Abstract
Progesterone, a key female sex hormone with pleiotropic functions in maintenance of pregnancy, has profound effects on regulation of immune responses. We report here a novel function of progesterone in regulation of naïve cord blood (CB) fetal T cell differentiation into key regulatory T cell subsets. Progesterone drives allogeneic activation-induced differentiation of CB naive, but not adult peripheral blood (PB), T cells into immune suppressive T regulatory cells (Tregs), many of which express FoxP3. Compared to those induced in the absence of progesterone, the FoxP3+ T cells induced in the presence of progesterone highly expressed memory T cell markers. In this regard, the Treg compartment in progesterone-rich CB is enriched with memory type FoxP3+ T cells. Moreover, CB antigen presenting cells were more efficient in inducing FoxP3+ T cells than their PB counterparts. Another related function of progesterone that we discovered was to suppress the differentiation of CB CD4+ T cells into inflammation-associated Th17 cells. Progesterone enhanced activation of STAT5 in response to IL-2 while it decreased STAT3 activation in response to IL-6, which is in line with the selective activity of progesterone in generation of Tregs versus Th17 cells. Additionally, progesterone has a suppressive function on the expression of the IL-6 receptor by T cells. The results identified a novel role of progesterone in regulation of fetal T cell differentiation for promotion of immune tolerance.
Introduction
Progesterone (P4)2 is a multifunctional female sex hormone, promoting the development of mammary glands, ovulation, implantation of embryos, and maintenance of pregnancy (1). P4 is produced by granulosa cells and corpus luteum in the ovary. During pregnancy, the placenta becomes a major organ for P4 production. P4 can be produced also by cord blood (CB)2 erythroblasts and is present at high levels in CB (2, 3). While the mechanism is still unclear, P4 is implicated in dampening immune responses to fetal and maternal antigens (4). P4 is metabolized into 17-hydroxyprogesterone, which can be eventually converted into other nuclear hormones including glucocorticoids, testosterone, and estradiol (5). P4 or its metabolic derivatives work through a number of different receptors. These include nuclear progesterone receptors (PR-A, PR-B and PR-C), membrane progestin receptors (mPRα, mPRβ, and mPRγ), progesterone receptor membrane component-1 and 2 (PGRMC1 and PGRMC2), and glucocorticoid receptor (GR) (6–11). It has been documented that PRs and mPRs are expressed by T cells (10, 12, 13).
T regulatory cells (Tregs)2 are heterogeneous and are functionally defined by their suppressive function on non-Treg cells. Tregs can be classified into natural and induced (i) Tregs. Some Tregs co-express FoxP3 and CD25 and have been studied extensively. Tregs play important roles in suppression of inflammatory and autoimmune responses (14–17). FoxP3+ Tregs are increased during pregnancy in both maternal and fetal systems in response to allogeneic antigens (18, 19). TGFβ1 and IL-2 play central roles in induction of induced FoxP3+ (iFoxP3+)2 Tregs (20–22). Fetal T cells have an increased propensity to differentiate into Tregs in a TGFβ1-dependent fashion (23, 24), and fetal T cell progenitor cells can more efficiently make Tregs compared to adult progenitor cells (25). Signal transducers and activators of transcription (STAT) 5A/B are involved in the IL-2-dependent induction of FoxP3+ T cells but restrain the generation of Th17 cells (26, 27). Th17 cells are defined by their ability to produce IL-17 and are associated with immune responses to extracellular pathogens and tissue-specific autoimmune diseases (28, 29). Th17 cells are mostly induced in the periphery from naïve T cells upon antigen priming. It has been well established that inflammatory cytokines such as IL-6, IL-23, IL-1β and TNF-α promote the generation of Th17 cells during antigen priming (30). Transcription factors, such as STAT3, RORγt, RORα and aryl hydrocarbon receptor (AHR), are implicated in Th17 cell development (28, 30–32).
Because P4 is greatly increased in CB and fetal circulation and plays important roles in suppression of immune responses, we investigated the possibility that P4 is actively involved in generation of Tregs and Th17 cells from CB naïve T cells. Our study revealed novel functions of P4 in regulation of immune responses. P4 induces naïve CB T cell differentiation into immune-suppressive Tregs, but it suppresses their differentiation into inflammatory Th17 cells. Interestingly, the P4-mediated induction of Tregs efficiently occurs with CB naïve T cells but not with adult peripheral blood (PB)2 T cells. We provide potential mechanisms involving STAT3/5 proteins and IL-6R in selective regulation of CB T cell differentiation. Potential impacts of this regulatory pathway on establishment of immune tolerance are discussed.
Materials and Methods
Preparation of cells
Mononuclear cells were prepared by density gradient centrifuge on histopaque 1077 (Sigma-Aldrich, St. Louis, MO) from human CB and adult PB as described previously (33). CD4+ T cells were isolated by the CD4+ T cell isolation kit (Miltenyi Biotec Inc. Auburn, CA) and further processed for depletion of CD25+ T cells. The isolated CB CD4+CD25− naïve T cells routinely had purity greater than 98%. For isolation of PB CD4+ CD45RA+CD45RO− naïve T cells, total CD4+ T cells were further depleted with CD45RO+, CD69+, and CD25+ T cells as described previously (34). For allogeneic antigen presenting cells, CD3−CD56− CB or adult PB mononuclear cells were used after irradiation (2000 Rad). The use of human CB and PB for this study has been approved by the institutional review board at Purdue University.
In vitro T cell differentiation in response to P4
Neonatal CB and adult PB naive CD4+ T cells were activated with one of the T cell activators including anti-CD3 & anti-CD28-coated beads (5 µl/million cells; Miltenyi Biotec Inc), phytohemagglutinin (PHA, 5 µg/ml), or irradiated CD3−CD56− allogeneic PB or CB mononuclear cells as antigen presenting cells for 6–7 days. IL-2 (25 U/ml) was added for generation of Tregs. All experiments were performed with phenol red-free RPMI medium supplemented with charcoal/dextran-treated fetal bovine serum (10%; HyClone). Depending on the activity of each batch, TGFβ1 was used in the concentration range from 25 to 500 pg/ml to induce FoxP3 at a moderate level (~30%). The cultured T cells were examined for expression of the FoxP3 protein by intracellular staining with anti-FoxP3 antibody (clone 236A/E7 for human, eBioscience, San Diego, CA) and of FoxP3 mRNA by real time PCR with the primers listed in Supplemental Table 1.
For induction of Th17 cells, CB naive CD4+ T cells were activated with anti-CD3/CD28 beads (Miltenyi Biotec Inc) for 6–7 days in RPMI medium supplemented with FBS (10%), IL-23 (25 ng/ml; R&D Systems), IL-1β (10 ng/ml; PeproTech, Inc. Rocky Hill, NJ), TGFβ1 (1 ng/ml; PeproTech), anti-IL-4 (10 µg/ml; MP4-25D2, BioLegend Inc), and anti-IFNγ (10 µg/ml; MD-1, BioLegend Inc). P4 was added at 2 µg/ml from the beginning of the culture. T cells were stained with anti-CD4 (RPA-T4) and then activated with PMA (50 ng/ml) and ionomycin (1 µM) for 4 h in the presence of monensin (2 µg/ml) before staining with antibodies to IL-17 and IFNγ as previously described (35).
Flow cytometry to determine surface antigen phenotype and STAT phosphorylation
Fresh or cultured T cells were examined for expression of CD4 (RPA-4), FoxP3 (PCH101), CD45RA (HI100), CD45RO (UCHL1), CD49f (GoH3), CD58 (1C3), CD45RB (MT4), CD25 (BC96), CD62L (DREG56), and CTLA4 (BNI3). A BD Canto II was used to determine the expression of the antigens. For detection of phosphorylated STAT5A/B or STAT3, human CB CD4+ T cells were first activated for 20 hours with anti-CD3/CD28 beads (Miltenyi Biotec Inc) and then stimulated with hIL-2 (200 U/ml) or hIL-6 (50 ng/ml) for 30 min. The cells were then fixed in 4% paraformaldehyde for 30 min followed by permeabilization in BD Phosflow Perm buffer III. Permeabilized cells were stained with anti-STAT5A/B (pY694) or anti-STAT3 (pY705) antibody (BD Biosciences, clone 47 and 4/P-STAT3) along with an anti-CD4 antibody.
Assessment of suppressive activity of the P4-induced FoxP3+ T cells
CB CD4+CD25− T cells (responders, 5×104 cells/well) and indicated cultured T cells as suppressors were co-cultured in 96-well plates for 5 days at indicated ratios in the presence of anti-CD3/CD28 beads. The suppressors were prepared by a culture with IL-2 (25 U/ml) for 6–7 days. Additionally, TGFβ1 (1 ng/ml) and/or P4 (2 µg/ml) were added as indicated. CB CD4+CD25− T cells were labeled with CFSE and co-cultured with indicated numbers of suppressors. Dilution of CFSE was determined by flow cytometry.
