Abstract
Aims. Patients with diabetic nephropathy are reported to have a high prevalence of left ventricular structural and functional abnormalities. This study was designed to assess the determinants of left ventricular mass index (LVMI) and left ventricular ejection fraction (LVEF) in diabetic patients at various stages of chronic kidney disease (CKD). Methods. This cross-sectional study enrolled 285 diabetic patients with CKD stages 3 to 5 from our outpatient department of internal medicine. Clinical and echocardiographic parameters were compared and analyzed. Results. We found a significant stepwise increase in LVMI (P < 0.001), LVH (P < 0.001), and LVEF <55% (P = 0.013) and a stepwise decrease in LVEF (P = 0.038) corresponding to advance in CKD stages. Conclusions. Our findings suggest that increases in LVMI and decreases in LVEF coincide with advances in CKD stages in patients with diabetes.
1. Introduction
Diabetic nephropathy is one of the major complications of diabetes mellitus (DM) and one of the major reasons for renal replacement therapy [1]. The leading cause of morbidity and mortality in patients with diabetic nephropathy is cardiovascular disease [2]. Cardiovascular risk in this population can partially be attributed to an increase of traditional risk factors among people with DM but may also be related to the risk factors for coexisting chronic kidney disease (CKD), such as proteinuria, fluid retention, anemia, oxidative stress, and chronic inflammatory state [2–4].
There are a number of hemodynamic and metabolic disturbances that affect the structure and function of heart in patients with diabetic nephropathy. The major factors that contribute to further heart failure in diabetic patients include cardiac microangiopathy, neuropathy of the cardiac autonomous nervous system, disturbed metabolism, and fatty degeneration of the myocardium [5]. These patients are reported to have a high prevalence of decreased left ventricular systolic function and increased left ventricular mass index (LVMI) resulting from pressure and volume overload [6, 7]. Echocardiographic measures of left ventricular function and structure have been reported to predict adverse cardiovascular outcomes in a variety of populations [8, 9]. Therefore, it is important to detect and treat abnormal geometry and dysfunction of heart early. However, little is known about the relation between the severity of left ventricular geometry and dysfunction and renal function impairment in diabetic patients. The aim of this study was to compare the LVMI and left ventricular ejection fraction (LVEF) among diabetic patients with various degrees of renal insufficiency and identify the independent risk factors associated with increased LVMI and decreased LVEF in this population.
2. Subjects and Methods
2.1. Study Patients and Design
The study was conducted in a regional hospital in southern Taiwan. In total, 285 diabetic patients with CKD stages 3 to 5 were enrolled consecutively from our outpatient department of internal medicine from January 2007 to May 2010. Patients with evidence of kidney damage lasting for more than 3 months were classified into CKD stage 3, 4, or 5 groups based on estimated glomerular filtration rate (eGFR) level (mL/min/1.73 m2) of 30 to 59, 15 to 29, and <15, respectively, as recommended in the National Kidney Foundation-Kidney Disease Outcomes Quality Initiative (K/DOQI) guidelines [10]. Patients with significant mitral valve disease and inadequate image visualization were excluded. The protocol for this study was approved by our institutional review board, and all enrolled patients gave written informed consent.
2.2. Evaluation of Cardiac Structure and Function
The echocardiographic examination was performed using VIVID 7 (General Electric Medical Systems, Horten, Norway), with the participant respiring quietly in the left decubitus position. The echocardiographers were blind to patient data. Two-dimensional and two-dimensional guided M-mode images were recorded from the standardized view points. The echocardiographic measurements included aortic root diameter, left atrial diameter (LAD), left ventricular internal diameter in diastole (LVIDd), and left ventricular internal diameter in systole (LVIDs), LVEF, peak early transmitral filling wave velocity (E), and peak late transmitral filling wave velocity (A). Left ventricular mass was calculated using the Devereux-modified method [11]. LVMI was calculated by dividing left ventricular mass by body surface area. Left ventricular hypertrophy (LVH) was defined when LVMI exceeded 134 g/m2 and 110 g/m2 for men and women, respectively [12]. Systolic function was assessed by measuring ejection fraction of left ventricle. Systolic dysfunction was defined as LVEF <55%. Diastolic function was estimated by measuring the E/A ratio; a value of <1.0 was considered diastolic dysfunction.
