Abstract
Resveratrol (trans-3,5,4’-trihydroxystilbene), a polyphenol found in red wine, has multiple beneficial activities that are similar to caloric restriction. In this study, we analyzed the effect of resveratrol on the gonadotropin genes, follicle-stimulating hormone (FSHβ) and luteinizing hormone (LHβ) in LβT2 immortalized mouse gonadotrope cells. Resveratrol specifically inhibited activin-induced FSHβ mRNA and protein expression, and reduced activin-stimulated Smad2/3 phosphorylation. Knockdown of SirT1 gene expression or SirT1 inhibition did not block repression of FSHβ expression or suppression of Smad2/3 phosphorylation, but did increase p53 acetylation. Taken together, our results suggest that resveratrol down-regulates Smad2/3 phosphorylation and suppresses FSHβ expression via a SirT1-independent pathway.
Keywords: resveratrol, FSH, Smad2/3, repression
1. Introduction
Trans-resveratrol (3,5,4’-trihydroxystilbene) is a polyphenol found in red wine and a variety of plant roots that has been extensively studied for its antioxidant, estrogenic, anti-inflammatory and longevity-enhancing effects [1]. It has been shown to mimic caloric restriction and has beneficial effects in neurological diseases [2], diabetes [3, 4], heart diseases [5]. At the molecular level, resveratrol activates the sirtuin, SirT1. Sirtuins are a family of highly conserved nicotinamide adenine dinucleotide (NAD+)-dependent deacetylases, named after the Saccharomyces cerevisiae gene, silent information regulation-2 (Sir2). Sirtuins act as sensors of cellular energy [6] and regulate lifespan in many species [7]. SirT1 deacetylates a number of transcription factors and coactivators involved in cell growth, differentiation, metabolism and mitochondrial function [8]. Resveratrol increases SirT1 activity by decreasing the Km for acetylated synthetic substrates [7] but its activity against endogenous substrates is somewhat controversial [9, 10].
Resveratrol action is more complicated, however, as it also has SirT1-independent effects activating the AMP-dependent kinase (AMPK), a key regulator of cellular and whole-body energy homeostasis, and neurite outgrowth [11]. AMPK is activated by depletion of cellular ATP resulting in an elevated AMP/ATP ratio [12]. Resveratrol also regulates mitogen-activated protein kinase (MAPK) signaling [13], inhibits cyclooxygenases [14] and subsequently modulates a broad range of biological process such as inflammation [15, 16] and proliferation [13, 17]. Furthermore, resveratrol is a phytoestrogen and functions as a mixed agonist/antagonist on both the estrogen receptor alpha (ERα) and ERβ [18, 19]. It also regulates lipid homeostasis by activating ATP-binding cassette transporters ABCA1 and ABCG1 via the transcription factor LXR-α [20].
As resveratrol is being widely consumed as a dietary supplement, it is important to know whether this compound has any potential effects on reproductive fitness. Therefore the aim of this study was to explore the effects of resveratrol on pituitary gonadotropin hormone expression and secretion as pituitary gonadotropes are central to the regulation of reproduction.
2. Materials and Methods
2.1 Materials and Cell Culture
Resveratrol was purchased from A.G. Scientific, Inc (San Diego, CA). Resveratrol was dissolved at 10 mM in ethanol then aliquoted and frozen at −80 °C. Aliquots were thawed, used then discarded to prevent oxidation of the compound. Kinases inhibitors SB203580, SB202190, JNK II inhibitor, PD98059 and compound C were obtained from Calbiochem (La Jolla, CA). Inhibitors were dissolved in DMSO and stored at −80°C. The specific SirT1 activator SRT1720 was from Sirtris Pharmaceuticals Inc. (Cambridge, MA), and SirT1 inhibitors Ex-242, Ex-243 and Ex-635 [21] were from Elixir Pharmaceuticals (Cambridge, MA). Activin A was purchased from R&D Systems. Antibodies to phospho-p38, phospho-AMPK, phospho-JNK, phospho-ERK, Smad2/3, phospho-Smad2, phospho-Smad3, SirT1, and acetylated-p53 were from Cell Signaling Technology (Denvers, MA); antibodies to Smad7 were from IMGENEX (San Diego, CA). Mouse LβT2 cells were cultured in DMEM (containing 4.5 g/L glucose) containing 10% fetal bovine serum and 1% Penicillin/Streptomycin and 1% Glutamax. Cell starvation media contains 10% DMEM plus 0.1% BSA. LβT2 cells were starved overnight and treated with or without 12.5 ng/ml activin A, or as otherwise stated. Resveratrol or SRT1720 was added for the indicated time and concentration.
