Abstract
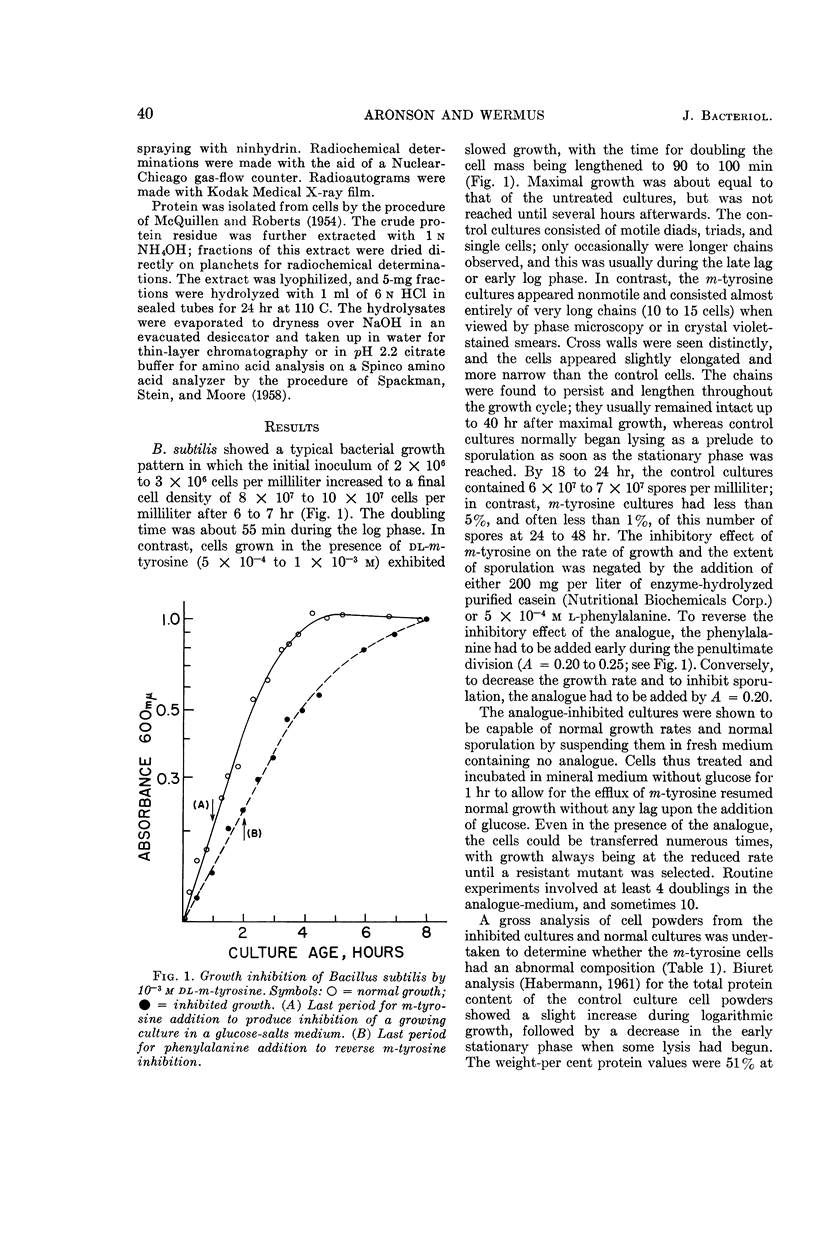
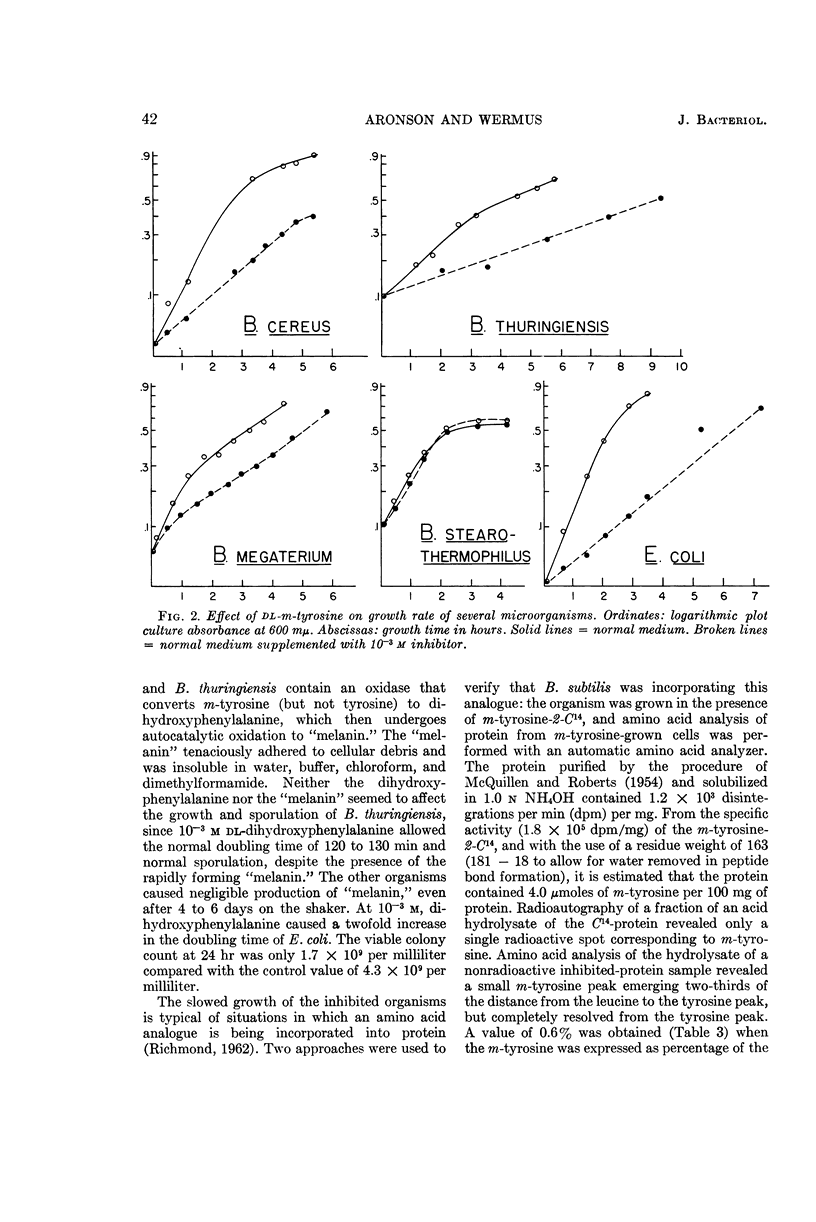
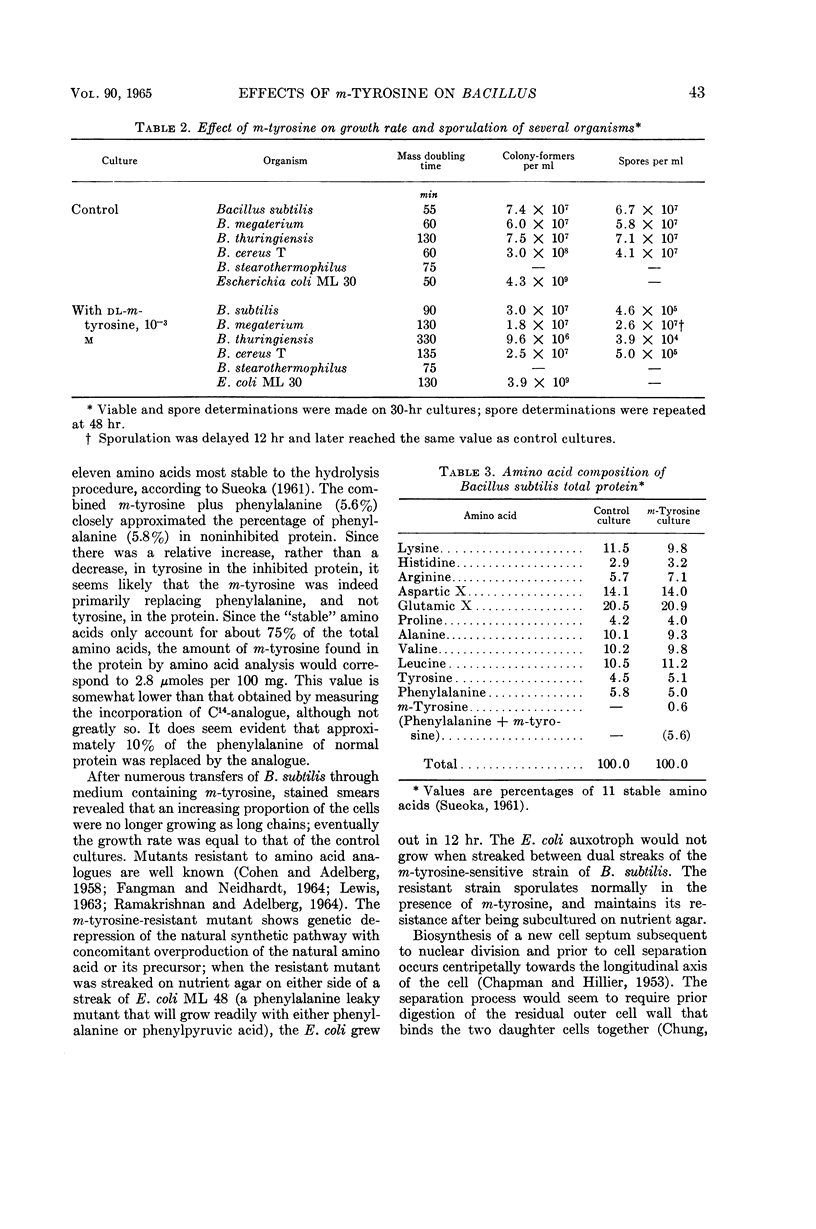
Aronson, John N. (Arizona State University, Tempe), and Gerald R. Wermus. Effects of m-tyrosine on growth and sporulation of Bacillus species. J. Bacteriol. 90:38–46. 1965.—The aromatic amino acid analogue, dl-β-(3-hydroxyphenyl)alanine (m-tyrosine), reduced sporulation of a strain of Bacillus subtilis to less than 5% of that of control cultures in a glucose-salts minimal medium. The mass-doubling time increased twofold, but maximal growth equivalent to that of control cultures was eventually attained. A decreased growth rate could be maintained in the presence of the analogue for more than 10 doublings, despite incorporation of m-tyrosine-2-C14 in place of some of the protein phenylalanine. The organism proliferated to chain lengths of 10 to 15 cells. These cells persisted for many hours after maximal growth had been reached, in contrast to normal cultures which had begun to autolyze and sporulate. The response to m-tyrosine of strains of B. cereus, B. thuringiensis, and B. megaterium was like that of B. subtilis. In addition, B. thuringiensis and B. cereus converted m-tyrosine to dihydroxyphenylalanine, which was further oxidized to a melaninlike substance. Growth of a strain of B. stearothermophilus was not slowed by m-tyrosine, but a strain of Escherichia coli grew at a reduced rate.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARBU E., LEE K. Y., WAHL R. Contenu en bases puriques et pyrimidiques des acides désoxyribonucléiques des bactéries. Ann Inst Pasteur (Paris) 1956 Aug;91(2):212–224. [PubMed] [Google Scholar]