2.3. Collection of Demographic, Medical, and Laboratory Data
Demographic and medical data, including age, gender, smoking history (ever versus never), and comorbid conditions, were garnered from medical records or interviews with patients. Study subjects were defined as having DM if their fasting blood glucose levels were greater than 126 mg/dL or they were taking hypoglycemic agents to control blood glucose levels. Similarly, participants were defined as having hypertension if their systolic blood pressures were ≥140 mmHg or diastolic blood pressure ≥90 mmHg or they were taking antihypertensive drugs. Coronary artery disease was defined if they had a history of typical angina with positive stress test, angiographically documented coronary artery disease, and old myocardial infarction or they had undergone coronary artery bypass surgery or angioplasty. Cerebrovascular disease was defined if they had a history of cerebrovascular incidents such as cerebral bleeding and infarction. Congestive heart failure was defined based on the Framingham criteria. Body mass index was calculated as the ratio of weight in kilograms divided by square of height in meters. Blood and urine samples were obtained within 1 month of enrollment. Laboratory data were measured from fasting blood samples using an autoanalyzer (Roche Diagnostics GmbH, D-68298 Mannheim COBAS Integra 400). Serum creatinine was measured by the compensated Jaffé (kinetic alkaline picrate) method in a Roche/Integra 400 Analyzer (Roche Diagnostics, Mannheim, Germany) using a calibrator traceable to isotope-dilution mass spectrometry [13]. The value of eGFR was calculated using the 4-variable equation in the Modification of Diet in Renal Disease (MDRD) study [14]. The HbA1c was measured by Prismus CLC 385 automated analyzer. Proteinuria was examined by dipsticks (Hema-Combistix, Bayer Diagnostics). A test result of 1+ or more was defined as positive. In addition, information regarding patient medications including aspirin, angiotensin converting enzyme inhibitors (ACEIs), angiotensin II receptor blockers (ARBs), non-ACEI/ARB antihypertensive drugs, and HMG-CoA reductase inhibitors (statins) during the study period was obtained from medical records.
2.4. Statistical Analysis
Data are expressed as percentages or mean ± standard deviation or median (25th–75th percentile) for triglyceride. Multiple comparisons among the study groups were performed by one-way analysis of variance (ANOVA) followed by post hoc test adjusted with a LSD correction. The relationship between two continuous variables was assessed by a bivariate correlation method (Pearson's correlation). Linear regression analysis was used to identify the factors associated with LVMI and LVEF. Significant variables in univariate analysis were selected for multivariate analysis. P value less than 0.05 was considered significant. All statistical operations were performed using SPSS 12.0 for Windows (SPSS Inc. Chicago, USA).
3. Results
As can be seen in Table 1, a summary of clinical characteristics organized by CKD stage, we studied 285 nondialyzed CKD patients (174 men and 111 women, mean age 66.4 ± 11.6 years). The prevalence of LVH and LVEF < 55% was 62.5% and 10.5%, respectively. Stepwise increases in the prevalence of a history of hypertension, cerebrovascular disease, and congestive heart failure, pulse pressure, uric acid, phosphorous, calcium-phosphorous product, proteinuria, and percentage of non-ACEI/ARB antihypertensive drug use and stepwise decreases in the diastolic blood pressure, albumin, hemoglobin, eGFR, and calcium corresponded to advancement in CKD from stage 3 to 5. In addition, there was a significant trend for a stepwise increase in the LAD, LVIDd, LVIDs, LVMI, and the prevalence of LVH and LVEF < 55% and a stepwise decrease in the LVEF corresponding to advancement in CKD from stage 3 to 5. Figure 1 shows the significant trend for a stepwise increase in LVMI (a) and the prevalence of LVH (b) corresponding to the advancement in CKD from stage 3 to 5. Figure 2 shows the significant trend for a stepwise decrease in LVEF (a) and a stepwise increase in the prevalence of LVEF < 55% (b) corresponding to the advancement in CKD from stage 3 to 5.
Table 1.
Clinical characteristics of patients among different stages of CKD.