Impact of P4 on T cell differentiation into iTregs in the presence of IL-6
Human CB naïve cells were activated with anti-CD3/CD28 beads together with or without P4 (2 µg/ml) in RPMI medium containing charcoal/dextran-treated FBS (10%), hIL-2 (25 U/ml) and TGFβ1 for 6–7 days. hIL-6 and/or anti-IL-6R neutralizing antibody (MAB227, 10 µg/ml) were added to the culture. The cultured cells were harvested and stained with anti-hCD4, anti-hCD25 and anti-hFoxP3 antibodies. The expression of FoxP3 was determined by Canto II.
Immunofluorescence staining and confocal microscopy
The cultured T cells were stained with antibodies for surface CD4 (clone RPA-T4) and intracellular FoxP3 (clone 236A/E7). The cells were cyto-spinned on slide glasses, and the expression of surface CD4 and nuclear FoxP3 was documented by a confocal microscopy (Zeiss LSM 710).
Microarray gene expression analysis
Human CB and PB naïve T cells were cultured for 5 days with anti-CD3/CD28 beads (Miltenyi Biotec) in the presence or absence of P4 (2 µg/ml) and/or TGFβ1 (150 pg/ml) in the RPMI medium supplemented with charcoal/dextran-treated FBS (10%) and hIL-2 (25 U/ml). The cultured cells were harvested and the total RNA was isolated using RNeasy kit (Qiagen). Total RNA, isolated from the cultured CD4+ T, was hybridized to Human Genome U133 Plus 2.0 Array chips (Affymetrix, Inc.) by Purdue Genomics Laboratory. These arrays contain over 54,000 cDNA spots corresponding to human sequence verified transcripts. Raw intensity values were obtained with the GCOS software (Affymetrix, Inc.) and normalized with the expression values of a housekeeping gene (GAPDH). Selection and filtering of high quality genes were based on a two-fold or greater differential in expression up or down between two conditions of comparison. Further selection was based on reproducibility between technical replicates, and transcripts without consistent results or with signal intensity lower than 50 were dismissed. The gene expression values were visualized with the multiplot module of the GenePattern genomic analysis platform (www.broad.mit.edu/cancer/software/genepattern) and TreeView module from EisenSoftware (http://rana.lbl.gov/EisenSoftware.htm). The raw and processed array data have been deposited at the GEO database (http://www.ncbi.nlm.nih.gov/geo/) (submission numbers: GSE22014, GSE22015, and GSE22025).
Real-time PCR analysis of mRNA expression
cDNA was made and real-time quantitative PCR was performed on a 7300 Sequence Detection System (Applied Biosystems, Foster City, CA) using the SYBR green Master Mix (Applied Biosystems) as described previously (36). Normalized expression was calculated based on Ct values with β-actin used as an internal control. The primers used for real time PCR are shown in Supplemental Table 1.
Statistical analysis
All experiments were performed multiple times to confirm their reproducibility. For combined data from multiple experiments, student’s paired 2-tailed t test was used. p values < or = 0.05 were considered significant. For combined data, the error bars are SEM. For the data that are difficult to combine, representative data are shown with error bars showing differences between duplicated measurements.
Results
P4 promotes the differentiation of human cord blood naive T cells into Tregs with a highly regulatory function
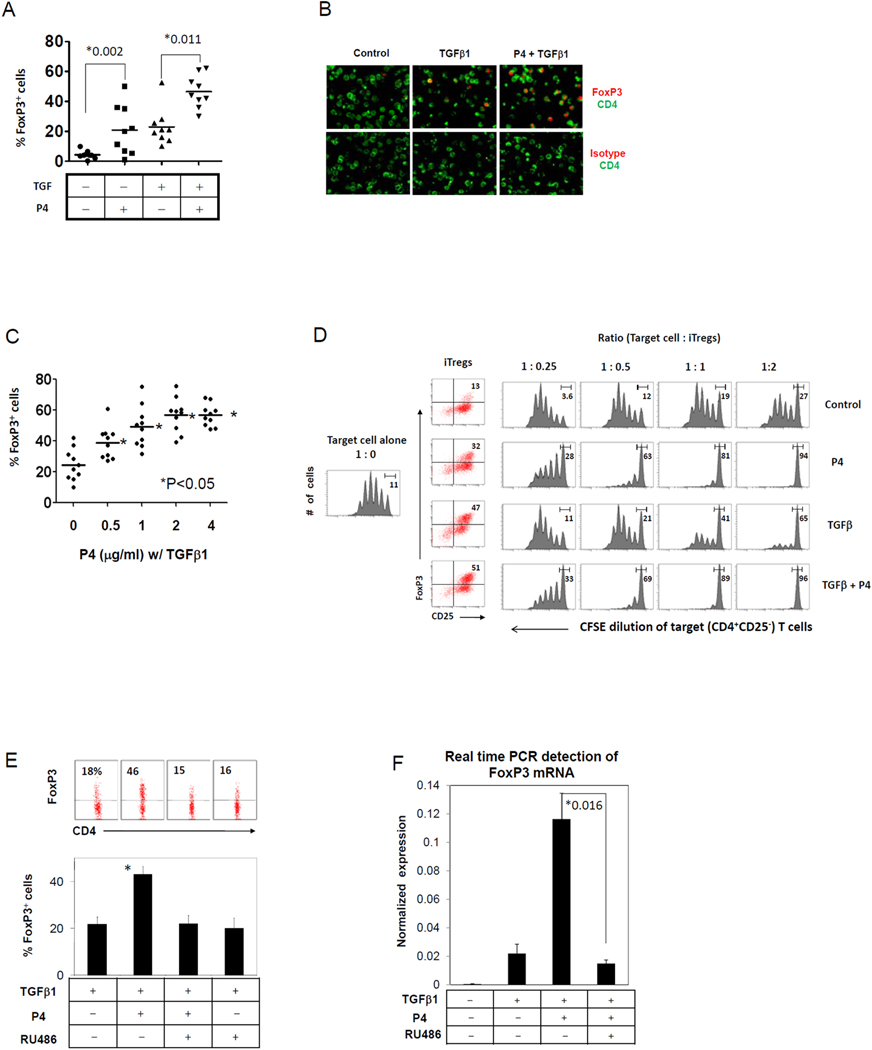
To determine if P4 enhances FoxP3+ Treg differentiation, CB naive T cells were activated in the presence or absence of P4. We supplemented the culture with TGFβ1 at a minimally active concentration, which is known to induce iFoxP3+ T cells. P4 had a positive effect on generation of iFoxP3+ T cells (Fig.1A). The FoxP3 induction in response to P4 occurred also in the absence of exogenous TGFβ1 in some experiments but it was more reliable and efficient in the presence of TGFβ1. The FoxP3 protein expressed in response to P4 was localized to the nuclei of T cells as expected (Fig.1B). The effect of P4 was a dose-dependent one, peaking at around 2 µg/ml of P4 (Fig.1C).
Fig. 1. P4 drives the differentiation of CB CD4+ T cells into highly suppressive Tregs.
(A) Treg-depleted CB fetal (CD4+CD25−) T cells were activated with beads coated with antibodies to CD3 and CD28, P4 (2 µg/ml) and IL-2 for 7 days, and frequencies of FoxP3+ T cells were determined by flow cytometry. TGFβ1 was added as indicated. (B) Microscopic detection of FoxP3 expression in CB T cells stimulated in the presence of P4 and/or TGFβ1. (C) A dose-dependent response of T cells to P4 in FoxP3 induction in the presence of TGFβ1. (D) The suppressive function of control and P4-treated CB T cells on the proliferation of CFSE-labeled CB CD4+CD25− naive T cells were examined. To prepare suppressors, CB naïve (CD4+CD25−) T cells were cultured in the presence of IL-2 (control), or IL-2 and P4 (P4) for 6–7 days. TGFβ1 was added as indicated. The suppressors were cultured with CFSE-labeled target T cells for 5 days and the CFSE intensity of target cells was determined by flow cytometry. RU486, a PR antagonist, was effective in blocking the expression of FoxP3 protein (E) and mRNA (F) induced by P4. Combined data are shown in the graphs in Fig. 1A (n=9), 1C (n=10), 1E (n=7), and 1F (n=3). *Significant differences between the two groups with P values or from the TGFβ1-alone control group.
It has been reported that induced human FoxP3+ T cells with TGFβ1 lack the suppressive activity characteristic for Tregs (37). Therefore, simple induction of FoxP3 appears not sufficient to make human T cells into Tregs, which is better defined functionally. Because of this reason, we investigated if the P4-induced FoxP3+ T cells have a real Treg-like suppressive function. We determined the ability of P4-induced Tregs to suppress the proliferation of target T cells (CFSE-stained CB CD4+CD25− T cells). Compared to IL-2-treated T cells, P4+IL-2-treated T cells displayed a potent suppressive activity. Similarly, P4 and TGFβ1-treated T cells displayed a greater suppressing activity on the proliferation of target (CD4+CD25−) T cells compared to TGFβ1 alone-treated T cells (Fig.1D). RU486, an antagonist for several different progesterone receptors, fully suppressed the P4-induced expression of the FoxP3 protein and transcription of the FoxP3 gene (Fig.1E and F).