- BUETOW D. E., LEVEDAHL B. H. Decline in the cellular content of RNA, protein and dry weight during the logarithmic growth of Euglena gracilis. J Gen Microbiol. 1962 Sep;28:579–584. doi: 10.1099/00221287-28-4-579. [DOI] [PubMed] [Google Scholar]
- CHAPMAN G. B., HILLIER J. Electron microscopy of ultra-thin sections of bacteria I. Cellular division in Bacillus cereus. J Bacteriol. 1953 Sep;66(3):362–373. doi: 10.1128/jb.66.3.362-373.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHUNG K. L., HAWIRKO R. Z., ISAAC P. K. CELL WALL REPLICATION. I. CELL WALL GROWTH OF BACILLUS CEREUS AND BACILLUS MEGATERIUM. Can J Microbiol. 1964 Feb;10:43–48. doi: 10.1139/m64-007. [DOI] [PubMed] [Google Scholar]
- COHEN G. N., ADELBERG E. A. Kinetics of incorporation of p-fluorophenylalanine by a mutant of Escherichia coli resistant to this analogue. J Bacteriol. 1958 Sep;76(3):328–330. doi: 10.1128/jb.76.3.328-330.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN M., MONOD J. Purification et proprietes de la beta-galactosidase (lactase) d'Escherichia coli. Biochim Biophys Acta. 1951 May;7(1):153–174. doi: 10.1016/0006-3002(51)90013-3. [DOI] [PubMed] [Google Scholar]
- DURHAM N. N. INHIBITION OF MICROBIAL GROWTH AND SEPARATION BY D-SERINE, VANCOMYCIN, AND MITOMYCIN C. J Bacteriol. 1963 Sep;86:380–386. doi: 10.1128/jb.86.3.380-386.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FANGMAN W. L., NEIDHARDT F. C. DEMONSTRATION OF AN ALTERED AMINOACYL RIBONUCLEIC ACID SYNTHETASE IN A MUTANT OF ESCHERICHIA COLI. J Biol Chem. 1964 Jun;239:1839–1843. [PubMed] [Google Scholar]