| Characteristics | Stage 3 (n = 99) |
Stage 4 (n = 99) |
Stage 5 (n = 87) |
P for trend | All patients (n = 285) |
|---|---|---|---|---|---|
| Age (year) | 66.3 ± 12.4 | 68.4 ± 10.7 | 64.1 ± 11.5† | 0.039 | 66.4 ± 11.6 |
| Male gender (%) | 75.8 | 58.6* | 47.1* | <0.001 | 61.1 |
| Smoking history (%) | 32.3 | 36.4 | 28.7 | 0.540 | 32.6 |
| Hypertension (%) | 79.8 | 81.8 | 97.7∗† | 0.001 | 86.0 |
| Coronary artery disease (%) | 13.1 | 13.1 | 18.4 | 0.514 | 14.7 |
| Cerebrovascular disease (%) | 10.1 | 21.2* | 27.6* | 0.009 | 19.3 |
| Congestive heart failure (%) | 10.1 | 15.2 | 28.7∗† | 0.003 | 17.5 |
| Systolic blood pressure (mmHg) | 144.5 ± 21.2 | 141.2 ± 20.0 | 148.3 ± 23.7† | 0.089 | 144.6 ± 21.8 |
| Diastolic blood pressure (mmHg) | 82.4 ± 12.3 | 77.4 ± 11.9* | 76.7 ± 14.1* | 0.005 | 78.9 ± 13.0 |
| Pulse pressure (mmHg) | 62.1 ± 16.7 | 63.8 ± 11.7 | 71.7 ± 19.9∗† | 0.001 | 65.7 ± 18.2 |
| Body mass index (kg/m2) | 26.2 ± 4.0 | 26.3 ± 3.5 | 25.0 ± 3.8∗† | 0.043 | 25.9 ± 3.8 |
|
| |||||
| Laboratory parameters | |||||
| Albumin (g/L) | 41.3 ± 3.5 | 39.9 ± 3.9* | 37.3 ± 4.5∗† | <0.001 | 39.6 ± 4.3 |
| Fasting glucose (mmol/L) | 8.2 ± 3.2 | 8.0 ± 3.8 | 8.3 ± 4.7 | 0.896 | 8.1 ± 3.9 |
| HbA1c (%) | 7.5 ± 1.4 | 8.1 ± 2.1* | 7.4 ± 1.8† | 0.032 | 7.7 ± 1.8 |
| Triglyceride (mmol/L) | 1.8 (1.1–2.4) | 1.8 (1.4–2.6) | 1.8 (1.2–2.7) | 0.173 | 1.8 (1.2–2.6) |
| Total cholesterol (mmol/L) | 5.0 ± 1.1 | 5.1 ± 1.3 | 5.2 ± 1.4 | 0.544 | 5.1 ± 1.3 |
| Hemoglobin (g/L) | 128.8 ± 18.7 | 115.4 ± 19.2* | 93.1 ± 13.4∗† | <0.001 | 113.2 ± 22.7 |
| Baseline eGFR (mL/min/1.73 m2) | 40.5 ± 6.6 | 23.1 ± 4.5* | 10.3 ± 3.0∗† | <0.001 | 25.2 ± 13.3 |
| Calcium (mmol/L) | 2.4 ± 0.2 | 2.4 ± 0.2 | 2.3 ± 0.2∗† | <0.001 | 2.4 ± 0.2 |
| Phosphate (mmol/L) | 1.1 ± 0.2 | 1.3 ± 0.2* | 1.6 ± 0.4∗† | <0.001 | 1.3 ± 0.3 |
| Calcium-phosphorous product (mmol2/L2) | 2.8 ± 0.5 | 3.1 ± 0.6* | 3.6 ± 0.8∗† | <0.001 | 3.1 ± 0.7 |
| Uric acid (μmol/L) | 456.7 ± 113.3 | 505.4 ± 138.6* | 530.8 ± 143.3* | 0.001 | 496.5 ± 135.1 |
| Proteinuria (%) | 47.5 | 75.5* | 98.9∗† | <0.001 | 72.9 |
|
| |||||
| Medications | |||||
| Aspirin use (%) | 30.2 | 32.3 | 34.5 | 0.826 | 32.2 |
| ACEI and/or ARB use (%) | 80.2 | 83.3 | 63.1∗† | 0.003 | 76.1 |
| Non-ACEI/ARB antihypertensive drug use (%) | 67.7 | 80.8* | 94.3∗† | <0.001 | 80.4 |
| Statin use (%) | 36.5 | 29.2 | 31.0 | 0.532 | 32.2 |
|
| |||||
| Echocardiographic data | |||||
| Aortic root diameter (cm) | 3.3 ± 0.4 | 3.3 ± 0.4 | 3.2 ± 0.4 | 0.262 | 3.3 ± 0.4 |
| LAD (cm) | 3.7 ± 0.6 | 3.9 ± 0.6 | 4.1 ± 0.6∗† | < 0.001 | 3.9 ± 0.6 |
| LVIDd (cm) | 4.8 ± 0.7 | 4.9 ± 0.8 | 5.1 ± 0.7∗† | 0.005 | 4.9 ± 0.8 |
| LVIDs (cm) | 2.9 ± 0.7 | 3.1 ± 0.8 | 3.3 ± 0.8∗† | 0.002 | 3.1 ± 0.8 |
| LVMI (g/m2) | 129.5 ± 43.5 | 139.1 ± 52.4 | 167.1 ± 45.9∗† | <0.001 | 144.3 ± 49.8 |
| LVH (%) | 44.4 | 61.6* | 83.9∗† | <0.001 | 62.5 |
| LVEF (%) | 69.0 ± 11.1 | 67.0 ± 11.7 | 64.5 ± 13.2* | 0.038 | 66.9 ± 12.1 |
| LVEF < 55% (%) | 4.0 | 11.1* | 17.2* | 0.013 | 10.5 |
| E/A < 1 (%) | 78.9 | 84.9 | 75.0 | 0.250 | 79.8 |
CKD: chronic kidney disease; eGFR: estimated glomerular filtration rate; ACEI: angiotensin converting enzyme inhibitor; ARB: angiotensin II receptor blocker; LAD: left atrial diameter; LVIDd: left ventricular internal diameter in diastole; LVIDs: left ventricular internal diameter in systole; LVMI: left ventricular mass index; LVH: left ventricular hypertrophy; LVEF: left ventricular ejection fraction; E: peak early transmitral filling wave velocity; A: peak late transmitral filling wave velocity.