Adult peripheral blood naïve T cells are resistant to P4-induced conversion into FoxP3+ T cells
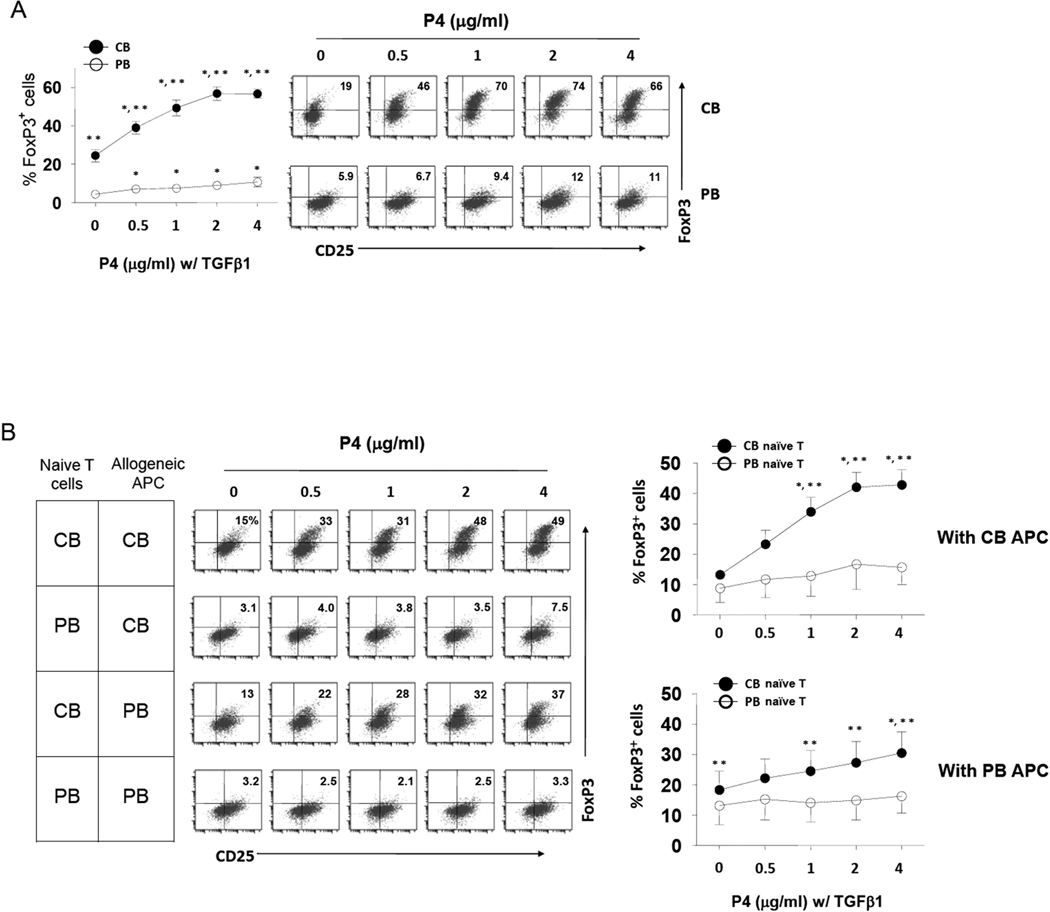
It has been previously reported that CB is a better source of Treg progenitors compared to PB (38). We examined if CB T cells are different from PB T cells in response to P4. Compared to the highly sensitive CB T cells, adult PB naïve T cells were largely unresponsive to P4 (Fig.2A).
Fig. 2. CB, but not adult PB, naïve T cells differentiate into FoxP3+ Tregs in response to P4.
(A) CB naive T cells and adult PB naïve CD4+ T cells were compared for their response to P4. T cells were activated with beads coated with antibodies to CD3 and CD28, P4 (2 µg/ml) and IL-2 for 7 days. TGFβ1 was added to all cultures. After culture, frequencies of FoxP3+ T cells among CD4+ T cells were determined by flow cytometry (n=8–10). (B) P4 promotes allogeneic antigen priming-induced differentiation of CB T cells into FoxP3+ T cells. Naïve CD4+ T cells isolated from the indicated sources were co-cultured with allogeneic APC (irradiated mononuclear cells) obtained from CB or PB for 7 days in the presence of indicated concentrations of P4 (n=4–6). Significant differences from the control point (0 µg/ml)* or from PB**.
To determine if P4 can selectively promote the induction of FoxP3+ T cells from CB versus PB naïve T cells during allogeneic responses between T cells and antigen presenting cells (APC), we co-cultured naïve CD4+ T cells and irradiated T-cell-depleted allogeneic CB or PB mononuclear cells isolated from different individuals (Fig.2B). P4 significantly increased the induction of FoxP3+ T cells during the allogeneic priming of CB T cells. In contrast, adult PB naïve T cells were again poorly responsive. CB APC were more potent than adult APC in induction of P4-induced FoxP3+ T cells (Fig.2B).
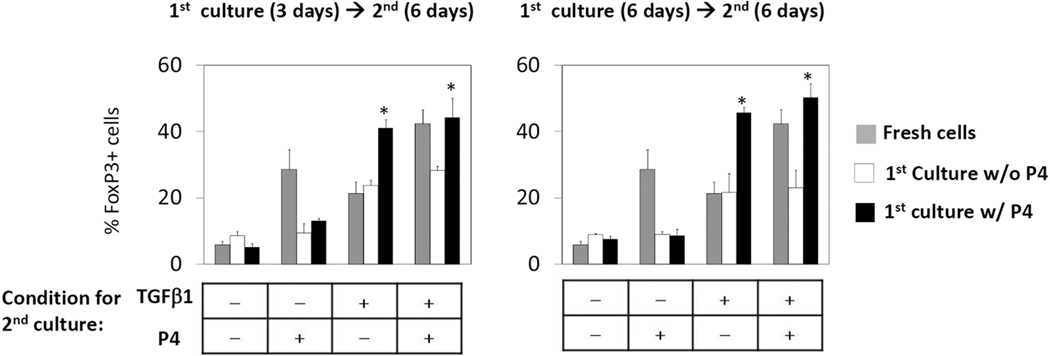
Next, we asked if CB naïve T cells would remain responsive to P4 even after previous antigen priming events. To address this, CB T cells were cultured first with or without P4 and then recultured with P4 and/or TGFβ1 (Fig.3). P4 was unable to induce FoxP3 expression once the CB naïve T cells were pre-activated. Initial exposure to P4 was critical to induce FoxP3+ T cells at high levels by P4 upon subsequent activation with TGFβ1 alone or P4 and TGFβ1. Three days of initial P4 exposure was sufficient to induce this effect. The results suggest that CB T cells indeed lose the ability to respond to P4 and TGFβ1 if they were previously activated in the absence of P4.
Fig. 3. Impact of P4 on stability of FoxP3 expression in T cells.
CB naïve CD4+CD25− T cells were activated with beads coated with antibodies to CD3 and CD28, P4 (2 µg/ml) and IL-2 for 3 or 6 days and then recultured with P4 and/or TGFβ1 for 6 days. Anti-CD3/28 and IL-2 were used to activate the T cells. FoxP3 expression was measured following the second culture. Combined data from 3–4 experiments are shown. *Significant differences from the respective control groups (no P4).
