Abstract
Mitochondrial outer membrane proteins have been found to be ubiquitinated and degraded by the proteasome. This process shares at least one component of the ERAD pathway of ER membrane protein degradation, the AAA ATPase cdc48/p97/VCP, thought to extract integral membrane proteins from the lipid bilayer and chaperone them to the proteasome. Proteasomal degradation of the outer mitochondrial membrane protein Mcl-1 regulates apoptosis whereas Parkin-mediated ubiquitination and degradation of Mitofusins can inhibit mitochondrial fusion and promote mitophagy. The breadth of outer mitochondrial membrane ubiquitin/proteasome substrates and the physiological relevance of their turnover is only beginning to be understood.
1. Introduction: mitochondrial protein quality systems
Mitochondria are the primary cellular sites of energy production. In addition, various vital cellular events including apoptosis, Ca2+ buffering, and macromolecule synthesis are also regulated by mitochondria. To eliminate surplus or dysfunctional mitochondrial proteins, or whole damaged organelles that can negatively influence cellular homeostasis, regulated mitochondrial biogenesis and clearance is required. Thus, to counteract continuously occurring accumulation of defective components of mitochondria and functional deterioration of these organelles a number of mitochondrial protein quality control mechanisms operate in the cell.
It has been known for several decades that within the mitochondrial matrix, descendents of bacterial ATP-stimulated mitochondrial proteases, including PIM1/Lon [1,2] i-AAA and the m-AAA proteases (e.g. YME1L1 [3] and paraplegin [4]), mediate the turnover of inner mitochondrial membrane (IMM) proteins. These proteases are essential for various aspects of mitochondrial function, including mtDNA maintenance, mitochondrial fusion and formation of mitochondrial respiratory complexes [1–4]. Since, aging- or disease-linked impairments of these proteases have been suggested to contribute to mitochondrial failure and subsequent cell deterioration (for reviews see [5,6]), they are thought to serve as important mitochondrial protein quality control systems.
Studies published more recently indicate that dynamic remodeling of mitochondrial membranes, mainly through fusion and fission of these organelles, serves as another essential mitochondrial quality system (for reviews see [7–9]). It has been proposed that mixing of mitochondrial contents possibly through cycles of fusion and fission can serve as a mechanism diluting local mitochondrial defects (through fusion) [10,11], as well as eliminating damaged organelles from the mitochondrial network (through fission and inhibition of fusion), and thus priming them for autophagosomal degradation [12, **13,14]. Supporting a critical role of mitochondrial membrane dynamics, impairments of mitochondrial fusion and/or fission lead to mitochondrial and cellular dysfunction (for reviews see [7–9]).
In addition to the above described quality control systems, recent evidence also indicates that the ubiquitin (Ub)/proteasome system controls mitochondrial proteostasis, either by regulating mitochondrial protein turnover, or controlling mitochondrial protein activities, and can therefore be considered as a mitochondrial quality control mechanism. Although, as we will discuss later, some studies support a role for the Ub/proteasome system in regulation of intra-mitochondrial proteins (e.g. those localized in the IMM), the majority of evidence points to the importance of ubiquitination and proteasomal degradation in the control of the outer mitochondrial membrane (OMM) proteostasis.
2. Outer mitochondrial membrane associated degradation (OMMAD)
Given that the OMM serves as a barrier separating mitochondria from the cytosol and plays vital roles for mitochondrial function, including regulation of metabolism, apoptosis and mitochondrial membrane dynamics, the quality control of OMM-associated proteins is likely to be of great importance for cell function. Notably, it has been shown that the majority of known OMM-associated substrates of the Ub/proteasome system are proteins central for the regulation of either apoptosis or mitochondrial membrane dynamics.
Proteins regulating mitochondrial steps in apoptosis
The most extensively studied OMM associated substrate of the Ub/proteasome system is an anti-apoptotic protein in the Bcl-2 family, Mcl-1. Under normal growth conditions the half-life of Mcl1 has been estimated to be in the range of ~40–60 min [15,16]. Upon induction of apoptosis Mcl1 is rapidly degraded in a Ub and proteasome-dependent manner [15,17]. The apoptotic degradation of Mcl1, as well as its turnover in non-apoptotic cells, is regulated by the counteracting activities of the HECT-domain-containing Ub ligase ARF-BP1/Mule (Mcl-1 Ub ligase E3) [18], and the deubiquitinase Usp9x [19]. Expression levels of ARF-BP1/Mule and Usp9x appears to be critical for the maintenance of proper cellular balance of anti- and pro-apoptotic proteins, and contributes to cell sensitivity to apoptosis and is linked to tumor formation [18, *19].