- FOSTER J. W., PERRY J. J. Intracellular events occurring during endotrophic sporulation in Bacillus mycoides. J Bacteriol. 1954 Mar;67(3):295–302. doi: 10.1128/jb.67.3.295-302.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HABERMANN V. The effect of 6-azauracil on microorganisms inhibited by chloramphenicol. Biochim Biophys Acta. 1961 Apr 29;49:204–211. doi: 10.1016/0006-3002(61)90884-8. [DOI] [PubMed] [Google Scholar]
- KERSTEN W., KERSTEN H. [On the mode of action of actinomycins. II. Formation of surplus desoxyribonucleic acid in Bacillus subtilis]. Hoppe Seylers Z Physiol Chem. 1962 May 4;327:234–242. doi: 10.1515/bchm2.1962.327.1.234. [DOI] [PubMed] [Google Scholar]
- KRONISH D. P., MOHAN R. R., SCHWARTZ B. S. DISTRIBUTION OF RADIOACTIVITY IN AUTOLYZED CELL WALL OF BACILLUS CEREUS DURING SPHEROPLAST FORMATION. J Bacteriol. 1964 Mar;87:581–587. doi: 10.1128/jb.87.3.581-587.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOMINSKI I., CAMERON J., WYLLIE G. Chaining and unchaining Streptococcus faecalis; a hypothesis of the mechanism of bacterial cell separation. Nature. 1958 May 24;181(4621):1477–1477. doi: 10.1038/1811477a0. [DOI] [PubMed] [Google Scholar]
- MILLET J., AUBERT J. P. [The metabolism of glutamic acid in the course of sporulation in Bacillus megaterium]. Ann Inst Pasteur (Paris) 1960 Feb;98:282–290. [PubMed] [Google Scholar]
- McQUILLEN K., ROBERTS R. B. The utilization of acetate for synthesis in Escherichia coli. J Biol Chem. 1954 Mar;207(1):81–95. [PubMed] [Google Scholar]