2.2 Quantitative real-time PCR
In experiments to test whether resveratrol alters basal gonadotropin gene expression, LβT2 cells were starved overnight then treated with increasing doses of resveratrol (25 – 100 µM) for 4 h. For experiments to test whether resveratrol or SRT1720 alters activin-stimulated gonadotropin expression, LβT2 cells were starved overnight in the presence or absence of 12.5 ng/ml activin A before addition of 100 µM resveratrol or 10 µM SRT1720 for a further 4 h. To test whether resveratrol or SRT1720 prevents the acute activin induction of FSHβ, LβT2 cells were starved overnight then extensively washed to remove any endogenously secreted activin before adding 12.5 ng/ml activin and 100 µM resveratrol or 10 µM SRT1720 simultaneously for 6 h.
In all experiments, RNA was extracted from LβT2 cells with RNA Bee (Tel-Test, Friendswood, TX) according to the manufacturer’s instructions. One µg total RNA was reverse transcribed using a High Capacity cDNA synthesis kit (Applied Biosystem Inc., Foster City, CA). Quantitative real-time PCR was performed by using the iQ SYBR Green Mastermix PCR Kit (Biorad, Hercules, CA) using the following primers: FSHβ forward, GACAGCTGACTGCACAGGAC; FSHβ reverse, CAATCTTACGGTCTCGTATACC; LHβ forward, CTGTCAACGCAACTCTGG; LHβ reverse, ACAGGAGGCAAAGCAGC; the ribosomal protein RPL19 forward, TCATGGAGCACATCCACAAG; and RPL19 reverse, GTGCTTCCTTGGTCTTAGAC. QPCR was carried out under the following conditions: 95 °C for 5 min, followed by 40 cycles at 95 °C for 15 sec, 56 °C for 30 sec, and 72 °C for 30 sec. Each sample was assayed in triplicate or quadruplicate, and the experiment was repeated three to five times. Replicates were averaged and divided by the mean value of the control gene RPL19 in the same sample. After each run, a melting curve analysis was performed to confirm that a single amplicon was generated in each reaction. Data are presented as relative mRNA level compared to basal untreated cells after normalization to RPL19.
2.3 Western blotting
To determine the time course of kinase activation, starved LβT2 cells were stimulated with 25 µM resveratrol for 1–24 h then cells were rinsed with PBS twice and lysed with lysis buffer [20 mM Tris-HCl (pH 7.4), 140 mM NaCl, 0.5% Nonidet P-40, 0.5 mM EDTA, with protease inhibitors (aprotinin, pepstatin, and leupeptin at 10 µg/ml each), and 1 mM phenylmethylsulfonyl fluoride]. For the inhibitor studies, cells were pretreated with vehicle, 10 µM Compound C to inhibit AMPK, 10 µM SB203580 to inhibit p38MAPK, 10 µM JNKII inhibitor to inhibit JNK, or 20 µM PD98059 to inhibit ERK, 50 µM Ex-243 to inhibit SirT1, or 50 µM Ex-242 as a control for Ex-243. For stimulation of cells with agonists, LβT2 cells were starved overnight in the presence or absence of 12.5 ng/ml activin A before addition of 100 µM resveratrol for a further 4 h.
In all cases, protein concentrations were determined with Bradford reagent (Bio-Rad), and an equal amount of protein per sample was loaded on SDS-PAGE gels. After proteins had been resolved by electrophoresis and transferred to polyvinylidene difluoride membrane, they were probed with specific primary antibodies. The bands were detected with secondary antibodies linked to horseradish peroxidase and enhanced chemiluminescence reagent (Amersham Pharmacia, Piscataway, NJ). Western blots were quantified by GeneGnome Bio Imaging chemiluminescence reader (Syngene, Frederick, MD).