In addition to Mcl1, turnover of other mitochondria-associated Bcl-2 family proteins, including Bax and Bcl-2 [20, *21,22,23], is also under Ub/proteasome control. Bax, a pro-apoptotic Bcl-2 family protein, is mainly localized in the cytosol in an apoptotically inactive form (6A7-epitope negative) and it moves to mitochondria upon pro-apoptotic trigger-induced change in its conformation (6A7-positive) [24–26]. Proteasome-dependent degradation of Bax occurs specifically on the mitochondria [23], suggesting that the apoptotic conformation of Bax might be recognized by the Ub conjugation machinery, and serve as a degradation signal preventing the accumulation of potentially dangerous apoptotically-active Bax in healthy cell mitochondria. Baxβ, a 24-kD splice variant of Bax that has shorter half-life, and is a more efficient pro-apoptotic protein than the more abundant 21-kD Baxα, is continuously degraded in a proteasome-dependent manner in non-apoptotic cells [22]. Furthermore, as shown by Benard et al., degradation of 6A7-positive Baxα is also proteasome-dependent and is regulated by IBRDC2, an IBR-type RING domain E3 Ub ligase [*21]. Based on these data, it has been proposed that a Ub-dependent apoptosis checkpoint safeguards mitochondria from Bax-dependent damage, and cells from unprompted apoptosis [*21,22]. Aside from Ub/proteasome-dependent regulation of Mcl-1 and Bax, several examples of other mitochondria-localized and mitochondria-interacting E3 Ub ligases, or Ub/proteasome-dependent regulatory events that influence mitochondrial steps in apoptosis have been described. BRCA1-associated RING domain 1 (BARD1) partially localizes to mitochondria, and it has been proposed that the apoptotic function of BARD1 is associated with stimulation of Bax oligomerization at mitochondria [27]. Furthermore, ARTS a pro-apoptotic mitochondrial protein is regulated through Ub/proteasome degradation [28]. Like Bax, high cellular levels of ARTS protein sensitize cells toward apoptosis, and in healthy cells ARTS levels are kept low through constant Ub-mediated degradation [28]. Moreover, in addition to Mcl-1 and Bax, ubiquitination of other Bcl-2 family proteins, including Bcl-2 [29], and a truncated form of Bid [30], regulates their expression and activity. Altogether, these data indicate a direct role for the Ub/proteasome system in the regulation of mitochondrial steps in apoptosis, and therefore place the Ub/proteasome system as a critical mitochondrial quality control system.
Mitochondrial membrane dynamics and mitochondrial autophagy
In Eukaryotic cells, turnover of Mitofusins (Mfn), integral GTPases of the OMM required for mitochondrial fusion (reviewed in [8,9]), is also mediated by the Ub/proteasome system [31–35]. Fzo1p, a yeast homologue of Mfn [36] is modified with proteasome-targeting Lys-48-linked Ub chains [31,32,34], and the proteasome inhibitor, MG132 [33] as well as aberrations in proteolytic activity (pre1 and pre2) of the 20S proteasomal core particle or the ATPase subunit (cim3 and cim5) of the 19S regulatory complex of the proteasome [34,35] suppressed the degradation of Fzo1p. In mammalian cells Mfn1 is relatively unstable, with a half-life estimated in a range of ~4–6hr [**37]. Mammalian cell culture studies reveal that, like Fzo1p in yeast, Mfn1 and Mfn2 are stabilized by proteasome inhibition [**13,**37]. In addition, consistent with Ub-dependence, accumulation of ubiquitinated forms of these proteins is detected in proteasome inhibitor-treated cells [**13,**37].
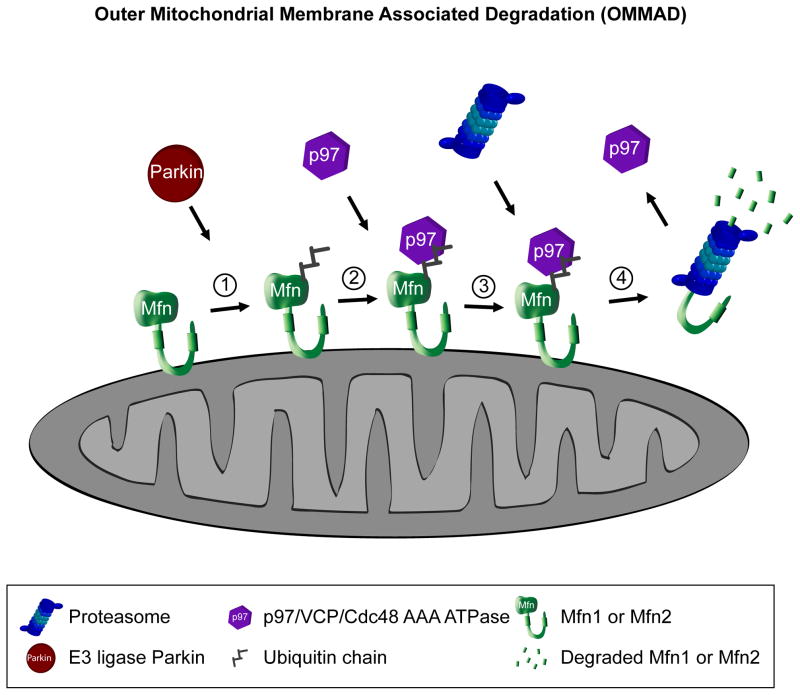
Under mitochondrial stress, Ub/proteasome-dependent degradation of mammalian Mfns [**13,*38] and the D. melanogaster homologue dMfn [39,40] is mediated by Parkin, an IBR-type RING domain E3 Ub ligase. Parkin translocates to functionally impaired mitochondria [41], and prior to initiating their removal by mitochondria-specific autophagy, Mfns are ubiquitinated and degraded in a proteasome-dependent manner [**13,**37,42]. Yet, since in cells deficient in Parkin expression the turnover of Mfns is also regulated by Ub/proteasome system [**13,*38], it is likely that, in addition to Parkin, another E3 Ub ligase mediates ubiquitination of these proteins. Interestingly, since inhibition of the proteasome suppresses Parkin-dependent autophagy of dysfunctional mitochondria [**13], it is likely that degradation of certain OMM-associated protein(s) initiates mitochondrial assembly of autophagy components (Fig. 1). Since Parkin can initiate mitochondria-specific autophagy in Mfn1/Mfn2−/− DKO cells [**13], OMM-associated substrates of the Ub/proteasome system other than Mfns likely serve this purpose. Consistent with this, it has been proposed that mitochondrial autophagy requires proteasomal degradation of the OMM associated voltage-dependent anion-selective channel 1 (VDAC1) [43]. Yet, since this process occurs in VDAC deficient cells [44], it is likely that degradation of other OMM-associated substrate(s) of the Ub/proteasome system is critical for initiation of mitochondria-specific autophagy.