P4-induced iFoxP3+ T cells are related to the memory type natural CB FoxP3+ T cells
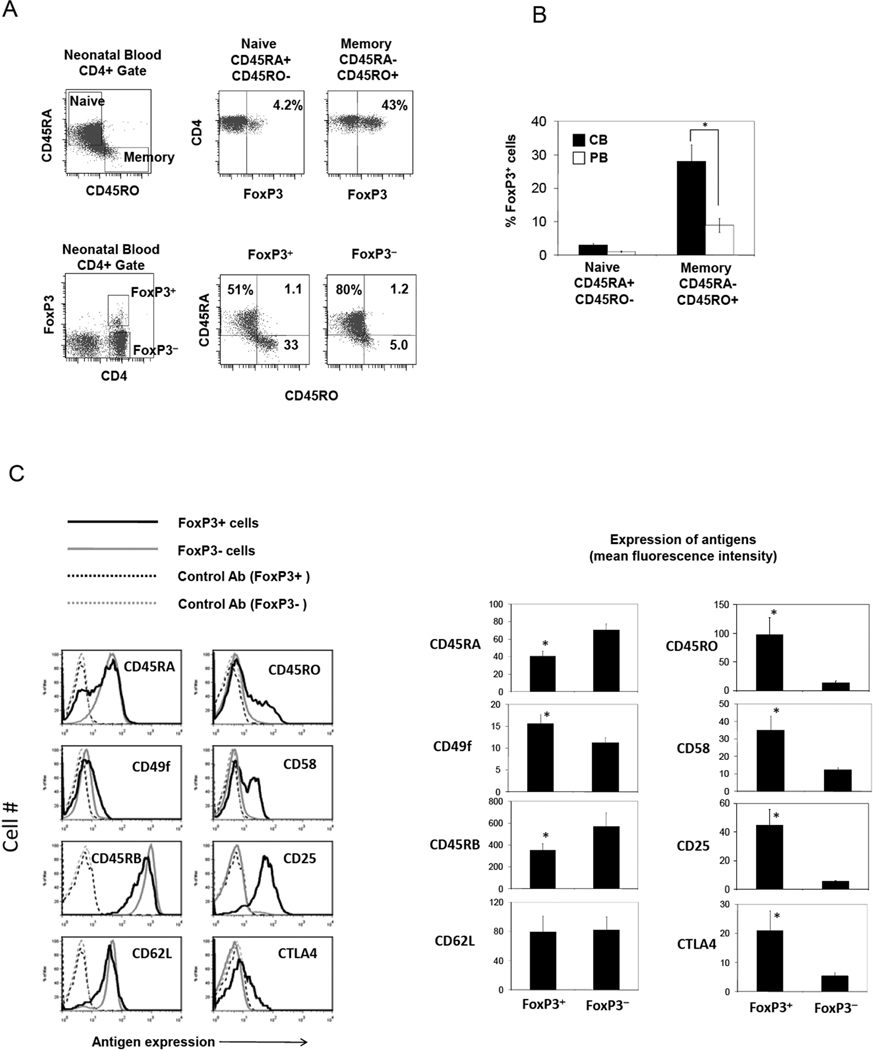
Human CB CD4+ T cells, although known to be mostly composed of naïve cells, contain a significant memory (CD45RA−CD45RO+) cell population, suggesting a history of ongoing antigen priming events in fetuses (Fig.4A). This can be induced preferentially by allogeneic immune responses between fetal T cells and maternal APC or fetal APC presenting maternal antigens. Interestingly, 30–40% of these memory T cells were FoxP3+ T cells (Fig.4A and B). This is considered very high compared to the low frequency (~10%) of FoxP3+ T cells among adult PB memory T cells (Fig.4B). While ~30% of FoxP3+ T cells were CD45RA− CD45RO+ memory T cells, only 1–5 % of FoxP3− T cells were CD45RA− CD45RO+ (Fig.4A). These results suggest that many CB FoxP3+ T cells may have been antigen-primed and assumed the memory T cell phenotype. These memory type CB FoxP3+ T cells expressed CD49f (integrin α6) and CD58 (LFA-3), the antigens typically associated with antigen-primed T cells (Fig.4C). Otherwise, these FoxP3+ T cells were CD25+CTLA4+, a phenotype typically associated with the FoxP3+ T cell population.
Fig. 4. The CB Treg compartment is enriched with memory type FoxP3+ T cells.
(A) Frequencies of naïve versus memory FoxP3+ and FoxP3− T cells in fresh CB. (B) Comparison of CB and adult PB in frequencies of naïve and memory FoxP3+ T cells. (C) Surface phenotype of CB FoxP3+ T cells. Combined data (n=4–8) are shown in a graph form. *Significant differences between paired groups or from FoxP3− T cells.
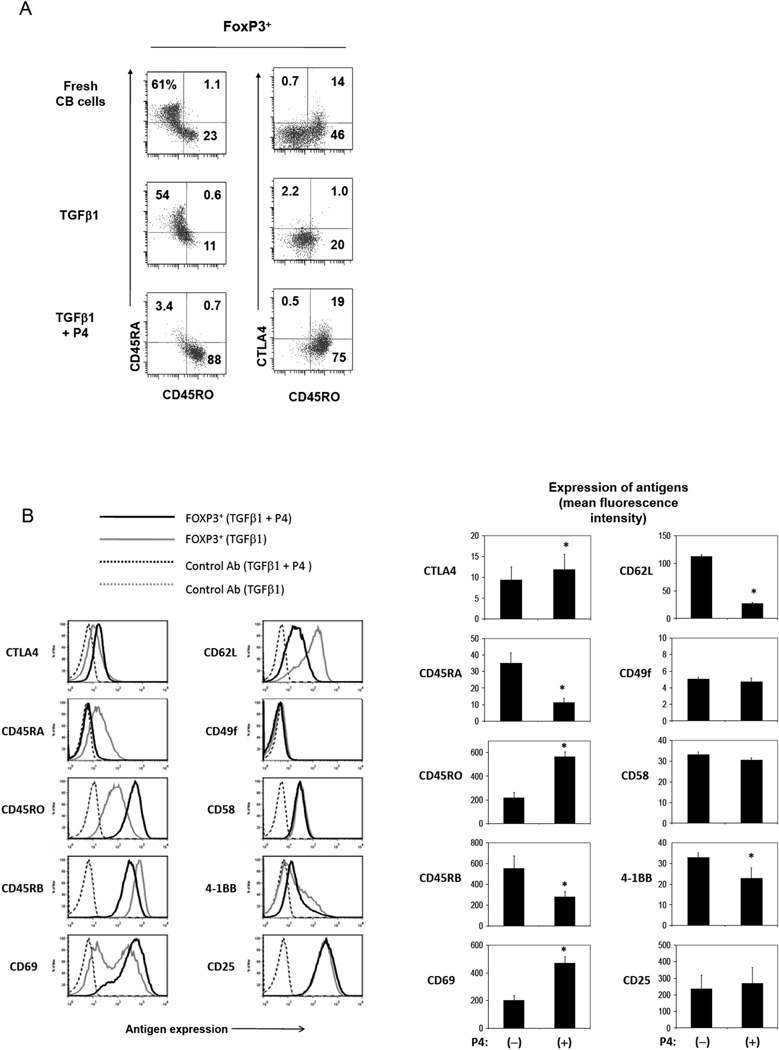
Most P4-induced iFoxP3+ T cells, but not control iFoxP3+ T cells generated in vitro, were CD45RO+CD45RA− cells (Fig.5A), and this phenotype is similar to the memory type FoxP3+ T cells naturally present in CB (Fig.4). In contrast, the T cells antigen-primed without exogenous P4 (but in the presence of TGFβ1) were mostly T cells of intermediary CD45RO−CD45RA− phenotype, suggesting that P4 has a profound regulatory effect on expression of the naïve and memory cell-associated antigens as well. Moreover, P4-induced FoxP3+ T cells were higher than control iFoxP3+ T cells in expression of CTLA4 and CD69, but lower in expression of CD45RB and CD62L, a feature consistent with recently activated memory FoxP3+ T cells (Fig.5B). Expression of another Treg-associated molecule 4-1BB was somewhat decreased on these cells compared to control iFoxP3+ T cells.
Fig. 5. P4 promotes the induction of memory type FoxP3+ T cells.
(A) Emergence of CD45RA− CD45RO+ FoxP3+ T cells from CB naïve CD4+ T cells in response to P4 in vitro. (B) Expression of various surface antigens by the P4-induced or control iFoxP3+ T cells. CB naïve CD4+CD25− T cells were activated with beads coated with antibodies to CD3 and CD28, P4 (2 µg/ml) and IL-2 for 7 days. Combined data for surface antigens (n=4) expressed by control and P4-induced FoxP3+ T cells are shown in a graph form. *Significant differences from the control group (no P4).