2.4 siRNA knockdown of SirT1
siRNA oligos for control scrambled RNA and SirT1 ON-TARGET plus SMARTpool were from Dharmacon Inc. siRNAs were micro-corporated into LβT2 cells using a microporator (Harvard Instruments, Cambridge, MA) at concentration of 5 µM. Cells were cultured for 72 hours after microporation before the starvation and stimulation for subsequent experiments.
2.5 FSH/LH Secretion Assay
LβT2 cells were seeded in 6-well dishes. Cells were serum starved overnight in DMEM containing 0.1% BSA and then treated with 100 µM resveratrol and 25 ng/ml activin for 9 h. After agonist treatment, cells were washed with DMEM containing 0.1% BSA three times and incubated in the same media (300 µl/well) to allow secretion. The conditioned medium was collected and centrifuged to remove the residual cell debris. Cells were lysed in RIPA buffer (300 µl/well), and cellular protein concentrations were measured. Mouse FSH and LH in both conditioned media and cell lysates were measured by the Ligand Core at the Center for Research in Reproduction at the University of Virginia. All values were normalized to total cellular protein concentrations.
2.6 Statistical Analysis
Data were analyzed by one way-ANOVA followed by Tukey post hoc tests. Individual pair-wise comparisons were performed using two-tailed t test. Analysis was performed using Excel (Microsoft, Redmond, WA) or Prizm (GraphPad Software, Inc., San Diego, CA). Unless otherwise stated, graphs show the mean and standard error. Letters indicate statistical significance (p<0.05), bars with the same letter are not significantly different.
3. Results
3.1 Resveratrol represses FSHβ expression in LβT2 cells
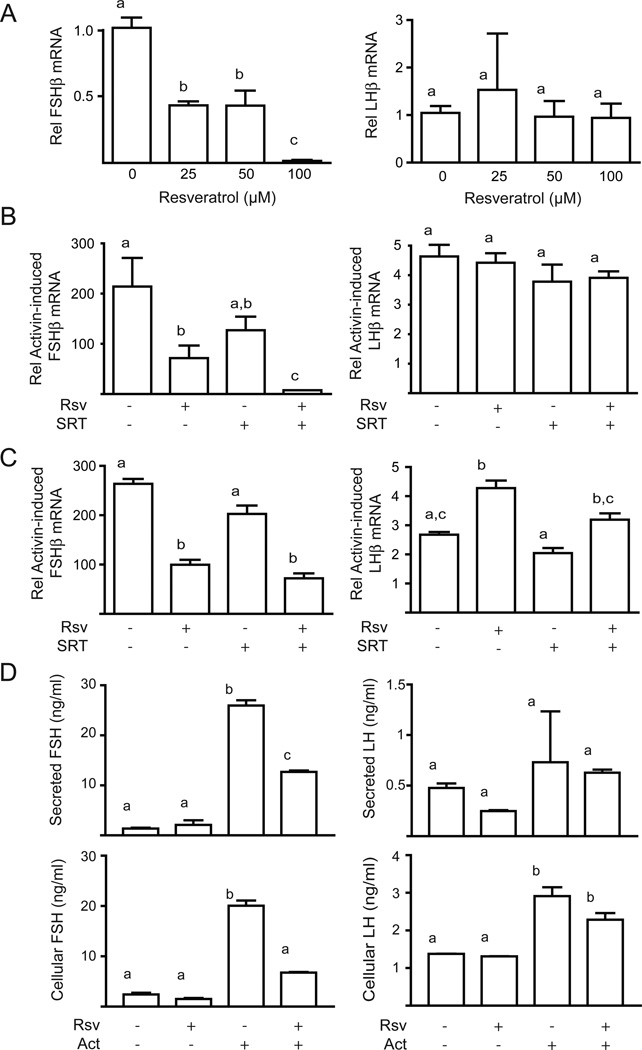
The initial experiments tested whether resveratrol alters basal gonadotropin gene expression. LβT2 cells were starved overnight then treated with increasing doses of resveratrol (25 – 100 µM) for 4 h. Resveratrol caused a dose-dependent decrease in follicle-stimulating hormone β (FSHβ) mRNA but had no effect on luteinizing hormone β (LHβ) mRNA (Figure 1A). We then tested the effect of resveratrol on activin-stimulated gonadotropin gene expression, as activin is the major driver for FSHβ expression. Activin induced a dramatic increase in FSHβ mRNA (200-fold), whereas the induction of LHβ mRNA was more modest (4-fold). Resveratrol reduced activin-induced FSHβ mRNA levels but had no effect on LHβ levels (Figure 1B). As resveratrol has been shown to be a SirT1 activator, we tested the effect of the specific SirT1 activator SRT1720 on FSHβ expression. The effect of SRT1720 was weaker than resveratrol and did not reach significance, however, simultaneous addition of both agonists had an additive effect (Figure 1B). As observed for resveratrol, SRT1720 did not repress LHβ expression.