Figure.
In addition to Mfns, Drp1 a large GTPase essential for mitochondrial fission (for reviews see [9,21]) is also targeted by Ub-conjugation. Based on co-immunoprecipitation experiments, it has been proposed that MARCH5 (also known as Mitol or MARCH-V) [45–47], a mitochondria-associated RING-finger E3 Ub ligase promotes ubiquitination of Drp1. Yet, in contrast to Mfns, MARCH5-mediated ubiquitination of Drp1 is not required for Drp1 degradation [45,46], but rather regulates Drp1 activity. Although RNAi downregulation, as well as overexpression of wild type or RING-inactive mutants of MARCH5, did not induce any detectable changes in the levels of Drp1 [45,46], expression of RING-inactive mutants of MARCH5 inhibited subcellular trafficking of Drp1 associated with abnormal elongation and interconnection of mitochondria [45]. It is important to examine the possibility that degradation of other mitochondrial protein(s), is regulated by MARCH5-dependent ubiquitination, and that this in turn affects cellular trafficking of Drp1. Notably, a large-scale proteomic study of Ub-modified proteins in yeast revealed that Dnm1p, a yeast homologue of Drp1 is also ubiquitinated [48], suggesting that Ub-dependent regulation of Drp1 might be evolutionally conserved. Clearly, further mechanistic studies are required to reveal the significance of the Ub/proteasome system in Drp1/Dnm1p and mitochondrial fission regulation.
Non-OMM Ub/proteasomal substrates
Mammalian sperm mitochondria that are preordained for degradation during normal development are tagged with Ub inside the oocyte cytoplasm and later subjected to proteolysis [49]. Notably, prohibitin, an IMM-associated protein, is ubiquitinated in sperm mitochondria [50]. Prohibitins regulate the stability of IMM proteins by protecting them from degradation by the IMM associated, Ub-independent protein degradation systems. Therefore, the Ub-dependent prohibitin turnover regulation might coordinate Ub-dependent and Ub-independent proteolytic quality control mechanisms in the mitochondria. Margineantu et al. [51] shows that a number of non-OMM mitochondrial proteins, including the OSCP subunit of mitochondrial F1F0-ATPase can be detected as Ub conjugates. Furthermore, since proteasome inhibition also induced an accumulation of certain IMM-localized proteins (e.g. vital for the respiratory function of mitochondria: COXI, III, IV, OSCP) [51] one might suggest that in addition to the OMM-associated proteins, the Ub/proteasome system also frequently regulates the turnover of IMM-associated proteins.
3. Molecular steps of OMMAD
CDC48/p97/VCP-mediated retrotranslocation of the OMM proteins
Since most known OMM-associated substrates of the Ub/proteasome system are integral membrane proteins, with one (e.g. Mcl1) or more (e.g. Mfns have two) transmembrane domains inserted in the OMM, these proteins likely need to be extracted from the OMM prior to proteasomal degradation. Consistently, in addition to ubiquitination machinery and the proteasome, participation of other factors is required for OMMAD.
