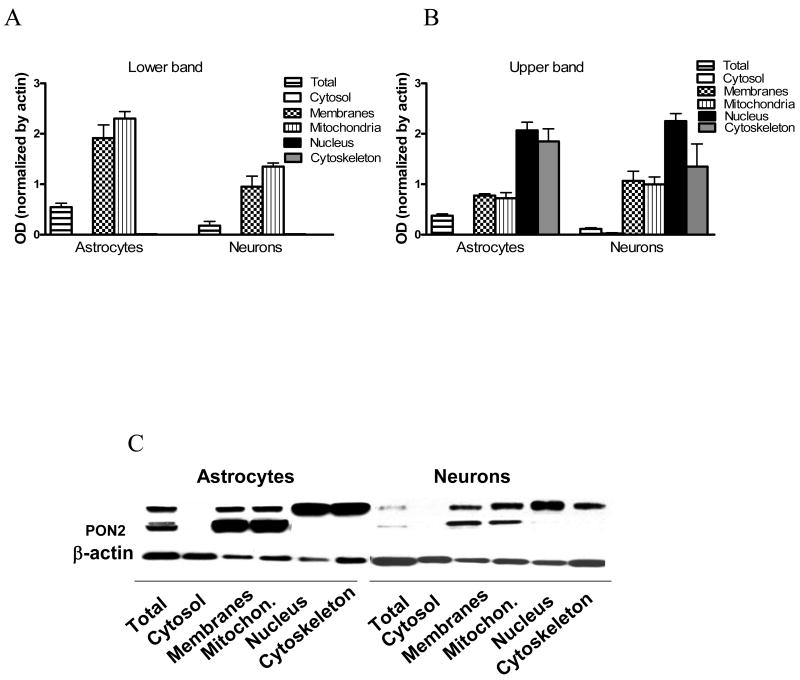
To determine the subcellular distribution of PON2 protein, cerebellar granule neurons and cerebellar astrocytes were fractionated according to Cox and Emily, (2006), with some modifications (Huang et al., 2010), and PON2 levels were measured in cellular fractions [cytosol, membranes (microsomes), nucleus, mitochondria, and cytoskeleton] by Western blot. The subcellular distribution of PON2 was similar in astrocytes and neurons. However, differences were found in the localization of the 43 kDa lower band (the putative PON2) and the upper band (the putative PON2 alternate isoform). As shown in Fig. 7, in both cell types, the highest levels of 43 kDa PON2 were found in mitochondria, followed by membranes (microsomes). No PON2 was detected in the cytosolic, nuclear, or cytoskeletal fractions. In contrast, the upper-band PON2 isoform was expressed at highest levels in the nucleus and the cytoskeleton, neither of which contained significant levels of the 43 kDa band (Fig. 7A,B).
Fig. 7.
Distribution of PON2 protein in subcellular fractions of mouse cerebellar granule neurons and cerebellar astrocytes. A. PON2 levels (measured by Western blot) in subcellular fractions. Cell fractionation was carried out as described in Methods. Shown are quantifications of the lower band, corresponding to PON2 (A), and of the upper band (B). (mean ± SD; n = 3). C. Representative blots of results shown in Fig. 7A and 7B.