P4 suppresses the generation of Th17 cells
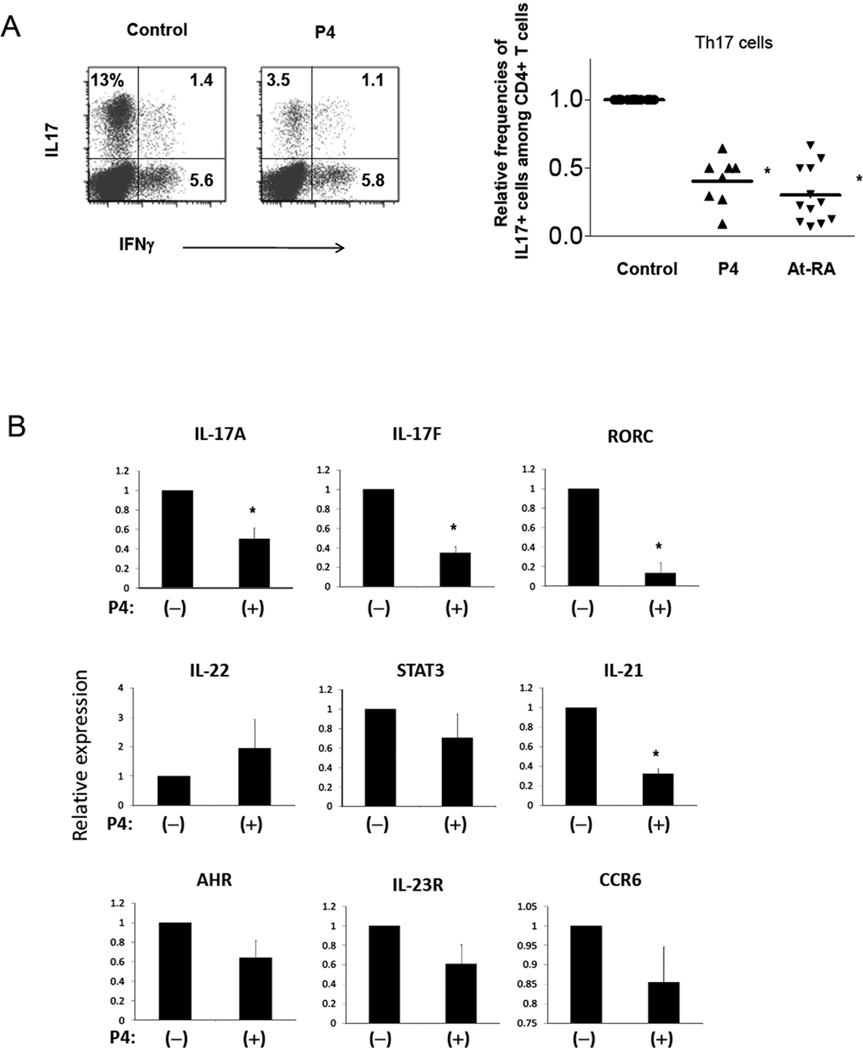
Th17 cells and FoxP3+ T cells, although very different in their functions, are related in their differentiation. Both of the populations are induced by TGFβ1. However, they are different in that IL-2 promotes FoxP3+ T cells and suppresses Th17 cell differentiation, while inflammatory cytokines such as IL-6 that induce Th17 cells suppress FoxP3+ T cell development. We, therefore, examined if P4 has any role in differentiation of CB T cells into Th17 cells in response to inflammatory cytokines. For this, we cultured naïve CB CD4+ T cells in a Th17 cell induction condition in the presence and absence of P4. P4 dramatically reduced the conversion of naïve T cells into Th17 cells (Fig.6A). The effect of P4 is similar to that of retinoic acid which is known to suppress Th17 cells. Along with IL-17A, expression of Th17 cell-associated IL-17F, IL-21, RORC transcripts was decreased by P4 (Fig.6B). Although not statistically significant, other genes such as IL-23R, STAT3, AHR, and CCR6 were decreased too. Expression of IL-22, however, was not suppressed by P4.
Fig. 6. P4 is an effective suppressor of T cell differentiation into Th17 cells.
Treg-depleted CB T cells were cultured in a Th17 cell-induction condition (anti-CD3/CD28 beads or PHA in the presence of IL-23, IL-1β, TGFβ1, anti-IL-4, and anti-IFNγ) for 7 days with or without P4 (2 µg/ml). The relative frequencies of Th17 cells and Th1 cells (A) and expression of Th17-associated genes at the RNA level determined with a real-time PCR method (B) is shown. The combined data in panel A were obtained from 8–12, and the data in panel B from 3–5, independent experiments. *Significant differences from the control group.
Potential mechanisms for P4-regulation of T cell differentiation through modulation of STAT5, STAT3, and IL-6R
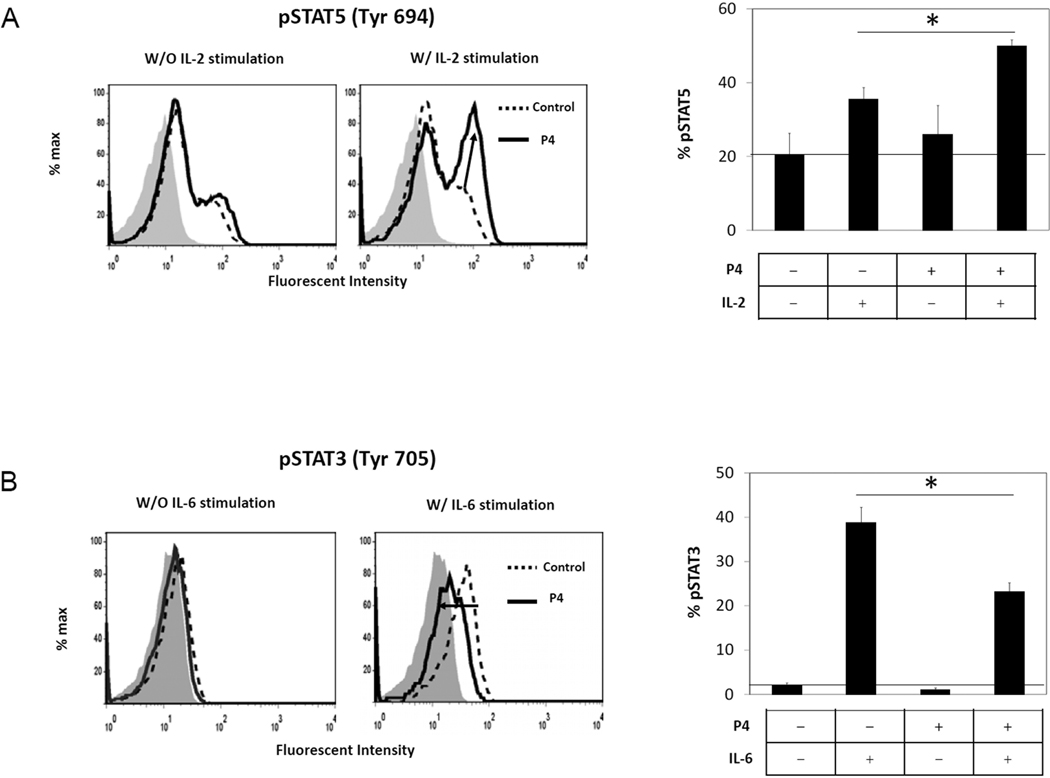
STAT5A/B proteins are key molecules that regulate T cell differentiation into FoxP3+ T cells and Th17 cells. Activation of STAT5 by IL-2 promotes the generation of FoxP3+ T cells at the expense of Th17 cells. We found that P4 increases the phosphorylation of STAT5A/B in the CB T cells induced by IL-2 (Fig.7A). P4 by itself, however, did not induce phosphorylation of STAT5A/B. We examined also the activation of STAT3 because this molecule is central to transmit signals to induce Th17 cells and suppress Tregs. Upon stimulation with IL-6, T cells cultured with P4 had decreased phosphorylation of STAT3 compared to the T cells cultured in the absence of P4 (Fig.7B). Thus, these results show that STAT3 and STAT5 are downstream targets of P4 for regulation of FoxP3+ T cells and Th17 cells.
Fig. 7. P4 enhances STAT5 activation in response to IL-2 while it suppresses STAT3 activation in response to IL-6.
CB CD4+CD25− T cells were activated with anti-CD3/CD28 beads in the presence or absence of P4 for 20 hours. The activated cells were stimulated with recombinant hIL-2 (200 U/ml) or hIL-6 (50 ng/ml) for 30 min and stained with an anti-STAT5 (pY694) or anti-STAT3 (pY705) antibody respectively. A control antibody was employed to identify the basal staining levels for the T cells which are shown as filled gray histograms. Representative and combined data (n=3) are shown. *Significant differences between the groups.