Recent studies have revealed that the cytosolic AAA ATPase CDC48/p97/VCP, that is required for extracting ubiquitinated proteins from the ER and other cellular membranes [52,53], regulates OMMAD both in mammalian and yeast cells [**13,**37, **54]. In mammals p97 is required for turnover of Mcl-1 and Mfn1, two OMM-associated proteins with relatively short half-lives [**13,**37]. Both of these proteins are stabilized on mitochondria in cells depleted of p97 activity [**37], suggesting that p97 acts directly on the OMM. A number of proteomic studies revealed that p97 associates with mitochondria in unstressed mammalian cells [55–57], further suggesting a widespread role for p97 in mitochondrial proteostasis. p97 is also required for Parkin-dependent stress-induced degradation of Mfns and subsequent autophagy of dysfunctional mitochondria [**13]. Notably, in Parkin expressing cells p97 accumulates on dysfunctional mitochondria [**13], confirming a direct mitochondrial role for this protein. Consistent with this, in response to mitochondrial stress, Cdc48 a yeast homologue of p97 is also recruited to the OMM [54]. Mitochondrial translocation of Cdc48 depends on, and occurs subsequently to mitochondrial translocation of Vms1 (VCP/Cdc48-associated mitochondrial stress-responsive 1) [**54]. Yeast cells depleted of Vms1, and therefore deficient in mitochondrial translocation of Cdc48, show progressive mitochondrial failure that is associated with increased sensitivity to mitochondrial stress, as well as a significant delay in the degradation rate of Fzo1p [**54]. Thus, the role for Cdc48/p97 in the regulation of OMM protein turnover appears to be conserved. Interestingly, the Cdc48/Vms1 protein complex also contained Npl4 protein. Since Npl4 is required for Cdc48/p97 mediated retrotranslocation of ubiquitinated proteins from membranes, including those following the endoplasmic reticulum associated degradation (ERAD) pathway [58], it appears that OMMAD shares a number of critical components with other Ub/proteasome dependent protein degradation pathways.
Mitochondria-associated deubiquitinases
Proteasomal degradation of polyubiquitinated proteins, as well as activities of mono- or Lys-63-chain poly- ubiquitinated proteins can be affected by activities of deubiquitinating proteases (DUBs) (for review see [59]). Until now, two mitochondria-associated DUBs have been identified [*19,60]. As discussed above Usp9x mediates deubiquitination, and regulates stability of Mcl1 [*19]. Usp9x partially localizes to the mitochondria where it binds Mcl1 and removes the proteasome targeting Lys 48-linked polyubiquitin chains [*19]. Notably, increased Usp9x expression correlates with increased Mcl1 protein in human follicular lymphomas and diffuse large B-cell lymphomas [*19]. Unlike Usp9x, Usp30, another mitochondria associated DUB [60] is specifically associated with the OMM. The mitochondrial substrates of Usp30 are currently unknown. Yet, since RNAi downregulation of Usp30 induces abnormal elongation and interconnection of mitochondria [60], it is likely that Usp30 mediates deubiquitination of certain proteins implicated in the regulation of mitochondrial membrane dynamics.
4. Future perspectives
As in the case of any developing research field, the studies summarized here are raising a number of questions regarding the scope and mechanism of Ub/proteasome system in mitochondrial homeostasis. For example, some published reports indicate a role for the Ub/proteasome in regulation of mitochondrial proteins localized in the inner mitochondrial compartments (e.g. IMM). However, the OMM is the barrier separating mitochondria from the cytosol, and the cytosol localized components of Ub/proteasome system. Thus, the mechanism of ubiquitination and subsequent movement of Ub-conjugated proteins from the IMM to the cytosol are currently unknown. Undoubtedly, further studies addressing this exciting research topic are needed. Furthermore, it is currently unknown whether misfolded proteins of the OMM, are processed by OMMAD in the same manner as misfolded ER proteins are targeted for proteasomal degradation by ERAD pathway. For example, the mechanisms by which mitochondrial proteins are recognized by Ub/proteasome system, as well as factors mediating this recognition and other steps of OMMAD need to be identified. Revealing additional molecular components as well as more detailed understanding of protein targets, mechanisms and roles of known mitochondrial components of the OMMAD pathway will likely help to address these issues.
Finally, the overall physiological significance of the OMMAD system should also be established. How the Ub/proteasome facilitates mitophagy, for example, remains enigmatic and important. Considering that mitochondrial dysfunctions are hallmarks of aging and various aging-linked diseases, one may assume that Up/proteasome system might be also important for elimination of misfolded mitochondrial proteins expected to accumulate in aging cells. The degrees to which aging-associated decline in Ub/proteasome activities contribute to mitochondrial dysfunctions need to be investigated.
Answering these, and many other emerging questions will likely shed more light on the significance of Ub/proteasome system in the regulation of mitochondrial function. Given the important role of mitochondria for cellular homeostasis, these should also improve general knowledge of mitochondrial biology, and reveal how mitochondrial dysfunctions contribute to disease.
Table 1.