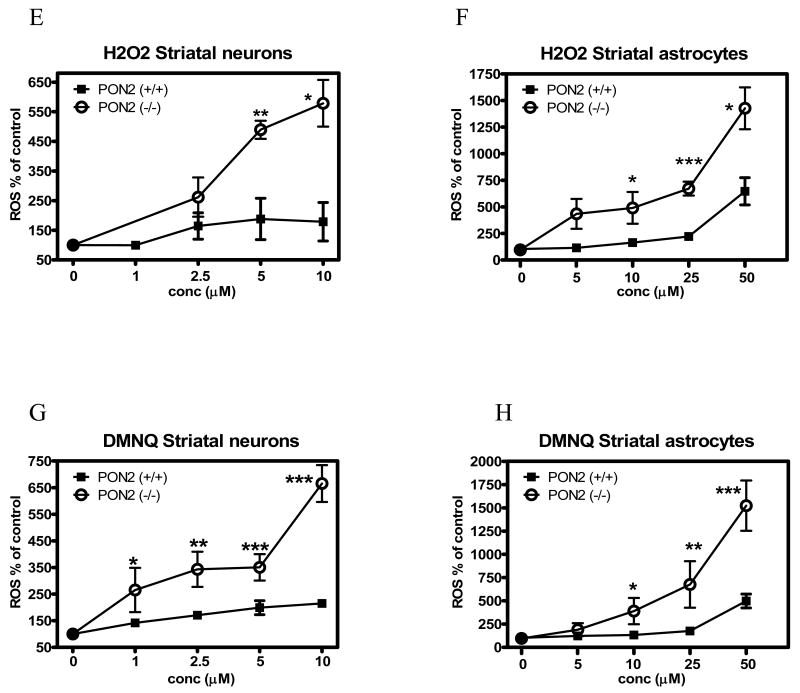
Susceptibility of cerebellar and striatal neurons and astrocytes to agents eliciting oxidative stress
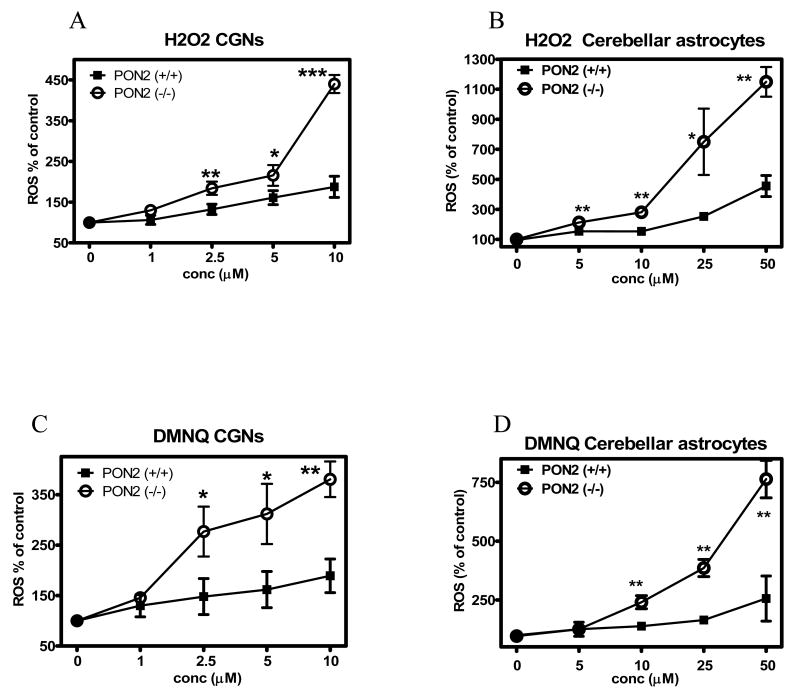
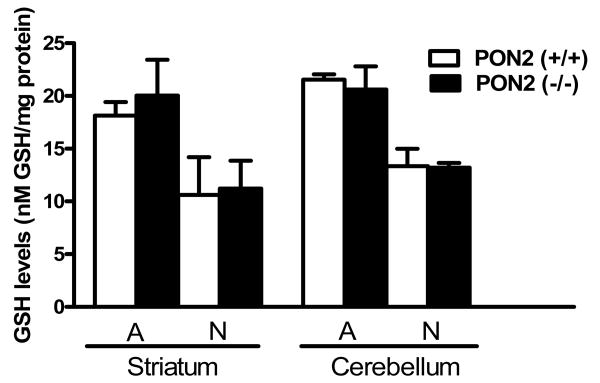
To provide an indication of whether PON2 exerts a protective effect toward oxidative stress in brain cells, as observed in other tissues and cell types, we investigated the cytotoxicity of two known oxidants, H2O2 and DMNQ, in cerebellar and striatal astrocytes, and in cerebellar granule neurons and striatal neurons, isolated from wild-type (PON2+/+) and PON2-deficient (PON-/-) mice (Table 1). In all instances, cells from PON2-/- mice were more susceptible to the toxicity of both compounds, by a factor of 5 to 11-fold. The differential susceptibility was higher in astrocytes than in neurons, and was higher in cells from striatum than in those from the cerebellum, in accordance with the relative levels of PON2 expression. The protection afforded by PON2 to neurons and astrocytes was related to its ability to scavenge reactive oxygen species (ROS) upon exposure to oxidants. Fig. 8 shows the levels of ROS induced by either H2O2 or DMNQ in astrocytes and neurons from striatum and cerebellum, isolated from PON2+/+ and PON2-/- mice. For both compounds, in all cell types from both brain regions, ROS levels were significantly higher in PON-/- mice (Fig. 8 A-H). Since glutathione (GSH) is a major determinant of cellular protection toward oxidative stress, we also measured GSH levels in these cells. As shown in Fig. 9, GSH levels did not differ between astrocytes and neurons isolated from striatum and cerebellum of PON2-/- and PON2+/+ mice.