Figure 1. Resveratrol represses FSHβ expression.
Panel A: Dose dependent effect of resveratrol on FSHβ and LHβ mRNA expression. Starved LβT2 cells were treated with increasing doses of resveratrol as indicated. Data are from three experiments performed in triplicate. Panel B: Resveratrol reverses the effect of chronic activin to increase FSHβ and LHβ mRNA expression. LβT2 cells were starved overnight with or without activin (Act), then stimulated with resveratrol (Rsv) or SRT1720 (SRT). Data are from five experiments performed in quadruplicate. Panel C: Resveratrol prevents activin induction of FSHβ and LHβ mRNA expression. Starved cells were treated acutely with activin and resveratrol or SRT1720. Data are from two experiments performed in duplicate. Panel D: Resveratrol inhibits activin-stimulated FSH and LH expression and secretion. Starved LβT2 cells were treated with resveratrol (Rsv) and activin (Act). Graphs show the mean and standard deviation LH and FSH levels in conditioned media or cell lysates from two experiments in triplicate.
The previous experiments showed that resveratrol could reverse elevated FSHβ expression due to activin pre-treatment, so we then tested whether resveratrol could prevent the activin induction by cotreating cells with activin and resveratrol. Activin induced FSHβ expression very strongly (250-fold) and again resveratrol significantly reduced activin-stimulated FSHβ mRNA levels (Figure 1C). The SirT1 activator SRT1720 did not reduce FSHβ mRNA and unlike the previous experiment did not have an additive effect with resveratrol (Figure 1C). In contrast to the earlier experiment, LHβ was stimulated by acute treatment with resveratrol and activin, but as before SRT1720 had no effect (Figure 1C).
We then tested whether resveratrol altered gonadotropin protein expression. Activin treatment increased cellular FSH levels 20-fold and resveratrol blunted this effect by 60% (Figure 1D). Similarly, activin increased FSH secretion 25-fold and resveratrol reduced this effect by 50%. Cellular LH levels, on the other hand, were only slightly induced by activin (3-fold) but secretion was unchanged (Figure 1D). Resveratrol had no effect on LH protein levels. Thus, the protein expression and secretion results were in agreement with the inhibition of FSHβ at transcriptional level.
