Abstract
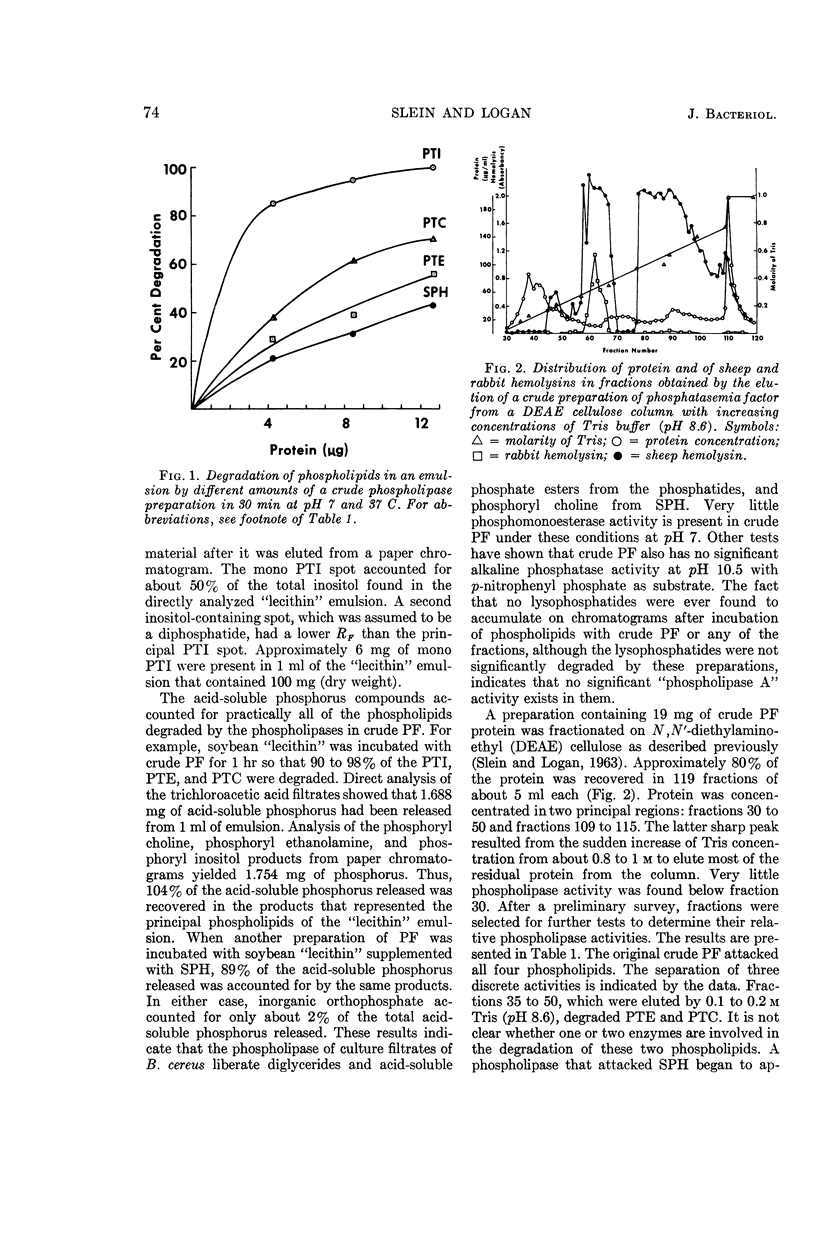
Slein, Milton W. (Fort Detrick, Frederick, Md.), and Gerald F. Logan, Jr. Characterization of the phospholipases of Bacillus cereus and their effects on erythrocytes, bone, and kidney cells. J. Bacteriol. 90:69–81. 1965.—Culture filtrates of Bacillus cereus contain phospholipases that split phosphoryl choline, phosphoryl ethanolamine, and phosphoryl inositol from the phospholipids phosphatidyl choline (PTC), sphingomyelin, phosphatidyl ethanolamine (PTE), and phosphatidyl inositol (PTI). It is possible that one enzyme catalyzes the degradation of PTE and PTC, but the other phospholipases appear to be separate entities. Some activity on phosphatidyl serine has also been noted. Quantitative paper chromatography has been used for characterizing the phospholipases that are separated on N,N′-diethylaminoethyl cellulose columns. A procedure for the analysis of inositol is included. A sensitive kidney cortex homogenate test is described that depends on the release of alkaline phosphatase for the measurement of phosphatasemia factor (PF) activity associated with the phospholipases. The effects of the phospholipases on erythrocytes, kidney, and bone cells are discussed. Hemolysin activity is inhibited by crude soybean “lecithin,” but hemolysis does not seem to be identical with PTE- or PTC-phospholipase activity. PF activity is also inhibited by the “lecithin.” Highest PF activity is associated with PTI-phospholipase. The phospholipase fractions differ in their sensitivities to trypsin. Phospholipases with similar properties have been obtained from culture filtrates of B. anthracis.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BANDURSKI R. S., AXELROD B. The chromatographic identification of some biologically important phosphate esters. J Biol Chem. 1951 Nov;193(1):405–410. [PubMed] [Google Scholar]
- BANGHAM A. D., DAWSON R. M. Electrokinetic requirements for the reaction between Cl. perfringens alpha-toxin (phospholipase C) and phospholipid substrates. Biochim Biophys Acta. 1962 May 7;59:103–115. doi: 10.1016/0006-3002(62)90701-1. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- BOHM P., RICHARZ G. Zur quantitativen Bestimmung von Inosit in Phosphatiden. Hoppe Seylers Z Physiol Chem. 1954;298(3-5):110–120. [PubMed] [Google Scholar]
- CONDREA E., DEVRIES A., MAGER J. HEMOLYSIS AND SPLITTING OF HUMAN ERYTHROCYTE PHOSPHOLIPIDS BY SNAKE VENOMS. Biochim Biophys Acta. 1964 Feb 24;84:60–73. doi: 10.1016/0926-6542(64)90101-5. [DOI] [PubMed] [Google Scholar]
- COSTLOW R. D. Lecithinase from Bacillus anthracis. J Bacteriol. 1958 Sep;76(3):317–325. doi: 10.1128/jb.76.3.317-325.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERLACH E., DEUTICKE B. EINE EINFACHE METHODE ZUR MIKROBESTIMMUNG VON PHOSPHAT IN DER PAPIERCHROMATOGRAPHIE. Biochem Z. 1963 Jul 26;337:477–479. [PubMed] [Google Scholar]