Table 1. Toxicity of oxidants in astrocytes and neurons from PON2+/+ and PON-/- mice.
| Cell type | PON2+/+ | PON2-/- | Ratio (95% CI) |
|---|---|---|---|
| IC50 (μM) | |||
| Striatal neurons | |||
| H2O2 | 9.6 ± 1.5 | 1.2 ± 0.4 | 8.7 (4.8-17.6) |
| DMNQ | 12.6 ± 1.6 | 1.7 ± 0.3 | 7.6 (5.3-10.8) |
| Cerebellar granule neurons | |||
| H2O2 | 6.2 ± 1.3 | 1.2 ± 0.7 | 5.2 (2.4-19.9) |
| DMNQ | 10.8 ± 1.8 | 2.3 ± 0.6 | 4.7 (2.9-8.4) |
| Striatal astrocytes | |||
| H2O2 | 42.0 ± 4.2 | 3.7 ± 0.9 | 11.3 (7.8-19.0) |
| DMNQ | 47.1 ± 1.3 | 5.6 ± 0.4 | 8.4 (7.5-9.6) |
| Cerebellar astrocytes | |||
| H2O2 | 34.1 ± 1.3 | 4.7 ± 1.0 | 7.3 (5.4-11.0) |
| DMNQ | 43.0 ± 1.4 | 6.1 ± 0.2 | 7.1 (6.6-7.6) |
Cell viability was measured by the MTT assay as described in Methods. Values of IC50 (μM) were determined from concentration-response curves using 4-5 concentrations of each compound in duplicate. Values are expressed as mean (± SD) of three separate experiments. All the IC50 values in PON2+/+ mice were significantly different (p<0.05) from the co respective ones in PON2-/- mice (one way ANOVA and Bonferroni Multiple Comparison Test). The 95% confidence intervals for each ratio were calculated by propagation of error for quotients. Ratios were not significantly different from each other.
Fig. 8.
Oxidative stress induced by H2O2 and by DMNQ in striatal and cerebellar astrocytes and in striatal neurons and cerebellar granule neurons from PON2+/+ and PON2-/- mice. Cells were incubated with different concentrations of each compound for 60 min and ROS levels were measured as described in Methods. A-D. Effects on cerebellar granule neurons (A, C) and cerebellar astrocytes (B, D). E-H. Effects on striatal neurons (E, G) and striatal astrocytes (F, H). Results are the mean (± SD) of three separate determinations. *Significantly different from PON2-/- mice, p<0.05.
Fig. 9.
Levels of glutathione (GSH) in striatal and cerebellar astrocytes, striatal neurons and cerebellar granule neurons from PON2+/+ and PON2-/- mice. Results are the mean (± SD) of three separate determinations.
Discussion
Results of the present study represent the first characterization on PON2 in mouse brain, and suggest that this enzyme exerts antioxidant actions in this tissue. In agreement with previous findings (Mochizuki et al., 1998; Ng et al., 2001; Rosenblatt et al., 2003; Levy et al., 2007; Stoltz et al., 2007; Marsillach et al., 2008; Precourt et al., 2009) we found that PON2 is expressed ubiquitously in mouse tissues. Highest levels were found in lung and small intestine, followed by heart and liver, while lower levels were found in testis, kidney and brain. A new finding is that PON2 protein levels were consistently higher, in all tissues examined, in female mice than in male mice. This may suggest a modulatory effect of estrogens on PON2 expression, which deserves further investigations. Of interest is that PON2 (as the other PONs) is capable of hydrolyzing several estrogen esters (Teiber et al., 2007). Furthermore, estrogens have been shown to increase PON1 activity both in humans (Sutherland et al., 2001), and in cultured hepatocytes (Ahmad and Scott, 2010). However, in the latter case, such increase was attributed to an increased stabilization of PON1 and not to an increased expression. In preliminary experiments, we have found that exposure of mouse striatal astrocytes to estradiol (20 μM for 24 h) caused a 70% increase in PON2 protein levels (Tait, Giordano, Costa, unpublished observation). The potential modulation of PON2 by sex hormones warrants further investigation, which is in progress.