Proteins implicated in the OMMAD pathway
| Protein | Biological function | Role in OMMAD/Targets | References |
|---|---|---|---|
| Parkin | IBR-domain E3 Ub ligase | ubiquitination of Mfn1, Mfn2 and VDAC1; induces mitochondria-specific auophagy | [13,41–43] |
| PINK1 | mitochondrial kinase | recruits Parkin to the mitochondria | [61,62] |
| MARCH5/MARCH-V/Mitol | mitochondrial RING-domain E3 Ub ligase | Binds and ubiquitinate Drp1 (mitochondrial fission factor) and Mitofusins (mitochondrial fusion factors); mitochondrial dynamics regulation | [45–47] |
| MULAN/MAPL | mitochondrial RING-domain E3 Ub ligase | mitochondrial dynamics regulation; reported also as E3 SUMO ligase targeting Drp1 | [63,64] |
| IBRDC2 | IBR-domain E3 Ub ligase | apoptosis regulation; targets Bax, a proapoptotic protein in Bcl2 family | [21] |
| Mule/ARF-BP1 | HECT domain E3 Ub ligase | ubiquitinates and regulates turnover of Mcl1, an antiapoptotic protein in Bcl2 family | [18] |
| USP9x | deubiquitinase | deubiquitinates and regulates turnover of Mcl1 | [19] |
| USP30 | mitochondrial deubiquitinase | regulates mitochondrial dynamics; substrates unknown | [60] |
| p97/CDC48 | AAA-ATPase; protein dislocase | regulates OMM protein turnover and mitochondria-specific autophagy in yeast and mammals | [13,37,54] |
| Vms1p | adaptor protein | cofactor of CDC48; regulates OMM protein and mitochondrial degradation in yeast | [54] |
| Npl4 | adaptor protein | cofactor of CDC48; regulates OMM protein and mitochondrial degradation in yeast | [54] |
| Proteasome | protein degradation complex | translocates to the mitochondria upon activation of Parkin-dependent, mitochondrial stress-induced mitophagy | [38] |
Highlights.
E3 ligases that can localize to mitochondria such as Parkin ubiquitinate outer mitochondrial membrane proteins.
Membrane spanning proteins localized to the outer mitochondrial membrane can be degraded by the ubiquitin proteosomal system.
p97, an AAA ATPase involved in membrane protein retrotranslocation mediates outer mitochondrial membrane protein degradation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Papers of particular interest, published within the annual period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Bender T, Lewrenz I, Franken S, Baitzel C, Voos W. Mitochondrial enzymes are protected from stress-induced aggregation by mitochondrial chaperones and the Pim1/LON protease. Mol Biol Cell. 2011 doi: 10.1091/mbc.E10-08-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Dyck L, Langer T. ATP-dependent proteases controlling mitochondrial function in the yeast Saccharomyces cerevisiae. Cell Mol Life Sci. 1999;56:825–842. doi: 10.1007/s000180050029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Griparic L, Kanazawa T, van der Bliek AM. Regulation of the mitochondrial dynamin-like protein Opa1 by proteolytic cleavage. J Cell Biol. 2007;178:757–764. doi: 10.1083/jcb.200704112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ishihara N, Fujita Y, Oka T, Mihara K. Regulation of mitochondrial morphology through proteolytic cleavage of OPA1. Embo J. 2006;25:2966–2977. doi: 10.1038/sj.emboj.7601184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Voos W. Mitochondrial protein homeostasis: the cooperative roles of chaperones and proteases. Res Microbiol. 2009;160:718–725. doi: 10.1016/j.resmic.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Ngo JK, Davies KJ. Importance of the lon protease in mitochondrial maintenance and the significance of declining lon in aging. Ann N Y Acad Sci. 2007;1119:78–87. doi: 10.1196/annals.1404.015. [DOI] [PubMed] [Google Scholar]
- 7.Lackner LL, Nunnari JM. The molecular mechanism and cellular functions of mitochondrial division. Biochim Biophys Acta. 2009;1792:1138–1144. doi: 10.1016/j.bbadis.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benard G, Karbowski M. Mitochondrial fusion and division: Regulation and role in cell viability. Semin Cell Dev Biol. 2009;20:365–374. doi: 10.1016/j.semcdb.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan DC. Mitochondria: dynamic organelles in disease, aging, and development. Cell. 2006;125:1241–1252. doi: 10.1016/j.cell.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 10.Chen H, Chomyn A, Chan DC. Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J Biol Chem. 2005;280:26185–26192. doi: 10.1074/jbc.M503062200. [DOI] [PubMed] [Google Scholar]