3.2 Resveratrol activates AMPK and MAP kinases JNK, ERK and p38MAPK
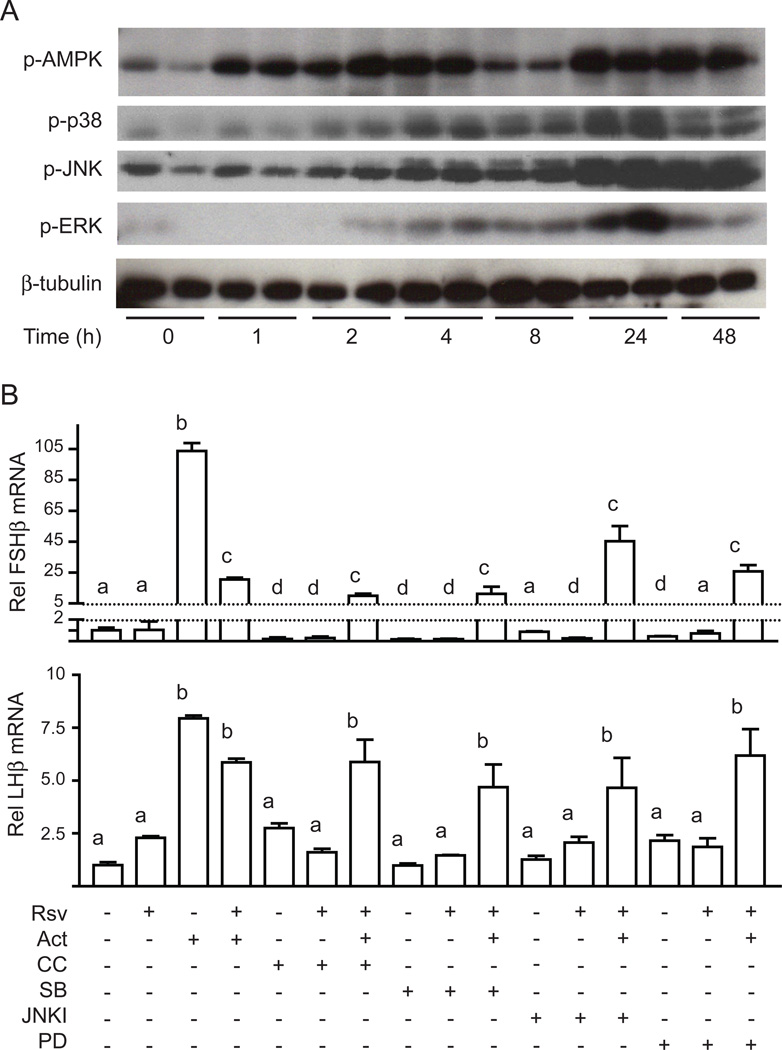
As gonadotropin gene expression is regulated by a number of signaling cascades, including the MAPK family of kinases, we investigated whether resveratrol activated or modulated these signaling pathways. Initially we tested whether resveratrol activates AMPK, a key kinase that regulates energy balance. In LβT2 cells, resveratrol caused a time-dependent increase in AMPK phosphorylation starting at 1 h, reaching a peak at 24 h and remaining elevated for 48 h (Figure 2A). A dose-response study performed at 4 h showed that 10–100 µM resveratrol was sufficient to activate AMPK maximally (Figure S1). We then assessed MAPKinase activation. Resveratrol caused a time-dependent activation of c-jun kinase (JNK), extracellular signal-regulated kinase (ERK) and p38MAPK (Figure 2A) with activation first apparent at 4 h and peaking at 24 h (Figure 2A). These results suggested a potential link between resveratrol and the regulation of gonadotropin gene expression.
Figure 2. Resveratrol activates AMPK, JNK, ERK, and p38MAPK.
Panel A: Time course of resveratrol activation of AMPK, JNK, ERK and p38. Cell lysates were immunoblotted for pAMPK, pJNK, pERK or p-p38MAPK. Panel B: Inhibitors to AMPK and MAP kinases do not prevent repression of FSHβ mRNA expression by resveratrol. The pharmacological inhibitors compound C (CC, AMPK inhibitor), SB203580 (SB, p38MAPK inhibitor), JNKII inhibitor (JNKI) or PD98059 (PD, MEK inhibitor) were added 30 min prior to resveratrol.
To address whether repression of FSHβ by resveratrol is mediated by AMPK or the MAPKs, we utilized specific pharmacological inhibitors at concentrations that we have previously shown to be effective in LβT2 cells to block phosphorylation of downstream targets such as Acetyl-CoA Carboxylase, Serum Response Factor, c-jun or Activating Transcription Factor-2. Pretreatment of cells with Compound C to inhibit AMPK, SB203580 to inhibit p38MAPK, JNKII inhibitor to inhibit JNK, or PD98059 to inhibit ERK, did not prevent the repression of FSHβ by resveratrol (Figure 2B). These inhibitors also had no effect on LHβ expression (Figure 2B). These results suggested that the repression of activin-induced FSHβ expression by resveratrol is likely mediated via molecular pathways other than AMPK and the MAPKs. Resveratrol has also been reported to act via the ER, retinoic acid receptor (RAR), retinoid-X receptor (RXR), aryl hydrocarbon receptor (ArhR), sulphonyl-urea receptor (SUR1), or cannabinoid receptor (CB1) but we were unable to document the involvement of any of these receptors in the repression of FSHβ using a variety of agonists and antagonists (Figure S2 and S3).