- NAKATA H. M. ORGANIC NUTRIENTS REQUIRED FOR GROWTH AND SPORULATION OF BACILLUS CEREUS. J Bacteriol. 1964 Nov;88:1522–1524. doi: 10.1128/jb.88.5.1522-1524.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORGEL L. E. ADAPTATION TO WIDE-SPREAD DISTURBANCE OF ENZYME FUNCTION. J Mol Biol. 1964 Jul;9:208–212. doi: 10.1016/s0022-2836(64)80101-7. [DOI] [PubMed] [Google Scholar]
- PREVIC E. P., BINKLEY S. B. SLOW EXPONENTIAL GROWTH OF ESCHERICHIA COLI IN PRESENCE OF RHO-FLUOROPHENYLALANINE. EFFECT OF THE ANALOG ON AROMATIC BIOSYNTHESIS. Biochim Biophys Acta. 1964 Jun 22;87:277–290. doi: 10.1016/0926-6550(64)90223-3. [DOI] [PubMed] [Google Scholar]
- RAMAKRISHNAN T., ADELBERG E. A. REGULATORY MECHANISMS IN THE BIOSYNTHESIS OF ISOLEUCINE AND VALINE. I. GENETIC DEREPRESSION OF ENZYME FORMATION. J Bacteriol. 1964 Mar;87:566–573. doi: 10.1128/jb.87.3.566-573.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHMOND M. H. The effect of amino acid analogues on growth and protein synthesis in microorganisms. Bacteriol Rev. 1962 Dec;26:398–420. doi: 10.1128/br.26.4.398-420.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHOCKMAN G. D. AMINO-ACID DEPRIVATION AND BACTERIAL CELL-WALL SYNTHESIS. Trans N Y Acad Sci. 1963 Dec;26:182–195. doi: 10.1111/j.2164-0947.1963.tb01241.x. [DOI] [PubMed] [Google Scholar]
- SMITH L. C., RAVEL J. M., LAX S. R., SHIVE W. THE EFFECTS OF PHENYLALANINE AND TYROSINE ANALOGS ON THE SYNTHESIS AND ACTIVITY OF 3-DEOXY-D-ARABINO-HEPTULOSONIC ACID 7-PHOSPHATE SYNTHETASES. Arch Biochem Biophys. 1964 May;105:424–430. doi: 10.1016/0003-9861(64)90026-8. [DOI] [PubMed] [Google Scholar]