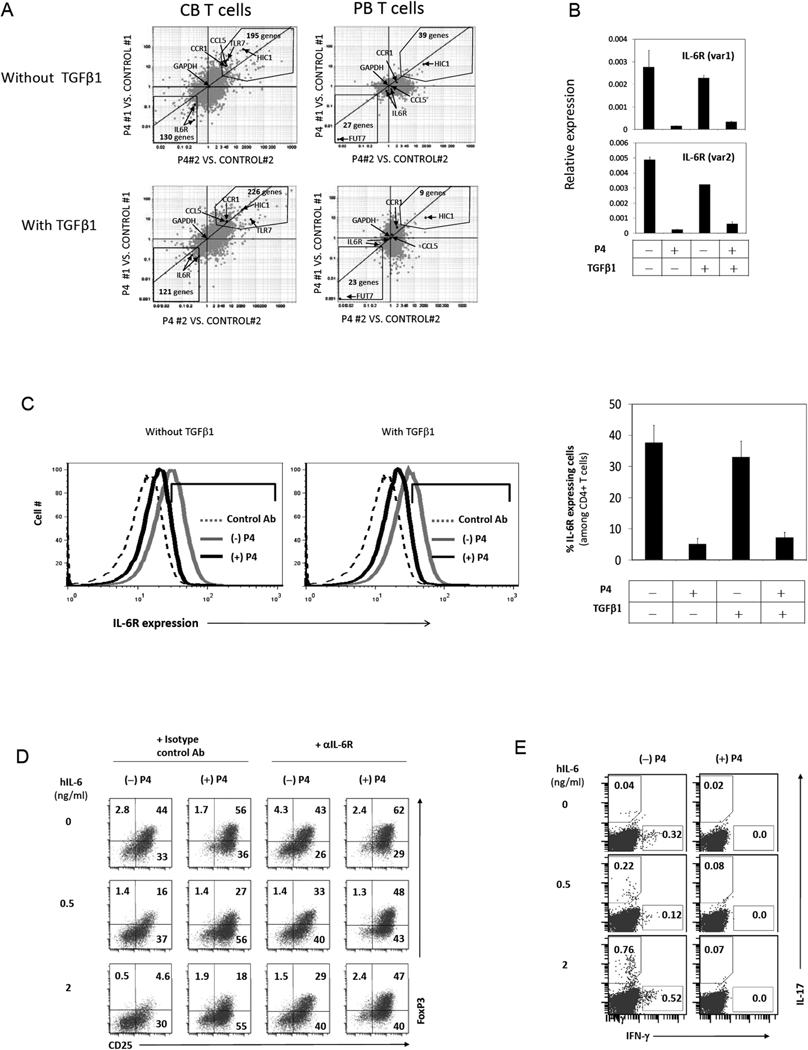
To gain more insights into the P4-regulation of T cell differentiation, we examined the global gene expression pattern of CB T cells regulated by P4. We found 325–347 genes up- or down-regulated by P4 either in the presence or absence of exogenous TGFβ1 (Fig.8A and Supplemental Fig.1A and B). PB T cells were relatively unresponsive with only 30–70 genes regulated by P4. IL-6 receptor (IL-6R) expression was greatly down-regulated by P4 in CB, but not PB, T cells (Fig.8A). IL-6R mediates the IL-6 signal, which suppresses the induction of FoxP3+ T cells but enhances the induction of Th17 cells (39, 40). We confirmed that IL-6R mRNA and protein are decreased by P4 (Fig.8B and C). Consistently, iFoxP3+ T cells were made even in the presence of IL-6 when P4 was added to the culture (Fig.8D). Also, the decrease in IL-6R expression by P4 accounts for the decreased induction of Th17 cells in response to IL-6 (Fig.8E).
Fig. 8. The function of P4 in induction of Tregs and suppression of Th17 cells is mediated through induction or suppression of distinct groups of genes.
(A) Multiplots showing P4-regulated genes. CB or PB naive T cells were activated with beads coated with antibodies to CD3 and CD28, P4 (2 µg/ml), and IL-2 for 5 days for the microarray study. The multiplots show groups of genes reproducibly up- or down-regulated in CB or PB T cells by P4. More detailed information is shown in Supplemental Figure 1. Expression of IL-6R is blocked by P4 in T cells at the RNA level (B) and the surface protein level (C). P4 promotes the generation of FoxP3+ T cells (D) but suppresses the induction of Th17 cells (E) in the presence of hIL-6. A representative data set out of 3 separate experiments is shown.
The following genes regulated by P4 have been verified by real time PCR (supplemental Fig.1C): growth factor independent 1 transcription repressor (GFI1, a suppressor of Th17 cell induction) (41), ankyrin repeat and SOCS box-containing 2 (ASB2, a regulator for degradation of SOCS proteins) (42), CD38 (a cyclic ADP ribose hydrolase), EBI3 (the common subunit of IL-27 and IL-35), TNFSF13B (also called BAFF or BLyS), leucine zipper transcription factor-like 1 (LZTFL1) (43), methyltransferase like 7A (METTL7A), hypermethylated in cancer 1 protein (HIC1), growth factor independent 1 transcription repressor (GFI1) (44), suppressor of cytokine signaling 1 (SOCS1), SOCS2, SOCS3, CC chemokine ligand 5 (CCL5), 2',5'-oligoadenylate synthetase 1 (OAS1), and interferon-induced protein with tetratricopeptide repeats 3 (IFIT3). Among these, SOCS1 and SOCS2 were down-regulated, while others are up-regulated. A more extensive list can be found in Supplemental Figure 1B.
Discussion
In this study, we investigated the P4 effect on CB fetal T cell differentiation into immune-suppressive Tregs and inflammatory Th17 cells. Through the study, we gained novel information regarding the function of P4 in regulation of T cell differentiation. First, we found that P4 has a positive effect on human naïve T cell differentiation into Tregs expressing FoxP3. Interestingly, CB T cells were highly responsive to P4 in this process while adult PB T cells were largely unresponsive to P4. Second, while P4 promotes the differentiation of naïve T cells into FoxP3+ T cells, P4 has an inhibitory effect on CB T cell differentiation into Th17 cells. P4 acts on neonatal T cells undergoing antigen priming and changes their gene expression program to steer the T cell differentiation into tolerogenic Tregs but suppresses the generation of potentially inflammatory effector T cells.
Several studies have proposed the link between nuclear hormones and FoxP3+ T cells. The best studied example so far is the role of retinoic acid in induction of FoxP3+ T cells (34, 45–47). Retinoic acid by itself is a weak inducer of FoxP3+ T cells but has a potent enhancing effect on TGFβ1-induced generation of FoxP3+ T cells. Reciprocally, retinoic acid has a suppressive effect on generation of Th17 cells. Also, P4 is implicated in induction and expansion of CD4+CD25+ Treg cells in the uterus of pregnant mice (48). Others reported that Tregs were decreased in the circulation of pregnant females and proposed that P4 may negatively regulate T cell conversion into Tregs (49). This study, however, failed to determine the direct effect of P4 on Treg-depleted naïve T cells and, thus, appears to be inconclusive. Also, the circulating Tregs may not faithfully represent the Tregs that are induced in response to P4 in specific tissues. Other nuclear hormones that are implicated in induction or expansion of Tregs are estrogen and vitamin D (50, 51). Therefore, nuclear hormone receptors as a group appear to play important roles in induction of immune tolerance.
Separate from the function of P4, we observed that antigen presenting cells of CB provide signals conducive for generation of Tregs. When CB T cells are antigen-primed by allogeneic APC, CB APC were significantly more efficient in inducing FoxP3+ T cells compared to PB APC. All of the characteristics of CB T cells and APC would work together to make the immune responses induced by CB cells more tolerogenic and less inflammatory.
Our results have implications in a number of biological processes. One important process is regulation of immune responses and T cell differentiation during fetal development. The serum concentration of P4 at near term pregnancy can reach ~150 ng/ml in maternal circulation, ~400 ng/ml in CB, and 300–3000 ng/ml in the human placenta tissue (3, 52). Thus, up to ~3000 ng/ml of P4 is considered within the physiological concentration range. Importantly, the doses of P4 that we used in this study are within this range. The P4-treated T cells were more suppressive than control iTregs induced with TGFβ1, and this suggests that P4 fundamentally changes the function of most T cells beyond induction of FoxP3 expression in some cells. P4 has the potential to suppress T cell responses in both pregnant females and fetus. The difficulty of adult T cells to become Tregs in response to P4, however, suggests that the P4 function is more important in fetus and newborns than pregnant females. Therefore, a hypothetical role for P4-induced induction of regulatory T cells and suppression of Th17 cells is to promote immune tolerance in the early life.
We identified increased STAT5 activation, and decreased STAT3 activation and IL-6R expression by P4 as potential mechanisms for the P4-regulated differentiation of T cells. STAT5 activation in response to IL-2 promotes the T cell differentiation into FoxP3+ T cells but restrains the process into Th17 cells. IL-6R, of course, is essential to receive the IL-6 signal, which is, in contrast to IL-2 and STAT5, a major signal to shift T cell differentiation toward the generation of Th17 cells away from FoxP3+ T cells. In this regard, it has been reported that P4 induces the expression and activation of STAT5 in breast cells (53, 54). STAT3 is a central player in delivering the signals from IL-6 and IL-23 in generation of Th17 cells and suppression of Tregs. Therefore, dampened activation of STAT3 in conjunction with enhanced activation of STAT5 would make the cell signaling system conducive for generation of Tregs and suppress Th17 cell development. It may not be just coincidental that decreased IL-6R is linked to retinoic acid-mediated regulation of T cell differentiation in mice as well (55). It appears to be that there are common mechanisms for the action of nuclear hormone receptor ligands in regulation of T cell differentiation. In addition to STAT3/5 and IL-6R, additional genes and molecules are likely to be involved in the regulation of fetal T cells by P4. In this regard, we identified many genes that are regulated by P4 through a genome-wide microarray study.