3.3 Resveratrol-mediated FSHβ repression is SirT-1 independent
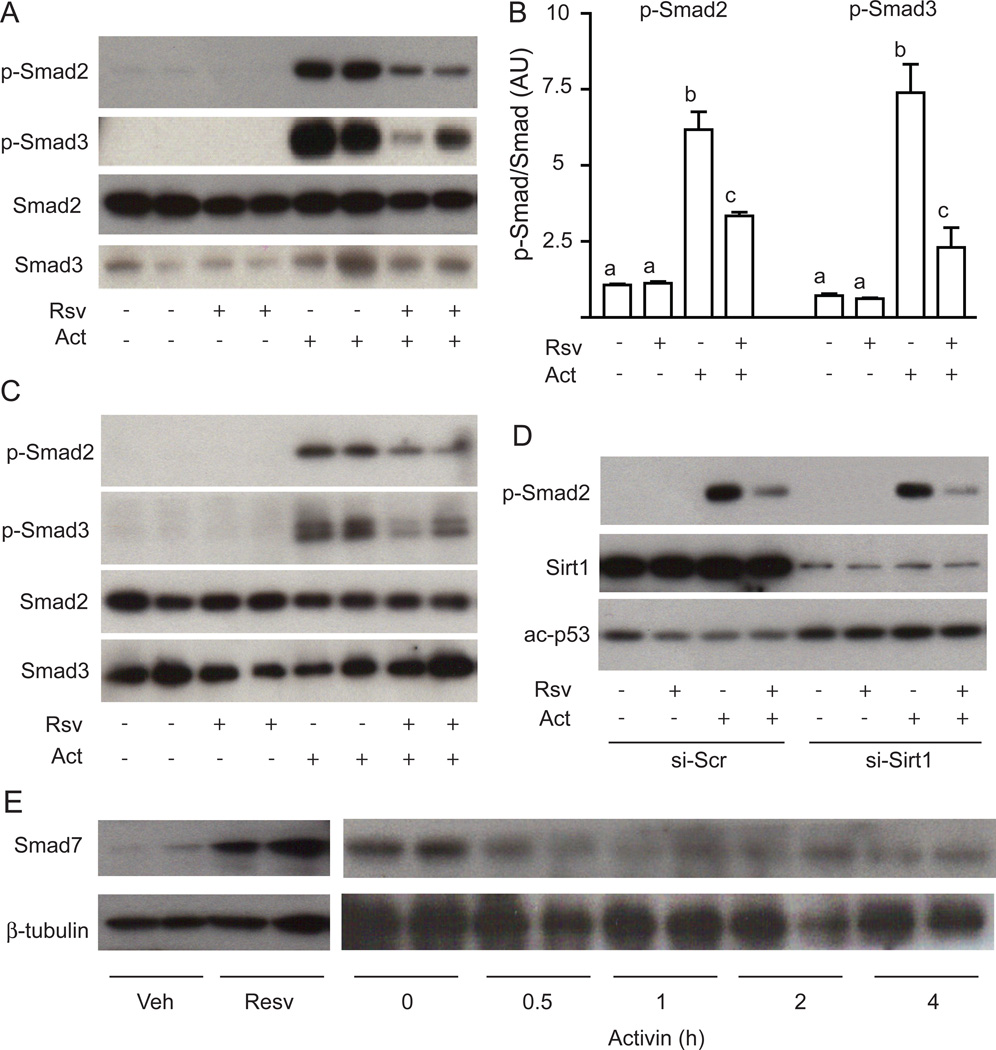
Resveratrol is thought to be a SirT1 activator but our data with SRT1720 did not mimic resveratrol, therefore we investigated whether SirT1 mediates the repressive effects of resveratrol FSHβ expression. To initially test the role of SirT1, we used small molecule inhibitors of SirT1, Ex-243 and Ex-635. We used an inactive analog Ex-242 as a negative control. The SirT1 inhibitor Ex-243 increased the acetylation of p53 (Figure 3A) verifying its inhibitory activity but did not increase FSHβ expression in the basal state nor did it alter the inhibition of activin-stimulated FSHβ (Figure 3B). The inactive analog Ex-242 had no effect as expected, and the second inhibitor Ex-635 also had no effect (Figure 3B). We then took a genetic approach to deplete SirT1. We verified that resveratrol treatment does not alter total SirT1 protein expression (Figure 4A). We introduced SirT1 siRNA oligos into LβT2 cells by microporation resulting in a >90% loss of endogenous SirT1 (Figure 4B). Resveratrol still repressed activin-induced FSHβ expression in the absence of SirT1 (Figure 4C). The SirT1 knockdown also did not alter LHβ expression as expected (Figure 4C).