- Saunders G. F., Campbell L. L., Postgate J. R. Base composition of deoxyribonucleic acid of sulfate-reducing bacteria deduced from buoyant density measurements in cesium chloride. J Bacteriol. 1964 May;87(5):1073–1078. doi: 10.1128/jb.87.5.1073-1078.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueoka N. CORRELATION BETWEEN BASE COMPOSITION OF DEOXYRIBONUCLEIC ACID AND AMINO ACID COMPOSITION OF PROTEIN. Proc Natl Acad Sci U S A. 1961 Aug;47(8):1141–1149. doi: 10.1073/pnas.47.8.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- WEBB J. M. A sensitive method for the determination of ribonucleic acid in tissues and microorganisms. J Biol Chem. 1956 Aug;221(2):635–649. [PubMed] [Google Scholar]
- WEBB M. The influence of magnesium on cell division. VI. The action of certain hydrolytic enzymes on the filamentous and chain forms of gram-positive rod-shaped organisms. J Gen Microbiol. 1951 Aug;5(3):496–501. doi: 10.1099/00221287-5-3-496. [DOI] [PubMed] [Google Scholar]
- WEINBERG E. D. MANGANESE REQUIREMENT FOR SPORULATION AND OTHER SECONDARY BIOSYNTHETIC PROCESSES OF BACILLUS. Appl Microbiol. 1964 Sep;12:436–441. doi: 10.1128/am.12.5.436-441.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WELKER N. E., CAMPBELL L. L. EFFECT OF CARBON SOURCES ON FORMATION OF ALPHA-AMYLASE BY BACILLUS STEAROTHERMOPHILUS. J Bacteriol. 1963 Oct;86:681–686. doi: 10.1128/jb.86.4.681-686.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]