- 11.Nakada K, Sato A, Hayashi J. Mitochondrial functional complementation in mitochondrial DNA-based diseases. Int J Biochem Cell Biol. 2009;41:1907–1913. doi: 10.1016/j.biocel.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 12.Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. Embo J. 2008;27:433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13**.Tanaka A, Cleland MM, Xu S, Narendra DP, Suen DF, Karbowski M, Youle RJ. Proteasome and p97 mediate mitophagy and degradation of mitofusins induced by Parkin. J Cell Biol. 2010;191:1367–1380. doi: 10.1083/jcb.201007013. This study provides evidence that proteasome and p97 medite Parkin-dependent degradation of mitofusins and are also required for Parkin-dependent mitophagy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Romanello V, Guadagnin E, Gomes L, Roder I, Sandri C, Petersen Y, Milan G, Masiero E, Del Piccolo P, Foretz M, et al. Mitochondrial fission and remodelling contributes to muscle atrophy. Embo J. 2010;29:1774–1785. doi: 10.1038/emboj.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nijhawan D, Fang M, Traer E, Zhong Q, Gao W, Du F, Wang X. Elimination of Mcl-1 is required for the initiation of apoptosis following ultraviolet irradiation. Genes Dev. 2003;17:1475–1486. doi: 10.1101/gad.1093903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang T, Kozopas KM, Craig RW. The intracellular distribution and pattern of expression of Mcl-1 overlap with, but are not identical to, those of Bcl-2. J Cell Biol. 1995;128:1173–1184. doi: 10.1083/jcb.128.6.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cuconati A, Mukherjee C, Perez D, White E. DNA damage response and MCL-1 destruction initiate apoptosis in adenovirus-infected cells. Genes Dev. 2003;17:2922–2932. doi: 10.1101/gad.1156903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhong Q, Gao W, Du F, Wang X. Mule/ARF-BP1, a BH3-only E3 ubiquitin ligase, catalyzes the polyubiquitination of Mcl-1 and regulates apoptosis. Cell. 2005;121:1085–1095. doi: 10.1016/j.cell.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 19*.Schwickart M, Huang X, Lill JR, Liu J, Ferrando R, French DM, Maecker H, O’Rourke K, Bazan F, Eastham-Anderson J, et al. Deubiquitinase USP9X stabilizes MCL1 and promotes tumour cell survival. Nature. 2010;463:103–107. doi: 10.1038/nature08646. This manuscript shows that stability of the OMM-associated Mcl1 and cell sensitivity to apoptosis is regulated by deubiquitinase USP9x. [DOI] [PubMed] [Google Scholar]
- 20.Azad N, Vallyathan V, Wang L, Tantishaiyakul V, Stehlik C, Leonard SS, Rojanasakul Y. S-nitrosylation of Bcl-2 inhibits its ubiquitin-proteasomal degradation. A novel antiapoptotic mechanism that suppresses apoptosis. J Biol Chem. 2006;281:34124–34134. doi: 10.1074/jbc.M602551200. [DOI] [PubMed] [Google Scholar]
- 21*.Benard G, Neutzner A, Peng G, Wang C, Livak F, Youle RJ, Karbowski M. IBRDC2, an IBR-type E3 ubiquitin ligase, is a regulatory factor for Bax and apoptosis activation. Embo J. 2010;29:1458–1471. doi: 10.1038/emboj.2010.39. This manuscript shows that Bax in apoptotically active conformation is recognised and tagged for proteasomal degradation by E3 Ub ligase IBRDC2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fu NY, Sukumaran SK, Kerk SY, Yu VC. Baxbeta: a constitutively active human Bax isoform that is under tight regulatory control by the proteasomal degradation mechanism. Mol Cell. 2009;33:15–29. doi: 10.1016/j.molcel.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 23.Liu FT, Agrawal SG, Gribben JG, Ye H, Du MQ, Newland AC, Jia L. Bortezomib blocks Bax degradation in malignant B cells during treatment with TRAIL. Blood. 2008;111:2797–2805. doi: 10.1182/blood-2007-08-110445. [DOI] [PubMed] [Google Scholar]
- 24.Hsu YT, Youle RJ. Bax in murine thymus is a soluble monomeric protein that displays differential detergent-induced conformations. J Biol Chem. 1998;273:10777–10783. doi: 10.1074/jbc.273.17.10777. [DOI] [PubMed] [Google Scholar]
- 25.Hsu YT, Wolter KG, Youle RJ. Cytosol-to-membrane redistribution of Bax and Bcl-X(L) during apoptosis. Proc Natl Acad Sci U S A. 1997;94:3668–3672. doi: 10.1073/pnas.94.8.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolter KG, Hsu YT, Smith CL, Nechushtan A, Xi XG, Youle RJ. Movement of Bax from the cytosol to mitochondria during apoptosis. J Cell Biol. 1997;139:1281–1292. doi: 10.1083/jcb.139.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tembe V, Henderson BR. BARD1 translocation to mitochondria correlates with Bax oligomerization, loss of mitochondrial membrane potential, and apoptosis. J Biol Chem. 2007;282:20513–20522. doi: 10.1074/jbc.M702627200. [DOI] [PubMed] [Google Scholar]
- 28.Lotan R, Rotem A, Gonen H, Finberg JP, Kemeny S, Steller H, Ciechanover A, Larisch S. Regulation of the proapoptotic ARTS protein by ubiquitin-mediated degradation. J Biol Chem. 2005;280:25802–25810. doi: 10.1074/jbc.M501955200. [DOI] [PubMed] [Google Scholar]