*P < 0.05 compared to stage 3; † P < 0.05 compared to stage 4.
Figure 1.
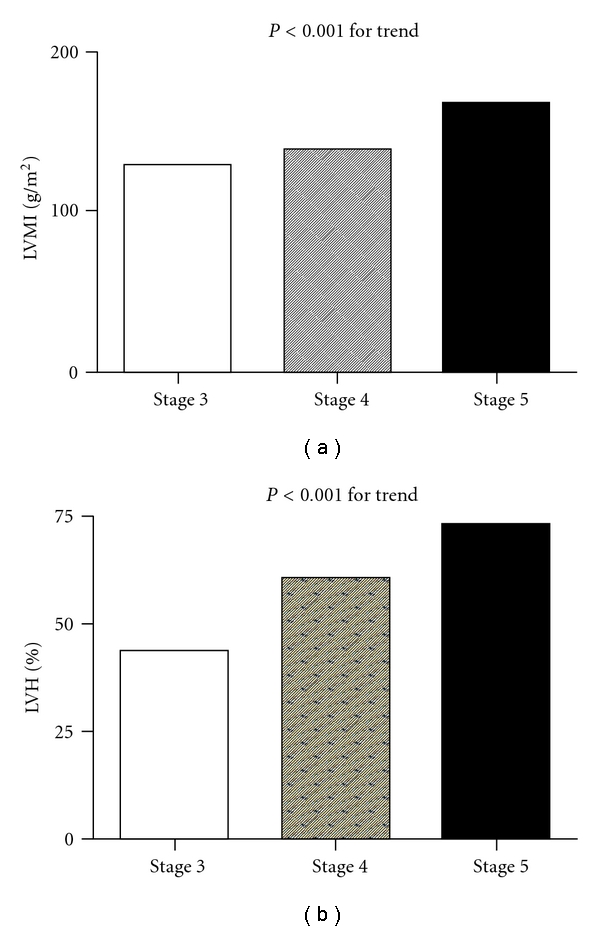
There was a significant trend for a stepwise increase in left ventricular mass index (LVMI) (P < 0.001 for trend) (a) and the prevalence of left ventricular hypertrophy (LVH) (44.4%, 61.6%, and 83.9%, resp.; P < 0.001 for trend) (b) corresponding to the advancement in chronic kidney disease from stage 3 to 5.
Figure 2.
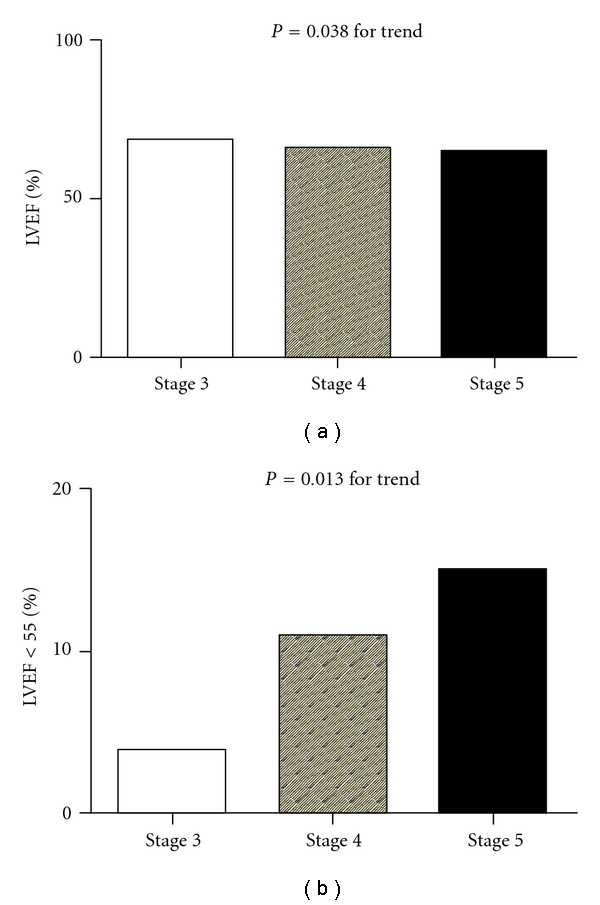
There was a significant trend for a stepwise decrease in left ventricular ejection fraction (LVEF) (P = 0.038 for trend) (a) and stepwise increase in the prevalence of LVEF < 55% (4.0%, 11.1%, and 17.2%, resp.; P < 0.013 for trend) (b) corresponding to the advancement in chronic kidney disease from stage 3 to 5.