Figure 3. SirT1 inhibition does not prevent repression of FSHβ expression.
Panel A: SirT1 inhibitor Ex-243 increases acetylation of p53. Cells were treated with Ex-242, Ex-243 or vehicle (Ctl) and then whole cell lysates were immunoblotted for acetylated p53. The membrane was stripped and reblotted for Smad2 to demonstrate equal protein loading. Panel B: SirT1 inhibition does not prevent the repressive effect of resveratrol on FSHβ mRNA expression. Cells were starved overnight with or without activin (Act) then Ex-242, Ex-243, Ex-635 or vehicle was added for 4 h and finally resveratrol (Rsv) was added for a further 4 h.
Figure 4. The repressive effect of resveratrol on FSHβ expression and Smad2 phosphorylation is independent of SirT1.
Panel A: Treatment of cells with increasing doses (25 µM, 50 µM and 100 µM) of resveratrol (Rsv) for 4 h does not change SirT1 protein levels. Panel B: SirT1 protein was knocked down by siRNA. Cell lysates were isolated from cells microporated with SirT1 (si-SirT1) or scrambled (si-Scr) siRNA and immunoblotted for SirT1. Panel C: Effect of SirT1 knockdown on FSHβ mRNA expression. Seventy-two hours after siRNA electroporation, cells were starved overnight with or without activin (Act) then stimulated with resveratrol (Rsv).
3.4 Resveratrol down-regulates Smad2/3 phosphorylation
Given that resveratrol’s effects were specific for FSHβ and not LHβ, we investigated activin signaling via Smad2/3, as these have been shown to regulate FSH expression. As expected, activin treatment causes a significant induction of Smad2 and Smad3 phosphorylation in LβT2 cells (Figure 5A). Subsequent treatment with resveratrol blunts the phosphorylation of Smad2 and Smad3 (Figure 5B). Similar results were obtained when resveratrol was added simultaneously with activin (Figure 5C). The observed decrease in Smad phosphorylation with resveratrol is consistent with the specific repression of activin-induced FSHβ expression. We then assessed whether the knockdown of SirT1 altered Smad phosphorylation. SirT1 was knocked down by microporation of SirT1 siRNA as before, but the knockdown did not prevent the inhibition of activin-mediated Smad2 phosphorylation by resveratrol (Figure 5D), although it did increase acetylation of p53, a known target of SirT1. We were unable to demonstrate acetylation of Smad2 and Smad3 by immunoprecipitation and immunoblotting experiments, and resveratrol did not alter acetylation of Smad2 and Smad3 (data not shown). This data suggests that the ability of resveratrol to regulate Smad phosphorylation is independent of SirT1.
Figure 5. Resveratrol represses activin-induced phosphorylation of Smad2 and Smad3.