- 29.Breitschopf K, Haendeler J, Malchow P, Zeiher AM, Dimmeler S. Posttranslational modification of Bcl-2 facilitates its proteasome-dependent degradation: molecular characterization of the involved signaling pathway. Mol Cell Biol. 2000;20:1886–1896. doi: 10.1128/mcb.20.5.1886-1896.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tait SW, de Vries E, Maas C, Keller AM, D’Santos CS, Borst J. Apoptosis induction by Bid requires unconventional ubiquitination and degradation of its N-terminal fragment. J Cell Biol. 2007;179:1453–1466. doi: 10.1083/jcb.200707063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neutzner A, Benard G, Youle RJ, Karbowski M. Role of the ubiquitin conjugation system in the maintenance of mitochondrial homeostasis. Ann N Y Acad Sci. 2008;1147:242–253. doi: 10.1196/annals.1427.012. [DOI] [PubMed] [Google Scholar]
- 32.Neutzner A, Youle RJ, Karbowski M. Outer mitochondrial membrane protein degradation by the proteasome. Novartis Found Symp. 2007;287:4–14. discussion 14–20. [PubMed] [Google Scholar]
- 33.Neutzner A, Youle RJ. Instability of the mitofusin Fzo1 regulates mitochondrial morphology during the mating response of the yeast Saccharomyces cerevisiae. J Biol Chem. 2005;280:18598–18603. doi: 10.1074/jbc.M500807200. [DOI] [PubMed] [Google Scholar]
- 34.Cohen MM, Leboucher GP, Livnat-Levanon N, Glickman MH, Weissman AM. Ubiquitin-proteasome-dependent degradation of a mitofusin, a critical regulator of mitochondrial fusion. Mol Biol Cell. 2008;19:2457–2464. doi: 10.1091/mbc.E08-02-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Escobar-Henriques M, Westermann B, Langer T. Regulation of mitochondrial fusion by the F-box protein Mdm30 involves proteasome-independent turnover of Fzo1. J Cell Biol. 2006;173:645–650. doi: 10.1083/jcb.200512079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hermann GJ, Thatcher JW, Mills JP, Hales KG, Fuller MT, Nunnari J, Shaw JM. Mitochondrial fusion in yeast requires the transmembrane GTPase Fzo1p. J Cell Biol. 1998;143:359–373. doi: 10.1083/jcb.143.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37**.Xu S, Peng G, Wang Y, Fang S, Karbowski M. The AAA-ATPase p97 is essential for outer mitochondrial membrane protein turnover. Mol Biol Cell. 2011;22:291–300. doi: 10.1091/mbc.E10-09-0748. This work demonstrates that steady state turnover and retrotranslocation of the proteasome-dependent OMM-associated substrates is regulated by the component of the ERAD pathway, an AAA-ATPase p97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38*.Chan NC, Salazar AM, Pham AH, Sweredoski MJ, Kolawa NJ, Graham RL, Hess S, Chan DC. Broad activation of the ubiquitin-proteasome system by Parkin is critical for mitophagy. Hum Mol Genet. 2011 doi: 10.1093/hmg/ddr048. This paper, along with the reference (13), provides evidence that proteasomal activity and degradation of mitochondrial substrates might be required for Parkin-dependent mitophagy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poole AC, Thomas RE, Yu S, Vincow ES, Pallanck L. The mitochondrial fusion-promoting factor mitofusin is a substrate of the PINK1/parkin pathway. PLoS ONE. 2010;5:e10054. doi: 10.1371/journal.pone.0010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ziviani E, Tao RN, Whitworth AJ. Drosophila parkin requires PINK1 for mitochondrial translocation and ubiquitinates mitofusin. Proc Natl Acad Sci U S A. 2010;107:5018–5023. doi: 10.1073/pnas.0913485107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol. 2008;183:795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gegg ME, Cooper JM, Chau KY, Rojo M, Schapira AH, Taanman JW. Mitofusin 1 and mitofusin 2 are ubiquitinated in a PINK1/parkin-dependent manner upon induction of mitophagy. Hum Mol Genet. 2010 doi: 10.1093/hmg/ddq419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Geisler S, Holmstrom KM, Skujat D, Fiesel FC, Rothfuss OC, Kahle PJ, Springer W. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat Cell Biol. 2010;12:119–131. doi: 10.1038/ncb2012. [DOI] [PubMed] [Google Scholar]
- 44.Narendra D, Kane LA, Hauser DN, Fearnley IM, Youle RJ. p62/SQSTM1 is required for Parkin-induced mitochondrial clustering but not mitophagy; VDAC1 is dispensable for both. Autophagy. 2010;6:1090–1106. doi: 10.4161/auto.6.8.13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karbowski M, Neutzner A, Youle RJ. The mitochondrial E3 ubiquitin ligase MARCH5 is required for Drp1 dependent mitochondrial division. J Cell Biol. 2007;178:71–84. doi: 10.1083/jcb.200611064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakamura N, Kimura Y, Tokuda M, Honda S, Hirose S. MARCH-V is a novel mitofusin 2- and Drp1-binding protein able to change mitochondrial morphology. EMBO Rep. 2006;7:1019–1022. doi: 10.1038/sj.embor.7400790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yonashiro R, Ishido S, Kyo S, Fukuda T, Goto E, Matsuki Y, Ohmura-Hoshino M, Sada K, Hotta H, Yamamura H, et al. A novel mitochondrial ubiquitin ligase plays a critical role in mitochondrial dynamics. Embo J. 2006;25:3618–3626. doi: 10.1038/sj.emboj.7601249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peng J, Schwartz D, Elias JE, Thoreen CC, Cheng D, Marsischky G, Roelofs J, Finley D, Gygi SP. A proteomics approach to understanding protein ubiquitination. Nat Biotechnol. 2003;21:921–926. doi: 10.1038/nbt849. [DOI] [PubMed] [Google Scholar]