As seen in Table 2 which summarizes our findings on the possible determinants of LVMI in our study patients, univariate analysis showed a significant positive correlation between LVMI and being male, a history of smoking, coronary artery disease, and congestive heart failure, advanced CKD stages, systolic blood pressure, pulse pressure, phosphorous, proteinuria, aspirin use, and non-ACEI/ARB antihypertensive drug use and negative correlation between LVMI and albumin, hemoglobin, calcium, and ACEI and/or ARB use. Further forward multivariate analysis revealed a significant correlation between increases in LVMI and being male, a history of congestive heart failure, advanced CKD stages, high systolic blood pressure, and low serum albumin level.
Table 2.
Determinants of left ventricular mass index (LVMI) in study patients.
| Characteristics | Univariate | Multivariate (forward) | ||
|---|---|---|---|---|
| Standardized coefficient β | P | Standardized coefficient β | P | |
| Age (year) | 0.012 | 0.842 | — | — |
| Male versus female | 0.117 | 0.048 | 0.211 | <0.001 |
| Smoking(ever versus never) | 0.126 | 0.033 | — | — |
| Coronary artery disease | 0.147 | 0.013 | — | — |
| Cerebrovascular disease | 0.024 | 0.681 | — | — |
| Congestive heart failure | 0.262 | <0.001 | 0.196 | 0.001 |
| CKD stage | 0.301 | <0.001 | 0.262 | <0.001 |
| Systolic blood pressure (mmHg) | 0.225 | <0.001 | 0.203 | <0.001 |
| Diastolic blood pressure (mmHg) | 0.095 | 0.118 | — | — |
| Pulse pressure (mmHg) | 0.202 | 0.001 | — | — |
| Body mass index (kg/m2) | 0.049 | 0.413 | — | — |
|
| ||||
| Laboratory parameters | ||||
| Albumin (g/L) | −0.321 | <0.001 | −0.132 | 0.032 |
| Fasting glucose (mmol/L) | 0.027 | 0.653 | — | — |
| HbA1c (%) | −0.052 | 0.389 | — | — |
| Triglyceride (Log mmol/L) | −0.034 | 0.570 | — | — |
| Cholesterol (mmol/L) | 0.047 | 0.436 | — | — |
| Hemoglobin (g/L) | −0.212 | <0.001 | — | — |
| Calcium (mmol/L) | −0.185 | 0.002 | — | — |
| Phosphate (mmol/L) | 0.182 | 0.002 | — | — |
| Calcium-phosphorous product (mmol2/L2) | 0.114 | 0.060 | — | — |
| Uric acid (μmol/L) | 0.117 | 0.052 | — | — |
| Proteinuria | 0.216 | <0.001 | — | — |
|
| ||||
| Medications | — | — | ||
| Aspirin use (%) | 0.190 | 0.001 | — | — |
| ACEI and/or ARB use (%) | −0.167 | 0.005 | — | — |
| Non-ACEI and/or ARB antihypertensive drug use (%) | 0.188 | 0.001 | — | — |
| Statin use (%) | −0.041 | 0.502 | — | — |
Values expressed as standardized coefficient β. Abbreviations are the same as Table 1.
Table 3 summarizes the results of our analysis of possible determinants of LVEF in our study patients. Univariate analysis showed a positive correlation between LVEF and albumin, calcium, and ACEI and/or ARB use and a negative correlation with being male, a history of coronary artery disease and congestive heart failure, advanced CKD stages, uric acid, phosphorous, aspirin use, and non-ACEI/ARB antihypertensive drug use. Further forward multivariate analysis revealed a correlation between decreased LVEF and being male, a history of coronary artery disease, advanced CKD stages, low serum albumin level, and ACEI and/or ARB use.
Table 3.