The results of the genome-wide microarray study show the differential responses between CB and PB T cells in response to P4. CB T cells were highly responsive in up or down-regulating genes, while PB T cells did not significantly change their gene expression program in response to P4. It would be interesting to study in the future how the genes in CB and PB T cells are differentially regulated at the molecular level. Among the genes up-regulated with P4, TNFSF13B/BAFF is known to expand CD4+CD25+Foxp3+ Tregs (56). EBI3, a subunit of IL35 and induced by P4, is a key effector cytokine of Tregs to suppress target cells (57, 58). SOCS1, which negatively regulates Tregs (59), was decreased by P4. CD38, important for homeostasis of Tregs through regulation of nicotinamide adenine dinucleotide (NAD) (60), was increased by P4. Some of the genes such as OAS1, RGS1 and TNF13B, highly induced in response to P4, were among the genes induced in Tregs derived from fetal stem and progenitor cells (25). Roles of the P4-regulated molecules in control of T cell differentiation and function remain to be determined.
Supplementary Material
Acknowledgments
The authors thank Drs. H. Schafer, S. Sinnott, and P. Genaris (OBGYN, IU Health Arnett) for their efforts in collecting CB specimens; and A. Feil for her excellent service with microarray hybridization and scanning (Purdue Genomics Core Facility).
Footnotes
This study was supported, in part, from grants from NIH (1R01AI074745, 1R56AI080769, and 1R01DK076616) and Crohn’s and Colitis Foundation of America to CHK.
Abbreviations: CB, cord blood; PB, peripheral blood; P4, progesterone; PR, progesterone receptor; iFoxP3+ cells, induced FoxP3+ cells; Tregs, T regulatory cells; iTregs, induced Tregs.
References
- 1.Spencer TE, Bazer FW. Biology of progesterone action during pregnancy recognition and maintenance of pregnancy. Front Biosci. 2002;7:d1879–d1898. doi: 10.2741/spencer. [DOI] [PubMed] [Google Scholar]
- 2.Loganath A, Peh KL, Chew PC, Wong YC, Ng SC. Evidence for progesterone synthesis by human umbilical cord blood erythrocytes. Biology of the neonate. 2000;78:13–16. doi: 10.1159/000014240. [DOI] [PubMed] [Google Scholar]
- 3.Hercz P, Ungar L, Siklos P. Perinatal progesterone in maternal-fetoplacental system during mature and premature deliveries. Acta obstetricia et gynecologica Scandinavica. 1988;67:233–235. doi: 10.3109/00016348809004210. [DOI] [PubMed] [Google Scholar]
- 4.Arck P, Hansen PJ, Mulac Jericevic B, Piccinni MP, Szekeres-Bartho J. Progesterone during pregnancy: endocrine-immune cross talk in mammalian species and the role of stress. Am J Reprod Immunol. 2007;58:268–279. doi: 10.1111/j.1600-0897.2007.00512.x. [DOI] [PubMed] [Google Scholar]
- 5.Jaffe RB. Biogenesis and metabolism of progesterone in the human fetus and placenta. Clinical obstetrics and gynecology. 1967;10:47–59. doi: 10.1097/00003081-196703000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Scarpin KM, Graham JD, Mote PA, Clarke CL. Progesterone action in human tissues: regulation by progesterone receptor (PR) isoform expression, nuclear positioning and coregulator expression. Nucl Recept Signal. 2009;7:e009. doi: 10.1621/nrs.07009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rohe HJ, Ahmed IS, Twist KE, Craven RJ. PGRMC1 (progesterone receptor membrane component 1): a targetable protein with multiple functions in steroid signaling, P450 activation and drug binding. Pharmacol Ther. 2009;121:14–19. doi: 10.1016/j.pharmthera.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cahill MA. Progesterone receptor membrane component 1: an integrative review. The Journal of steroid biochemistry and molecular biology. 2007;105:16–36. doi: 10.1016/j.jsbmb.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Bouchard P. Progesterone and the progesterone receptor. J Reprod Med. 1999;44:153–157. [PubMed] [Google Scholar]
- 10.Dressing GE, Goldberg JE, Charles NJ, Schwertfeger KL, Lange CA. Membrane progesterone receptor expression in mammalian tissues: a review of regulation and physiological implications. Steroids. 2011;76:11–17. doi: 10.1016/j.steroids.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kontula K, Paavonen T, Luukkainen T, Andersson LC. Binding of progestins to the glucocorticoid receptor. Correlation to their glucocorticoid-like effects on in vitro functions of human mononuclear leukocytes. Biochem Pharmacol. 1983;32:1511–1518. doi: 10.1016/0006-2952(83)90474-4. [DOI] [PubMed] [Google Scholar]
- 12.Ndiaye K, Poole DH, Pate JL. Expression and regulation of functional oxytocin receptors in bovine T lymphocytes. Biology of reproduction. 2008;78:786–793. doi: 10.1095/biolreprod.107.065938. [DOI] [PubMed] [Google Scholar]
- 13.Dosiou C, Hamilton AE, Pang Y, Overgaard MT, Tulac S, Dong J, Thomas P, Giudice LC. Expression of membrane progesterone receptors on human T lymphocytes and Jurkat cells and activation of G-proteins by progesterone. J Endocrinol. 2008;196:67–77. doi: 10.1677/JOE-07-0317. [DOI] [PubMed] [Google Scholar]
- 14.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 15.Xu L, Kitani A, Strober W. Molecular mechanisms regulating TGF-beta-induced Foxp3 expression. Mucosal Immunol. 2010;3:230–238. doi: 10.1038/mi.2010.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feuerer M, Hill JA, Mathis D, Benoist C. Foxp3+ regulatory T cells: differentiation, specification, subphenotypes. Nat Immunol. 2009;10:689–695. doi: 10.1038/ni.1760. [DOI] [PubMed] [Google Scholar]
- 17.Zhou X, Bailey-Bucktrout S, Jeker LT, Bluestone JA. Plasticity of CD4(+) FoxP3(+) T cells. Curr Opin Immunol. 2009;21:281–285. doi: 10.1016/j.coi.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nature immunology. 2004;5:266–271. doi: 10.1038/ni1037. [DOI] [PubMed] [Google Scholar]
- 19.Sasaki Y, Sakai M, Miyazaki S, Higuma S, Shiozaki A, Saito S. Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Molecular human reproduction. 2004;10:347–353. doi: 10.1093/molehr/gah044. [DOI] [PubMed] [Google Scholar]
- 20.Horwitz DA, Zheng SG, Wang J, Gray JD. Critical role of IL-2 and TGF-beta in generation, function and stabilization of Foxp3+CD4+ Treg. European journal of immunology. 2008;38:912–915. doi: 10.1002/eji.200738109. [DOI] [PubMed] [Google Scholar]
- 21.Peng Y, Laouar Y, Li MO, Green EA, Flavell RA. TGF-beta regulates in vivo expansion of Foxp3-expressing CD4+CD25+ regulatory T cells responsible for protection against diabetes. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:4572–4577. doi: 10.1073/pnas.0400810101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. The Journal of experimental medicine. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang CC, Satwani P, Oberfield N, Vlad G, Simpson LL, Cairo MS. Increased induction of allogeneic-specific cord blood CD4+CD25+ regulatory T (Treg) cells: a comparative study of naive and antigenic-specific cord blood Treg cells. Experimental hematology. 2005;33:1508–1520. doi: 10.1016/j.exphem.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 24.Mold JE, Michaelsson J, Burt TD, Muench MO, Beckerman KP, Busch MP, Lee TH, Nixon DF, McCune JM. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science (New York, N.Y. 2008;322:1562–1565. doi: 10.1126/science.1164511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mold JE, Venkatasubrahmanyam S, Burt TD, Michaelsson J, Rivera JM, Galkina SA, Weinberg K, Stoddart CA, McCune JM. Fetal and adult hematopoietic stem cells give rise to distinct T cell lineages in humans. Science (New York, N.Y. 2010;330:1695–1699. doi: 10.1126/science.1196509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen L, Cohen AC, Lewis DB. Impaired allogeneic activation and T-helper 1 differentiation of human cord blood naive CD4 T cells. Biol Blood Marrow Transplant. 2006;12:160–171. doi: 10.1016/j.bbmt.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 27.Zorn E, Nelson EA, Mohseni M, Porcheray F, Kim H, Litsa D, Bellucci R, Raderschall E, Canning C, Soiffer RJ, Frank DA, Ritz J. IL-2 regulates FOXP3 expression in human CD4+CD25+ regulatory T cells through a STAT-dependent mechanism and induces the expansion of these cells in vivo. Blood. 2006;108:1571–1579. doi: 10.1182/blood-2006-02-004747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 140:845–858. doi: 10.1016/j.cell.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 29.Weaver CT, Hatton RD. Interplay between the TH17 and TReg cell lineages: a (co-)evolutionary perspective. Nature reviews. 2009;9:883–889. doi: 10.1038/nri2660. [DOI] [PubMed] [Google Scholar]