The antibody used to detect PON2 identified two bands in some tissues (brain, testis, kidney), but not in others. The lower band represents a protein of ∼43 kD, which is the reported MW for PON2. Some previous studies had identified one band for PON2 in human macrophages, human endothelial cells, human and rat intestine, and Caco-2 cells (Ng et al., 2001; Shiner et al., 2004; Levy et al., 2007; Precourt et al., 2009). In contrast, two bands of PON2, with small differences in molecular weight, were found in Caco-2 cells, human and mouse gastrointestinal tracts, mouse liver, A549 cells, human umbilical vein derived cells, and human artery smooth muscle cells (Shamir et al., 2005; Ng et al., 2006; Horke et al., 2007; 2010). The detection of two bands for PON2 would suggest the presence of two isoforms of this enzyme, in accordance with the two splice variants of mRNA (Mochizuki et al., 1998; Horke et al., 2007; 2010). Furthermore, PON2 is known to have four putative N-linked gycosylation sites at asparagine residues (Stoltz et al. 2009). However, deglycosylation experiments showed that both putative isoforms were glycosylated, as indicated by a shift of both bands upon incubation with PNGase F (Fig. 10). Further studies are needed, involving purification of the upper band, and its analysis (e.g. by mass spectrometry), to identify its structural features and other potential post-translation modifications. This may be of relevance, given for example the findings of the subcellular distribution study (Fig. 7). Of note, nevertheless, is that neither band was detected in tissues from PON2 -/- mice (Fig. 1C; Ng et al., 2006).
Fig. 10.
Effect of enzymatic deglycosylation on PON2 in primary striatal astrocytes. Enzymatic deglycosylation with PNGase F was performed as described in Methods. Note that the MW of both bands shifted after 1 and 4 hr of PNGase incubation indicating that both native proteins are glycosylated.
Regional analysis of PON2 protein expression in the CNS indicated highest levels in nucleus accumbens, substantia nigra and striatum, three dopaminergic areas known to present high levels of oxidative stress due to their high iron content and to dopamine metabolism (Floor, 2000; Shadrina et al., 2010). Lower levels were found in other brain regions (cerebellum, cerebral cortex, hippocampus, brainstem), and levels in female mice were consistently higher than those found in male animals. The pattern of PON2 lactonase activity and of PON2 mRNA levels followed similar regional and gender profiles. Previously, PON2 mRNA levels were identified in whole brain from mice (Primo-Parmo et al., 1996; Ng et al., 2006) and humans (Mochizuki et al., 1998; Mackness et al., 2010); however, regional distribution had not been studied. PON1 was expressed at very low levels in brain regions and did not show any regional or gender difference, while PON3 was not detected in any brain area.
Western blot analysis of PON2 in astrocytes and neurons from different brain regions indicated that in each brain area, astrocytes displayed higher PON2 levels than neurons. This was confirmed by measurements of PON2 activity and of PON2 mRNA levels. The higher expression of PON2 in astrocytes is of interest, as these cells also have a higher GSH content than neurons (Rice and Russo-Menna, 1998; Giordano et al., 2008), underlying the importance of astrocytes in protecting the nervous system from oxidative stress. Cortical microglial cells also expressed PON2, at levels similar to neurons. In preliminary experiments, PON2 expression was also measured in two human cell lines, SH-SY5Y neuroblastoma and 132-1N1 astrocytoma cells. In contrast to mouse primary cells, levels of PON2 protein in the human cell lines were slightly higher in the neuroblastoma cells than in the astrocytoma cells (Tait, Giordano, Costa, unpublished)
Developmental analysis showed that PON2 protein levels in whole brain were highest before birth (GD 20) and early after birth (PND 1-7), and gradually declined with age, reaching a plateau of about 30% of the highest level between 1 and 2 months of age. This finding would suggest that PON2 may be relevant in early brain development to protect brain cells from oxidative stress (Sola et al., 2007; Blomgren et al., 2007; Hayashi, 2009). A more detailed regional study would be of interest to ascertain the expression of PON2 in different brain regions during development. Also of interest would be investigating the development of PON2 expression in other tissues, such as the liver, in which PON1 has been shown to be very low at birth, and to gradually increase with age (Li et al., 1997).