Determinants of left ventricular ejection fraction (LVEF) in study patients.
| Characteristics | Univariate | Multivariate (forward) | ||
|---|---|---|---|---|
| Standardized coefficient β | P | Standardized coefficient β | P | |
| Age (year) | 0.062 | 0.297 | — | — |
| Male versus female | −0.223 | <0.001 | −0.227 | <0.001 |
| Smoking(ever versus never) | −0.090 | 0.130 | — | — |
| Coronary artery disease | −0.169 | 0.004 | −0.153 | 0.008 |
| Cerebrovascular disease | −0.071 | 0.235 | — | — |
| Congestive heart failure | −0.155 | 0.009 | — | — |
| CKD stage | −0.151 | 0.011 | −0.173 | 0.007 |
| Systolic blood pressure (mmHg) | −0.038 | 0.528 | — | — |
| Diastolic blood pressure (mmHg) | −0.092 | 0.130 | — | — |
| Pulse pressure (mmHg) | 0.020 | 0.747 | — | — |
| Body mass index (kg/m2) | 0.010 | 0.871 | — | — |
|
| ||||
| Laboratory parameters | ||||
| Albumin (g/L) | 0.258 | < 0.001 | 0.188 | 0.003 |
| Fasting glucose (mmol/L) | −0.097 | 0.107 | — | — |
| HbA1c (%) | −0.040 | 0.509 | ||
| Triglyceride (Log mmol/L) | −0.039 | 0.518 | — | — |
| Cholesterol (mmol/L) | −0.069 | 0.247 | — | — |
| Hemoglobin (g/L) | 0.103 | 0.083 | — | — |
| Calcium (mmol/L) | 0.122 | 0.044 | ||
| Phosphate (mmol/L) | −0.168 | 0.005 | ||
| Calcium-phosphorous product (mmol2/L2) | −0.103 | 0.088 | — | — |
| Uric acid (μmol/L) | −0.146 | 0.015 | — | — |
| Proteinuria | −0.096 | 0.106 | — | — |
|
| ||||
| Medications | ||||
| Aspirin use (%) | −0.132 | 0.028 | — | — |
| ACEI and/or ARB use (%) | 0.203 | 0.001 | 0.143 | 0.014 |
| Non-ACEI and/or ARB antihypertensive drug use (%) | 0.048 | 0.422 | — | — |
| Statin use (%) | 0.004 | 0.951 | — | — |
Values expressed as standardized coefficient β. Abbreviations are the same as Table 1.
4. Discussion
In the present study, we evaluated the determinants of LVMI and LVEF in diabetic patients with various stages of CKD. We found a significant trend for a stepwise increase in LVMI and the prevalence of LVH and LVEF < 55% and a stepwise decrease in LVEF corresponding to advancement in CKD stage.
Patients with diabetic nephropathy have a high prevalence of LVH and left ventricular systolic dysfunction, two disorders that contribute majorly to increased risk of cardiovascular death [2–4]. Structural and functional abnormalities of the heart are common in patients with diabetic nephropathy because of pressure and volume overload [15, 16]. The prevalence of LVH ranges from 17% to 42% in patients with hypertension, 22% to 47% in patients with CKD, and 68.5% of dialysis patients, and LVH occurs in only 3.2% of the general population [8, 17, 18]. However, the prevalence of LVH in our study patients was relatively high (62.5%), which might be explained by the fact that all of the patients included in our study had diabetic nephropathy. The prevalence of left ventricular systolic dysfunction in patients with chronic renal insufficiency is approximately 7.6%–22% [8, 19]. In our patients, the prevalence of LVEF < 55% was 10.5%, which is compatible with previous findings.
DM, hypertension, and dyslipidemia are traditional cardiovascular risk factors. In addition to these traditional risk factors, patients with CKD may have other risk factors for increase cardiovascular risk such as inflammation, oxidative stress, anemia, metabolic disorders, calcium-phosphorous disorders, hypervolemia, and structural and functional abnormalities of heart, which may help to explain the high cardiovascular morbidity and mortality in such patients [20–25]. LVH, a common finding in patients with CKD, has been reported to advance with decreases in glomerular filtration rate [3]. Hillege et al. found that there was also a significant correlation between the deterioration of congestive heart failure and the progression of renal failure [26]. Our study found that, with the decrease of renal function, there was a significant trend for a stepwise increase in LVMI and the prevalence of LVH and LVEF < 55% and a stepwise decrease in LVEF in patients with diabetic nephropathy, which is consistent with the previous findings.
Low serum albumin level has been regarded as indicator of malnutrition. Malnutrition may worsen the outcome of CKD by aggravating existing inflammation and heart failure [27]. Hypoalbuminemia has been correlated with left ventricular structure and function [25, 28, 29]. Kursat et al. [25], evaluating the relationship between the degree of malnutrition and echocardiographic parameters in 72 hemodialysis patients, found that the malnutrition index, calculated using Subjective Global Assessment, had a positive correlation with left ventricular mass and index. They cited inadequate volume control as an explanation for their findings. Volume overload may substantially decrease energy and protein intake, suggesting a possible relation between volume overload and malnutrition. In addition, volume overload may increase the diastolic wall stress and in turn cause the development of LVH [25]. Trovato et al. [29], also investigating the correlation between heart failure and nutritional status in hemodialysis patients, reported an association between low serum albumin level and decreased LVEF. Our results consistently demonstrate independent association between low serum albumin levels and increased LVMI and decreased LVEF in patients with diabetic nephropathy.