- 49.Sutovsky P, Moreno RD, Ramalho-Santos J, Dominko T, Simerly C, Schatten G. Ubiquitin tag for sperm mitochondria. Nature. 1999;402:371–372. doi: 10.1038/46466. [DOI] [PubMed] [Google Scholar]
- 50.Thompson WE, Ramalho-Santos J, Sutovsky P. Ubiquitination of prohibitin in mammalian sperm mitochondria: possible roles in the regulation of mitochondrial inheritance and sperm quality control. Biol Reprod. 2003;69:254–260. doi: 10.1095/biolreprod.102.010975. [DOI] [PubMed] [Google Scholar]
- 51.Margineantu DH, Emerson CB, Diaz D, Hockenbery DM. Hsp90 inhibition decreases mitochondrial protein turnover. PLoS ONE. 2007;2:e1066. doi: 10.1371/journal.pone.0001066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ye Y, Meyer HH, Rapoport TA. The AAA ATPase Cdc48/p97 and its partners transport proteins from the ER into the cytosol. Nature. 2001;414:652–656. doi: 10.1038/414652a. [DOI] [PubMed] [Google Scholar]
- 53.Ye Y, Shibata Y, Yun C, Ron D, Rapoport TA. A membrane protein complex mediates retro-translocation from the ER lumen into the cytosol. Nature. 2004;429:841–847. doi: 10.1038/nature02656. [DOI] [PubMed] [Google Scholar]
- 54**.Heo JM, Livnat-Levanon N, Taylor EB, Jones KT, Dephoure N, Ring J, Xie J, Brodsky JL, Madeo F, Gygi SP, et al. A stress-responsive system for mitochondrial protein degradation. Mol Cell. 2010;40:465–480. doi: 10.1016/j.molcel.2010.10.021. This study demonstrates that, like in mammalian cells, stress-induced degradation of mitochondria in yeast relies on the components of the ubiquitin/proteasome system. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reifschneider NH, Goto S, Nakamoto H, Takahashi R, Sugawa M, Dencher NA, Krause F. Defining the mitochondrial proteomes from five rat organs in a physiologically significant context using 2D blue-native/SDS-PAGE. J Proteome Res. 2006;5:1117–1132. doi: 10.1021/pr0504440. [DOI] [PubMed] [Google Scholar]
- 56.Taylor SW, Fahy E, Zhang B, Glenn GM, Warnock DE, Wiley S, Murphy AN, Gaucher SP, Capaldi RA, Gibson BW, et al. Characterization of the human heart mitochondrial proteome. Nat Biotechnol. 2003;21:281–286. doi: 10.1038/nbt793. [DOI] [PubMed] [Google Scholar]
- 57.Zhang J, Li X, Mueller M, Wang Y, Zong C, Deng N, Vondriska TM, Liem DA, Yang JI, Korge P, et al. Systematic characterization of the murine mitochondrial proteome using functionally validated cardiac mitochondria. Proteomics. 2008;8:1564–1575. doi: 10.1002/pmic.200700851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ye Y, Meyer HH, Rapoport TA. Function of the p97-Ufd1-Npl4 complex in retrotranslocation from the ER to the cytosol: dual recognition of nonubiquitinated polypeptide segments and polyubiquitin chains. J Cell Biol. 2003;162:71–84. doi: 10.1083/jcb.200302169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wilkinson KD. DUBs at a glance. J Cell Sci. 2009;122:2325–2329. doi: 10.1242/jcs.041046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nakamura N, Hirose S. Regulation of mitochondrial morphology by USP30, a deubiquitinating enzyme present in the mitochondrial outer membrane. Mol Biol Cell. 2008;19:1903–1911. doi: 10.1091/mbc.E07-11-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Matsuda N, Sato S, Shiba K, Okatsu K, Saisho K, Gautier CA, Sou YS, Saiki S, Kawajiri S, Sato F, et al. PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J Cell Biol. 2010;189:211–221. doi: 10.1083/jcb.200910140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Narendra DP, Jin SM, Tanaka A, Suen DF, Gautier CA, Shen J, Cookson MR, Youle RJ. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 2010;8:e1000298. doi: 10.1371/journal.pbio.1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Braschi E, Zunino R, McBride HM. MAPL is a new mitochondrial SUMO E3 ligase that regulates mitochondrial fission. EMBO Rep. 2009;10:748–754. doi: 10.1038/embor.2009.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li W, Bengtson MH, Ulbrich A, Matsuda A, Reddy VA, Orth A, Chanda SK, Batalov S, Joazeiro CA. Genome-wide and functional annotation of human E3 ubiquitin ligases identifies MULAN, a mitochondrial E3 that regulates the organelle’s dynamics and signaling. PLoS ONE. 2008;3:e1487. doi: 10.1371/journal.pone.0001487. [DOI] [PMC free article] [PubMed] [Google Scholar]