- HANES C. S., ISHERWOOD F. A. Separation of the phosphoric esters on the filter paper chromatogram. Nature. 1949 Dec 31;164(4183):1107-12, illust. doi: 10.1038/1641107a0. [DOI] [PubMed] [Google Scholar]
- KERR S. E., KFOURY G. A. Chromatographic separation and identification of myo-inositol phosphates. Arch Biochem Biophys. 1962 Feb;96:347–353. doi: 10.1016/0003-9861(62)90419-8. [DOI] [PubMed] [Google Scholar]
- KUSHNER D. J., FELDMAN D. Characterization of the bacterial enzyme thromboplastinase. Biochim Biophys Acta. 1958 Dec;30(3):466–475. doi: 10.1016/0006-3002(58)90091-x. [DOI] [PubMed] [Google Scholar]
- LARNER J., JACKSON W. T., GRAVES D. J., STAMER J. R. Inositol dehydrogenase from Aerobacter aerogenes. Arch Biochem Biophys. 1956 Feb;60(2):352–363. doi: 10.1016/0003-9861(56)90437-4. [DOI] [PubMed] [Google Scholar]
- MAGASANIK B. Enzymatic adaptation in the metabolism of cyclitols in Aerobacter aerogenes. J Biol Chem. 1953 Dec;205(2):1007–1018. [PubMed] [Google Scholar]
- ROBINSON D. S., HARRIS P. M., POOLE J. C. Further studies on ethanolamine phosphatide and blood coagulation: the effect of a Bacillus cereus phosphatidase on the calcium time of citrated rat plasma. Q J Exp Physiol Cogn Med Sci. 1957 Jul;42(3):285–294. doi: 10.1113/expphysiol.1957.sp001264. [DOI] [PubMed] [Google Scholar]
- SLEIN M. W., LOGAN G. F., Jr Mechanism of action of the toxin of Bacillus anthracis. I. Effect in vivo on some blood serum components. J Bacteriol. 1960 Jul;80:77–85. doi: 10.1128/jb.80.1.77-85.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SLEIN M. W., LOGAN G. F., Jr Partial purification and properties of two phospholipases of Bacillus cereus. J Bacteriol. 1963 Feb;85:369–381. doi: 10.1128/jb.85.2.369-381.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slein M. W., Logan G. F. MECHANISM OF ACTION OF THE TOXIN OF BACILLUS ANTHRACIS II. : Alkaline Phosphatasemia Produced by Culture Filtrates of Various Bacilli. J Bacteriol. 1962 Feb;83(2):359–369. doi: 10.1128/jb.83.2.359-369.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISSBACH The enzymic determination of myo-inositol. Biochim Biophys Acta. 1958 Mar;27(3):608–611. doi: 10.1016/0006-3002(58)90393-7. [DOI] [PubMed] [Google Scholar]
- Ways P., Hanahan D. J. Characterization and quantification of red cell lipids in normal man. J Lipid Res. 1964 Jul;5(3):318–328. [PubMed] [Google Scholar]
- de GIER, VAN DEENEN L. Some lipid characteristics of red cell membranes of various animal species. Biochim Biophys Acta. 1961 May 13;49:286–296. doi: 10.1016/0006-3002(61)90128-7. [DOI] [PubMed] [Google Scholar]