One limitation of this study was that it had a cross-sectional design, and thus the predictors of cardiovascular events could not be evaluated. Further prospective studies are needed to confirm our findings.
In conclusion, our results found a significant trend for a stepwise increase in LVMI and the prevalence of LVH and LVEF < 55% and a stepwise decrease in LVEF corresponding to advancement in CKD stage in diabetic patients.
Disclosure
The authors have no financial interest in the information contained in the paper.
Acknowledgments
The research presented in this paper is supported by the Grant from Kaohsiung Municipal Hsiao-Kang Hospital (Kmhk-100), Kaohsiung Medical University, Kaohsiung, and the statistical work by the Department of Research Education and Training at Kaohsiung Municipal Hsiao-Kang Hospital.
References
- 1.Ritz E. Diabetic nephropathy. Saudi Journal of Kidney Diseases and Transplantation. 2006;17(4):481–490. [PubMed] [Google Scholar]
- 2.Ritz E. Heart and kidney: fatal twins? American Journal of Medicine. 2006;119(5) supplement 1:S31–S39. doi: 10.1016/j.amjmed.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 3.Levin A, Singer J, Thompson CR, Ross H, Lewis M. Prevalent left ventricular hypertrophy in the predialysis population: identifying opportunities for intervention. American Journal of Kidney Diseases. 1996;27(3):347–354. doi: 10.1016/s0272-6386(96)90357-1. [DOI] [PubMed] [Google Scholar]
- 4.Levin A, Thompson CR, Ethie J, et al. Left ventricular mass index increase in early renal disease: impact of decline in hemoglobin. American Journal of Kidney Diseases. 1999;34(1):125–134. doi: 10.1016/s0272-6386(99)70118-6. [DOI] [PubMed] [Google Scholar]
- 5.Veves A, Akbari CM, Primavera J, et al. Endothelial dysfunction and the expression of endothelial nitric oxide synthetase in diabetic neuropathy, vascular disease, and foot ulceration. Diabetes. 1998;47(3):457–463. doi: 10.2337/diabetes.47.3.457. [DOI] [PubMed] [Google Scholar]
- 6.Glowinska I, Grochowski J, Malyszko J. Cardiovascular complications in patients with diabetic nephropathy receiving pharmacological versus renal replacement therapy. Polskie Archiwum Medycyny Wewnętrznej. 2008;118(7-8):404–412. [PubMed] [Google Scholar]
- 7.Mataradzija A, Resic H, Rasic S, Kukavica N, Masnic F. Risk factors for development of cardiovascular complications in patients with chronic renal disease and diabetic nephropathy. Bosnian Journal of Basic Medical Sciences. 2010;10(supplement 1):S44–S50. doi: 10.17305/bjbms.2010.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eckardt KU, Scherhag A, Macdougall IC, et al. Left ventricular geometry predicts cardiovascular outcomes associated with anemia correction in CKD. Journal of the American Society of Nephrology. 2009;20(12):2651–2660. doi: 10.1681/ASN.2009060631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silaruks S, Sirivongs D, Chunlertrith D. Left ventricular hypertrophy and clinical outcome in CAPD patients. Peritoneal Dialysis International. 2000;20(4):461–466. [PubMed] [Google Scholar]
- 10.Levey AS, Coresh J, Bolton K, et al. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. American Journal of Kidney Diseases. 2002;39(2) supplement 1:S1–S266. [PubMed] [Google Scholar]
- 11.Devereux RB, Alonso DR, Lutas EM. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. American Journal of Cardiology. 1986;57(6):450–458. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 12.Devereux RB. Detection of left ventricular hypertrophy by M-mode echocardiography. Anatomic validation, standardization, and comparison to other methods. Hypertension. 1987;9(2):II19–II26. doi: 10.1161/01.hyp.9.2_pt_2.ii19. [DOI] [PubMed] [Google Scholar]
- 13.Vickery S, Stevens PE, Dalton RN, van Lente F, Lamb EJ. Does the ID-MS traceable MDRD equation work and is it suitable for use with compensated Jaffe and enzymatic creatinine assays? Nephrology Dialysis Transplantation. 2006;21(9):2439–2445. doi: 10.1093/ndt/gfl249. [DOI] [PubMed] [Google Scholar]