Cerebellar astrocytes and cerebellar granule neurons were utilized for a cellular subfractionation study, to determine the intracellular distribution of PON2. Some important findings have emerged from these experiments. First, PON2 levels in both cell types were highest in mitochondria, followed by microsomes. Second, intracellular distribution of the two PON2 isoforms was not homogeneous, as high levels of the putative splice variant were found in the nucleus and the cytoskeleton. In earlier studies in HeLa cells (Ng et al., 2001) and in Caco-2 cells (Shamir et al., 2005), PON2 had been described as a plasma membrane-associated protein, and in mouse macrophages PON2 was associated with the microsomal, but not the cytosolic fraction (Rosenblat et al., 2003). In EA.hy cells (human umbilical vein endothelial cells) Horke et al., (2007) found that PON2 was enriched in the nuclear envelope, and was also present in mitochondria and the endoplasmic reticulum, a finding later confirmed by the same investigators (Altenhofer et al., 2010). In a subsequent study in Caco-2 cells, Rothem et al., (2007) identified the endoplasmic reticulum as the major intracellular localization for PON2. In HeLa cells, PON2 protein was found highly-expressed in mitochondria, specifically in the inner mitochondrial membrane (Devarajan et al., 2010). In agreement with these findings, we found that in mouse cerebellar astrocytes and neurons the highest levels of PON2 protein were in the mitochondria and the microsomes. The finding that the nucleus and the cytoskeleton of both cell types expressed high levels of the putative alternative PON2 isoform and none of the 43 kDa isoform(Fig. 7) is still unclear, and warrants further investigation.
In several tissues, PON2 has been shown to exert an antioxidant effect (Ng et al., 2001). PON2 has been shown to antagonize oxidative stress induced by various factors in human and rat intestine (Levy et al., 2007), in human vascular endothelial cells (Horke et al. 2007), in lung epithelial carcinoma cells (Horke et al., 2010), in Caco-2/15 cells (Precourt et al., 2009), and in mouse macrophages (Rosenblatt et al., 2003). We compared the cytotoxicity of two oxidative stress-producing agents, H2O2 and DMNQ, in striatal and cerebellar astrocytes and neurons from wild type and from PON2-/- mice. As predicted, cells from PON2-/- mice were more susceptible to the toxicity of these compounds, and the degree of susceptibility was related to the relative PON2 content of wild type cells (i.e. the higher the PON2 levels, the higher the ratio of IC50s between wild-type and PON-/- mice). These findings were confirmed by direct measurements of ROS. As shown in Fig. 8, both compounds caused significantly higher levels of oxidative stress in astrocytes and neurons from PON2-/- mice than in wild type mice. Since glutathione (GSH) is a major determinant of cellular susceptibility to oxidative stress, we also measured GSH levels in all cell types. As shown in Fig. 9, GSH levels were identical in cells from wild type or PON2-/- mice, indicating that the observed differential susceptibility was due to the differential expression of PON2.
Mitochondria are a major source of free radical related oxidative stress (Higgins et al., 2010), and the preponderant localization of PON2 in mitochondria would support a role for this enzyme in protecting cells from oxidative damage. A recent study in HeLa cells has shown that PON2 binds to Coenzyme Q10 that associates with complex III in mitochondria, and that PON2 deficiency causes mitochondrial dysfunction (Devarajan et al., 2010). Furthermore, in human endothelial cells, PON2 has been shown to reduce, indirectly, but specifically, the release of superoxide from the inner mitochondrial membrane, without affecting levels of other radicals such as H2O2 and peroxynitrite (Altenhofer et al., 2010). Interestingly, the Cys311Ser mutation that influences lactonase activity (Stoltz et al., 2009) does not appear to affect PON2's antioxidant properties, suggesting independent hydrolytic and anti-oxidant functions (Altenhofer et al., 2010).
Acknowledgments
We thank Drs. Reddy, Lusis and Shih (Dept. of Medicine, Division of Cardiology, UCLA) for providing the PON2-/- mice, K. Dao and L. Tait for assistance in some of the experiments, and H.W. Wilkerson and F.M. Farin for mRNA measurements. This study was supported in part by grants from the National Institute of Environmental Health Sciences (P42ES04696, P30ES07033, and P50ES09601).