- 30.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annual review of immunology. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 31.Annunziato F, Cosmi L, Liotta F, Maggi E, Romagnani S. The phenotype of human Th17 cells and their precursors, the cytokines that mediate their differentiation and the role of Th17 cells in inflammation. International immunology. 2008;20:1361–1368. doi: 10.1093/intimm/dxn106. [DOI] [PubMed] [Google Scholar]
- 32.Nurieva RI, Dong C. Keeping autoimmunity in check: how to control a Th17 cell controller. Immunity. 2008;29:841–843. doi: 10.1016/j.immuni.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Lim HW, Broxmeyer HE, Kim CH. Regulation of trafficking receptor expression in human forkhead box P3+ regulatory T cells. J Immunol. 2006;177:840–851. doi: 10.4049/jimmunol.177.2.840. [DOI] [PubMed] [Google Scholar]
- 34.Kang SG, Lim HW, Andrisani OM, Broxmeyer HE, Kim CH. Vitamin A metabolites induce gut-homing FoxP3+ regulatory T cells. J Immunol. 2007;179:3724–3733. doi: 10.4049/jimmunol.179.6.3724. [DOI] [PubMed] [Google Scholar]
- 35.Lim HW, Lee J, Hillsamer P, Kim CH. Human Th17 cells share major trafficking receptors with both polarized effector T cells and FOXP3+ regulatory T cells. J Immunol. 2008;180:122–129. doi: 10.4049/jimmunol.180.1.122. [DOI] [PubMed] [Google Scholar]
- 36.Kang SG, Wang C, Matsumoto S, Kim CH. High and low vitamin A therapies induce distinct FoxP3+ T-cell subsets and effectively control intestinal inflammation. Gastroenterology. 2009;137:1391–1402. e1391–e1396. doi: 10.1053/j.gastro.2009.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tran DQ, Ramsey H, Shevach EM. Induction of FOXP3 expression in naive human CD4+FOXP3 T cells by T-cell receptor stimulation is transforming growth factor-beta dependent but does not confer a regulatory phenotype. Blood. 2007;110:2983–2990. doi: 10.1182/blood-2007-06-094656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Godfrey WR, Spoden DJ, Ge YG, Baker SR, Liu B, Levine BL, June CH, Blazar BR, Porter SB. Cord blood CD4(+)CD25(+)-derived T regulatory cell lines express FoxP3 protein and manifest potent suppressor function. Blood. 2005;105:750–758. doi: 10.1182/blood-2004-06-2467. [DOI] [PubMed] [Google Scholar]
- 39.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 40.Korn T, Mitsdoerffer M, Croxford AL, Awasthi A, Dardalhon VA, Galileos G, Vollmar P, Stritesky GL, Kaplan MH, Waisman A, Kuchroo VK, Oukka M. IL-6 controls Th17 immunity in vivo by inhibiting the conversion of conventional T cells into Foxp3+ regulatory T cells. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:18460–18465. doi: 10.1073/pnas.0809850105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu J, Davidson TS, Wei G, Jankovic D, Cui K, Schones DE, Guo L, Zhao K, Shevach EM, Paul WE. Down-regulation of Gfi-1 expression by TGF-beta is important for differentiation of Th17 and CD103+ inducible regulatory T cells. The Journal of experimental medicine. 2009;206:329–341. doi: 10.1084/jem.20081666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heuze ML, Guibal FC, Banks CA, Conaway JW, Conaway RC, Cayre YE, Benecke A, Lutz PG. ASB2 is an Elongin BC-interacting protein that can assemble with Cullin 5 and Rbx1 to reconstitute an E3 ubiquitin ligase complex. The Journal of biological chemistry. 2005;280:5468–5474. doi: 10.1074/jbc.M413040200. [DOI] [PubMed] [Google Scholar]
- 43.Kiss H, Kedra D, Kiss C, Kost-Alimova M, Yang Y, Klein G, Imreh S, Dumanski JP. The LZTFL1 gene is a part of a transcriptional map covering 250 kb within the common eliminated region 1 (C3CER1) in 3p21.3. Genomics. 2001;73:10–19. doi: 10.1006/geno.2000.6498. [DOI] [PubMed] [Google Scholar]
- 44.Phelan JD, Shroyer NF, Cook T, Gebelein B, Grimes HL. Gfi1-cells and circuits: unraveling transcriptional networks of development and disease. Current opinion in hematology. 2010;17:300–307. doi: 10.1097/MOH.0b013e32833a06f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science (New York, N.Y. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 46.Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. The Journal of experimental medicine. 2007;204:1765–1774. doi: 10.1084/jem.20070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. The Journal of experimental medicine. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mao G, Wang J, Kang Y, Tai P, Wen J, Zou Q, Li G, Ouyang H, Xia G, Wang B. Progesterone increases systemic and local uterine proportions of CD4+CD25+ Treg cells during midterm pregnancy in mice. Endocrinology. 2010;151:5477–5488. doi: 10.1210/en.2010-0426. [DOI] [PubMed] [Google Scholar]
- 49.Mjosberg J, Svensson J, Johansson E, Hellstrom L, Casas R, Jenmalm MC, Boij R, Matthiesen L, Jonsson JI, Berg G, Ernerudh J. Systemic reduction of functionally suppressive CD4dimCD25highFoxp3+ Tregs in human second trimester pregnancy is induced by progesterone and 17beta-estradiol. J Immunol. 2009;183:759–769. doi: 10.4049/jimmunol.0803654. [DOI] [PubMed] [Google Scholar]
- 50.Polanczyk MJ, Carson BD, Subramanian S, Afentoulis M, Vandenbark AA, Ziegler SF, Offner H. Cutting edge: estrogen drives expansion of the CD4+CD25+ regulatory T cell compartment. J Immunol. 2004;173:2227–2230. doi: 10.4049/jimmunol.173.4.2227. [DOI] [PubMed] [Google Scholar]
- 51.Jeffery LE, Burke F, Mura M, Zheng Y, Qureshi OS, Hewison M, Walker LS, Lammas DA, Raza K, Sansom DM. 1,25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J Immunol. 2009;183:5458–5467. doi: 10.4049/jimmunol.0803217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chiron Diagnostics ACS. Centaur Progesterone Assay Manual. 1998 [Google Scholar]
- 53.Santos SJ, Haslam SZ, Conrad SE. Estrogen and progesterone are critical regulators of Stat5a expression in the mouse mammary gland. Endocrinology. 2008;149:329–338. doi: 10.1210/en.2007-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Richer JK, Lange CA, Manning NG, Owen G, Powell R, Horwitz KB. Convergence of progesterone with growth factor and cytokine signaling in breast cancer. Progesterone receptors regulate signal transducers and activators of transcription expression and activity. The Journal of biological chemistry. 1998;273:31317–31326. doi: 10.1074/jbc.273.47.31317. [DOI] [PubMed] [Google Scholar]
- 55.Xiao S, Jin H, Korn T, Liu SM, Oukka M, Lim B, Kuchroo VK. Retinoic acid increases Foxp3+ regulatory T cells and inhibits development of Th17 cells by enhancing TGF-beta-driven Smad3 signaling and inhibiting IL-6 and IL-23 receptor expression. J Immunol. 2008;181:2277–2284. doi: 10.4049/jimmunol.181.4.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Walters S, Webster KE, Sutherland A, Gardam S, Groom J, Liuwantara D, Marino E, Thaxton J, Weinberg A, Mackay F, Brink R, Sprent J, Grey ST. Increased CD4+Foxp3+ T cells in BAFF-transgenic mice suppress T cell effector responses. J Immunol. 2009;182:793–801. doi: 10.4049/jimmunol.182.2.793. [DOI] [PubMed] [Google Scholar]
- 57.Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, Cross R, Sehy D, Blumberg RS, Vignali DA. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 58.Niedbala W, Wei XQ, Cai B, Hueber AJ, Leung BP, McInnes IB, Liew FY. IL-35 is a novel cytokine with therapeutic effects against collagen-induced arthritis through the expansion of regulatory T cells and suppression of Th17 cells. European journal of immunology. 2007;37:3021–3029. doi: 10.1002/eji.200737810. [DOI] [PubMed] [Google Scholar]
- 59.Zhan Y, Davey GM, Graham KL, Kiu H, Dudek NL, Kay TW, Lew AM. SOCS1 negatively regulates the production of Foxp3+ CD4+ T cells in the thymus. Immunol Cell Biol. 2009;87:473–480. doi: 10.1038/icb.2009.23. [DOI] [PubMed] [Google Scholar]
- 60.Hubert S, Rissiek B, Klages K, Huehn J, Sparwasser T, Haag F, Koch-Nolte F, Boyer O, Seman M, Adriouch S. Extracellular NAD+ shapes the Foxp3+ regulatory T cell compartment through the ART2-P2X7 pathway. The Journal of experimental medicine. 2010;207:2561–2568. doi: 10.1084/jem.20091154. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.