- 14.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of diet in renal disease study group. Annals of Internal Medicine. 1999;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 15.Paoletti E, Bellino D, Cassottana P, Rolla D, Cannella G. Left ventricular hypertrophy in nondiabetic predialysis CKD. American Journal of Kidney Diseases. 2005;46(2):320–327. doi: 10.1053/j.ajkd.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 16.Stewart GA, Gansevoort RT, Mark PB, et al. Electrocardiographic abnormalities and uremic cardiomyopathy. Kidney International. 2005;67(1):217–226. doi: 10.1111/j.1523-1755.2005.00072.x. [DOI] [PubMed] [Google Scholar]
- 17.Zoccali C. How important is echocardiography for risk stratification in follow-up of patients with chronic kidney disease? Nature Clinical Practice Nephrology. 2007;3(4):178–179. doi: 10.1038/ncpneph0441. [DOI] [PubMed] [Google Scholar]
- 18.Harnett JD, Parfrey PS, Griffiths SM, Gault MH, Barre P, Guttmann RD. Left ventricular hypertrophy in end-stage renal disease. Nephron. 1988;48(2):107–115. doi: 10.1159/000184887. [DOI] [PubMed] [Google Scholar]
- 19.Barrionuevo JDA, Vargas-Machuca MFG, Pulido FG, Sacaluga LG, Govantes MAG, Martinez-Martinez A. Transthoracic echocardiographic findings in patients with chronic kidney disease awaiting kidney transplantation. Transplantation Proceedings. 2010;42(8):3123–3125. doi: 10.1016/j.transproceed.2010.05.123. [DOI] [PubMed] [Google Scholar]
- 20.Olivero JJ, Nguyen PT. Chronic kidney disease: a marker of cardiovascular disease. Methodist DeBakey Cardiovascular Journal. 2009;5(2):24–29. doi: 10.14797/mdcj-5-2-24. [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez-Iturbe B, Correa-Rotter R. Cardiovascular risk factors and prevention of cardiovascular disease in patients with chronic renal disease. Expert Opinion on Pharmacotherapy. 2010;11(16):2687–2698. doi: 10.1517/14656561003796570. [DOI] [PubMed] [Google Scholar]
- 22.Zoccali C, Benedetto FA, Tripepi G, et al. Left ventricular systolic function monitoring in asymptomatic dialysis patients: a prospective cohort study. Journal of the American Society of Nephrology. 2006;17(5):1460–1465. doi: 10.1681/ASN.2005111240. [DOI] [PubMed] [Google Scholar]
- 23.Silberberg JS, Barre PE, Prichard SS, Sniderman SD. Impact of left ventricular hypertrophy on survival in end-stage renal disease. Kidney International. 1989;36(2):286–290. doi: 10.1038/ki.1989.192. [DOI] [PubMed] [Google Scholar]
- 24.Cintron G, Johnson G, Francis G, Cobb F, Cohn JN. Prognostic significance of serial changes in left ventricular ejection fraction in patients with congestive heart failure. The V-HeFT VA cooperative studies group. Circulation. 1993;87(supplement 6):VI17–VI23. [PubMed] [Google Scholar]
- 25.Kursat S, Tekce H, Ekmekci C, Colak HB, Alici T. Relationship between the degree of malnutrition and echocardiographic parameters in hemodialysis patients. Nephron Clinical Practice. 2007;106(3):c136–c142. doi: 10.1159/000103001. [DOI] [PubMed] [Google Scholar]
- 26.Hillege HL, Girbes AR, de Kam PJ, et al. Renal function, neurohormonal activation, and survival in patients with chronic heart failure. Circulation. 2000;102(2):203–210. doi: 10.1161/01.cir.102.2.203. [DOI] [PubMed] [Google Scholar]
- 27.Levin NW, Handelman GJ, Coresh J, Port FK, Kaysen GA. Reverse epidemiology: a confusing, confounding, and inaccurate term. Seminars in Dialysis. 2007;20(6):586–592. doi: 10.1111/j.1525-139X.2007.00366.x. [DOI] [PubMed] [Google Scholar]
- 28.Io H, Matsumoto M, Okumura K, et al. Predictive factors associated with left ventricular hypertrophy at baseline and in the follow-up period in non-diabetic hemodialysis patients. Seminars in Dialysis. 2011;24(3):349–354. doi: 10.1111/j.1525-139X.2010.00759.x. [DOI] [PubMed] [Google Scholar]
- 29.Trovato GM, Iannetti E, Catalano D, Squatrito R, Vitale M, Zuccala G. [Heart failure and nutritional status in hemodialysis] Recenti Progressi in Medicina. 2001;92(11):655–659. [PubMed] [Google Scholar]