Footnotes
Conflict of interest: The authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmad S, Scott JE. Estradiol enhances cell-associated paraoxonase 1 (PON1) activity in vitro without altering PON1 expression. Biochem Biophys Res Commun. 397:441–446. doi: 10.1016/j.bbrc.2010.05.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altenhofer S, Witte I, Teiber JF, Wilgenbus P, Pautz A, Li H, Daiber A, Witan H, Clement AM, Forstermann U, Horke S. One enzyme, two functions. PON2 prevents mitochondrial superoxide formation and apoptosis independent from its lactonase activity. J Biol Chem. 2010;285:24398–24403. doi: 10.1074/jbc.M110.118604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomgren K, Leist M, Groc L. Pathological apoptosis in the developing brain. Apoptosis. 2007;12:993–1010. doi: 10.1007/s10495-007-0754-4. [DOI] [PubMed] [Google Scholar]
- Cox B, Emili A. Tissue subcellular fractionation and protein extraction for use in mass-spectrometry-based proteomics. Nat Protoc. 2006;1:1872–1878. doi: 10.1038/nprot.2006.273. [DOI] [PubMed] [Google Scholar]
- Darte C, Beaufay H. Analytical subcellular fractionation of cultivated mouse resident peritoneal macrophages. J Exp Med. 1983;157:1208–1228. doi: 10.1084/jem.157.4.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devarajan A, Bourquard N, Hama S, Navab M, Grijalva VR, Morvardi S, Clarke C, Vergnes L, Reue K, Teiber JF, Reddy ST. Paraoxonase 2 deficiency alters mitochondrial function and exacerbates the development of atherosclerosis. Antiox Redox Signal. 2010 doi: 10.1089/ars.2010.3430. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganov DI, La Du BN. Pharmacogenetics of paraoxonase: a brief review. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:78–88. doi: 10.1007/s00210-003-0833-1. [DOI] [PubMed] [Google Scholar]
- Draganov DI, Teiber JF, Speelman A, Osawa Y, Sunahara R, La Du BN. Human paraoxonases (PON1, PON2 and PON3) are lactonases with overlapping and distinct substrate specificities. J Lipid Res. 2005;46:1239–1247. doi: 10.1194/jlr.M400511-JLR200. [DOI] [PubMed] [Google Scholar]
- Floor E. Iron as a vulnerability factor in nigrostriatal degeneration in aging and Parkinson's disease. Cell Mol Biol (Noisy-le-grand) 2000;46:709–720. [PubMed] [Google Scholar]
- Giordano G, White CC, McConnachie LA, Fernandez C, Kavanagh TJ, Costa LG. Neurotoxicity of domoic acid in cerebellar granule neurons in a genetic model of glutathione deficiency. Mol Pharmacol. 2006;70:2116–21126. doi: 10.1124/mol.106.027748. [DOI] [PubMed] [Google Scholar]
- Giordano G, Kavanagh TJ, Costa LG. Neurotoxicity of a polybrominated diphenyl ether mixture (DE-71) in mouse neurons and astrocytes is modulated by intracellular glutathione levels. Toxicol Appl Pharmacol. 2008;232:161–168. doi: 10.1016/j.taap.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M. Oxidative stress in developmental brain disorders. Neuropathology. 2009;29:1–8. doi: 10.1111/j.1440-1789.2008.00888.x. [DOI] [PubMed] [Google Scholar]
- Higgins GC, Beart PM, Shin YS, Chen MJ, Cheung NS, Nagley P. Oxidative stress: emerging mitochondrial and cellular themes and variations in neuronal injury. J Alzheimers Dis. 2010;20(Suppl. 2):S453–S473. doi: 10.3233/JAD-2010-100321. [DOI] [PubMed] [Google Scholar]
- Horke S, Witte I, Wilgenbus P, Kruger M, Starnd D, Forstermann U. Paraoxonase-2 reduces oxidative stress in vascular cells and decreases endoplasmic reticulum stress-induced caspase activation. Circulation. 2007;115:2055–2064. doi: 10.1161/CIRCULATIONAHA.106.681700. [DOI] [PubMed] [Google Scholar]
- Horke S, Witte I, Altenhoffer S, Wilgenbus P, Goldeck M, Forstermann U, Xiao J, Kramer GL, Haines DC, Chowdhary PK, Haley RW, Teiber JF. Paraoxonase-2 is down regulated by the Pesudomonas aeruginosa quorum-sensing signal N-(3-oxododecanyl)-L-homoserine lactone and attenuates oxidative stress induced by pyocyanin. Biochem J. 2010;426:73–83. doi: 10.1042/BJ20091414. [DOI] [PubMed] [Google Scholar]
- Huang SC, Giordano G, Costa LG. Comparative cytotoxicity and intracellular accumulation of five polybrominated diphenyl ether congeners in mouse cerebellar granule neurons. Toxicol Sci. 2010;114:124–132. doi: 10.1093/toxsci/kfp296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klintworth H, Garden G, Xia Z. Rotenone and paraquat do not directly activate microglia or induce inflammatory cytokine release. Neurosci Lett. 2009;462:1–5. doi: 10.1016/j.neulet.2009.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy E, Trudel K, Bendayan M, Seidman E, Delvin E, Elchebly M, Lavoie JC, Precourt LP, Amre D, Sinnett D. Biological role, protein expression, subcellular localization, and oxidative stress response of paraoxonase 2 in the intestine of human and rats. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1252–G1261. doi: 10.1152/ajpgi.00369.2007. [DOI] [PubMed] [Google Scholar]
- Li WF, Matthews C, Disteche CM, Costa LG, Furlong CE. Paraoxonase (Pon1) in mice: sequencing, chromosomal localization and developmental expression. Pharmacogenetics. 1997;7:137–144. doi: 10.1097/00008571-199704000-00007. [DOI] [PubMed] [Google Scholar]
- Li WF, Costa LG, Richter RJ, Hagen T, Shih DM, Tward A, Lusis AJ, Furlong CE. Catalytic efficiency determines the in vivo efficacy of PON1 for detoxifying organophosphates. Pharmacogenetics. 2000;10:767–779. doi: 10.1097/00008571-200012000-00002. [DOI] [PubMed] [Google Scholar]
- Mackness B, Beltran-Debon R, Aragones G, Joven J, Camps J, Mackness M. Human tissue distribution of paraoxonases 1 and 2 mRNA. IUBMB Life. 2010;62:480–482. doi: 10.1002/iub.347. [DOI] [PubMed] [Google Scholar]
- Marsillach J, Mackness B, Mackness M, Riu F, Beltran R, Joven J, Camps J. Immunohistochemical analysis of paraoxonase-1, 2 and 3 expression in normal mouse tissues. Free Rad Biol Med. 2008;45:146–157. doi: 10.1016/j.freeradbiomed.2008.03.023. [DOI] [PubMed] [Google Scholar]
- Marsillach J, Aragonès G, Mackness B, Mackness M, Rull A, Beltrán-Debón R, Pedro-Botet J, Alonso-Villaverde C, Joven J, Camps J. Decreased paraoxonase-1 activity is associated with alterations of high-density lipoprotein particles in chronic liver impairment. Lipids Health Dis. 2010;14:9, 46. doi: 10.1186/1476-511X-9-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki H, Scherer SW, Xi T, Nickle DC, Majer M, Huizenga JJ, Tsui LC, Prochazka M. Human PON2 gene at 7q21.3: cloning, multiple mRNA forms, and missense polymorphisms in the coding sequence. Gene. 1998;213:149–157. doi: 10.1016/s0378-1119(98)00193-0. [DOI] [PubMed] [Google Scholar]
- Ng CJ, Wadleigh DJ, Gangopadhyyay A, Hama S, Grijalva VR, Navab M, Fogelman AM, Reddy ST. Paraoxonase-2 is a ubiquitously expressed protein with antioxidant properties and is capable of preventing cell-mediated oxidative modification of low density lipoprotein. J Biol Chem. 2001;276:44444–44449. doi: 10.1074/jbc.M105660200. [DOI] [PubMed] [Google Scholar]
- Ng CJ, Bourquard N, Grijalva V, Hama S, Shih DM, Navab M, Fogelman AM, Lusis AJ, Young S, Reddy ST. Paraoxonase-2 deficiency aggravates atherosclerosis in mice despite lower apolipoprotein-B-containing lipoproteins. Antiatherogenic role for paraoxonase-2. J Biol Chem. 2006;281:29491–29500. doi: 10.1074/jbc.M605379200. [DOI] [PubMed] [Google Scholar]
- Precourt LP, Seidman E, Delvin E, Amre D, Deslandres C, Dominguez M, Sinnett D, Levy E. Comparative expression analysis reveals differences in the regulation of intestinal paraoxonase family members. Int J Biochem Mol Biol. 2009;41:1628–1637. doi: 10.1016/j.biocel.2009.02.013. [DOI] [PubMed] [Google Scholar]
- Primo-Parmo SL, Sorenson RC, Teiber J, La Du BN. The human serum paraoxonase/arylesterase gene (PON1) is one member of a multigene family. Genomics. 1996;33:498–507. doi: 10.1006/geno.1996.0225. [DOI] [PubMed] [Google Scholar]
- Ramsby ML, Makowski GS. Differential detergent fractionation of eukaryotic cells. Analysis by two-dimensional gel electrophoresis. Methods Mol Biol. 1999;112:53–66. doi: 10.1385/1-59259-584-7:53. [DOI] [PubMed] [Google Scholar]
- Reynolds A, Chad L, Mosley RL, Geldelman HE. Oxidative stress and the pathogenesis of neurodegenerative disorders. Int Rev Neurobiol. 2007;82:297–325. doi: 10.1016/S0074-7742(07)82016-2. [DOI] [PubMed] [Google Scholar]
- Rice ME, Russo-Menna I. Differential compartmentalization of brain ascorbate and glutathione between neurons and glia. Neuroscience. 1998;82:1213–1223. doi: 10.1016/s0306-4522(97)00347-3. [DOI] [PubMed] [Google Scholar]
- Rosenblatt M, Draganov D, Watson CE, Bisgaier CL, La Du BN, Aviram M. Mouse macrophage paraoxonase-2 activity is increased whereas cellular paraoxonase 3 activity is decreased under oxidative stress. Arterioscler Thromb Vasc Biol. 2003;23:468–474. doi: 10.1161/01.ATV.0000059385.95664.4D. [DOI] [PubMed] [Google Scholar]
- Rothem L, Hartman C, Dahan A, Lachter J, Eliakim R, Shamir R. Paraoxonases are associated with intestinal inflammatory diseases and intracellularly localized in the endoplasmic reticulum. Free Rad Biol Med. 2007;43:730–739. doi: 10.1016/j.freeradbiomed.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Sayre LM, Perry G, Smith MA. Oxidative stress and neurotoxicity. Chem Res Toxicol. 2008;21:172–188. doi: 10.1021/tx700210j. [DOI] [PubMed] [Google Scholar]
- Shadrina MI, Slominski PA, Limborska SA. Molecular mechanisms of pathogenesis of Parkinson's disease. Int Rev Cell Mol Biol. 2010;281:229–266. doi: 10.1016/S1937-6448(10)81006-8. [DOI] [PubMed] [Google Scholar]
- Shamir R, Hartman C, Karry R, Pavlotzky E, Eliakim R, Lachter J, Suissa A, Aviram M. Paraoxonases (PONs) 1, 2 and 3 are expressed in human and mouse gastrointestinal tract and in Caco-2 cell line: selective secretion of PON1 and PON2. Free Rad Biol Med. 2005;39:336–344. doi: 10.1016/j.freeradbiomed.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Shiner M, Fuhrman B, Aviram M. Paraoxonase 2 (PON2) expression is upregulated via a reduced-nicotinamide-adenine-dinucleotide-phosphate (NADPH)-dependent mechanism during monocytes differentiation into macrophages. Free Rad Biol Med. 2004;37:2052–2063. doi: 10.1016/j.freeradbiomed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Sola A, Rogido MR, Deulofeut R. Oxygen as a neonatal helath hazard: call for detente in clinical practice. Acta Pediatr. 2007;96:801–812. doi: 10.1111/j.1651-2227.2007.00287.x. [DOI] [PubMed] [Google Scholar]
- Stoltz DA, Ozer EA, Ng CJ, Yu JM, Reddy ST, Lusis AJ, Bourquard N, Parsek MR, Zabner J, Shih DM. Paraoxonase-2 deficiency enhances Psudomonas aeruginosa quorum sensing in murine tracheal epithelia. Am J Physiol Lung Cell Mol Physiol. 2007;292:L852–L860. doi: 10.1152/ajplung.00370.2006. [DOI] [PubMed] [Google Scholar]
- Stoltz DA, Ozer EA, Recker TJ, Estin M, Yang X, Shih DM, Lusis AJ, Zabner J. A common mutation in paraoxonase-2 results in impaired lactonase activity. J Biol Chem. 2009;284:35564–35571. doi: 10.1074/jbc.M109.051706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland WH, Manning PJ, de Jong SA, Allum AR, Jones SD, Williams SM. Hormone-replacement therapy increases serum paraoxonase arylesterase activity in diabetic postmenopausal women. Metabolism. 2001;50:319–324. doi: 10.1053/meta.2001.20201. [DOI] [PubMed] [Google Scholar]
- Teiber JF, Billecke SS, La Du BN, Draganov DI. Estrogen esters as substrates for human paraoxonases. Arch Biochem Biophys. 2007;461:24–29. doi: 10.1016/j.abb.2007.02.015. [DOI] [PubMed] [Google Scholar]
- Teiber JF, Horke S, Haines DC, Chowdhary PK, Xiao J, Kramer GL, Haley RW, Draganov DI. Dominant role of paraoxonases in inactivation of the Pseudomonas aeruginosa quorum-sensing signal N-(3-oxododecanoyl)-L-homoserine lactone. Infect Immun. 2008;76:2512–2519. doi: 10.1128/IAI.01606-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bos C, Roth J, Koch HG, Hartmann M, Sorg C. Phosphorylation of MRP14, an S100 protein expressed during monocytic differentiation, modulates Ca(2+)-dependent translocation from cytoplasm to membranes and cytoskeleton. J Immunol. 1996;156:1247–1254. [PubMed] [Google Scholar]