Panel A: Resveratrol reverses activin-induced phosphorylation of Smad2 and Smad3. LβT2 cells were starved overnight with or without activin (Act) then stimulated with resveratrol (Rsv). Cell lysates were immunoblotted for phospho-Smad2, phospho-Smad3, Smad2, or Smad3. Panel B: Quantification of Smad2 and Smad3 phosphorylation. Panel C: Resveratrol inhibits acute phosphorylation of Smad2 and Smad3. Starved cells were washed extensively then treated with resveratrol (Rsv) before stimulation with activin (Act). Cell lysates were immnoblotted for phospho-Smad2 and phospho-Smad3 as before. Panel D: Knockdown of SirT1 does not increase Smad2 phosphorylation. SirT1 or scrambled (Scr) siRNA oligos were microporated into LβT2 cells then cells were stimulated with or without activin (Act) then with resveratrol (Rsv). Cell lysates were immunoblotted for phospho-Smad2, SirT1, and acetylated p53. Panel E: Resveratrol induces Smad7 expression. Starved LβT2 cells were treated with resveratrol (Resv) or activin (Act). Cell lysates were immunoblotted for Smad7 then stripped and reblotted for β-tubulin.
Phosphorylation of the signaling Smads by the TGFβ family receptors can be antagonized by expression of the inhibitory Smads 6 and 7. Therefore, we determined whether resveratrol alters expression of Smad7 under conditions where we observe repression of FSHβ. An acute treatment with resveratrol showed a significant increase of Smad7 protein (Figure 5E). Acute activin treatment did not induce Smad7 expression (Figure 5E) although Smad7 expression can be induced by chronic activin signaling as part of a negative feedback loop (data not shown).
4. Discussion
Resveratrol has multiple reported beneficial effects on reproduction in animal models. Treatment of immature female rats with sub-cutaneous resveratrol increases uterine wet weight via thickening the columnar epithelial cells and the number of glands [22]. Resveratrol also prevents the teratogenic effects of dioxin in pregnant mice [23] and prevents embryonic stress in diabetic rats [24]. Not all studies concur, however, as prepubertal exposure of rats to 100 mg/kg resveratrol for 5 days results in early vaginal opening and irregular estrous cycles with a prolonged estrus phase [25] but oral treatment of outbred CD-1 mice with resveratrol (3 mg/l in drinking water) for four weeks had no effect on reproductive indices [26]. The beneficial effects of resveratrol are not limited to female rodents as resveratrol decreases oxidative stress in human sperm and rat germinal cells [27] and reestablishes spermatogenesis after testicular injury in rats [28]. Despite these promising effects, our understanding of its function and impact on reproduction and development, however, is very limited [29]. To this end, in this study we documented the effect of resveratrol on pituitary gonadotropin hormones.
We observed that resveratrol represses basal and activin-induced FSHβ gene expression and FSH protein synthesis and secretion in vitro. This inhibition appears to be independent of SirT1 as inhibition of SirT1 by RNAi knockdown or using a pharmacological inhibitor does not block the repressive effect and the inhibition is only partially mimicked by another SirT1 activator. We showed that resveratrol activates many signaling pathways that are known to be important for regulation of gonadotropin expression, such as ERK, JNK, and p38MAPK, but inhibition of these pathways is without effect [30, 31]. Furthermore, peak activation of the MAPKs also occurs after the effect of resveratrol to reduce FSHβ expression, so these pathways are unlikely to be involved. Another reported target for resveratrol is AMPK [32, 33]. Although resveratrol activates AMPK within 1 h, we do not believe that AMPK mediates the resveratrol effect as the AMPK inhibitor compound C does not prevent the repression of FSHβ by resveratrol. We were also unable to document the involvement of the ER, RAR, RXR, ArhR, SUR1, or CB1 receptors in the resveratrol effect, so the cellular target for resveratrol in these pituitary cells remains unknown. At the mechanistic level, the repression of FSHβ is likely mediated by an inhibition of Smad2 and Smad3 phosphorylation downstream of the activin receptor as these Smads are potent inducers of FSHβ expression [34]. This decreased phosphorylation was paralleled by an increase in Smad7 expression that may compete with the signaling Smads for the activin receptor [34–36]. We did not observe Smad2 and Smad3 acetylation in contrast to reports in other cells [37]. It remains to be determined whether the increase in Smad7 is transcriptional or post-transcriptional, and whether this accounts for the decreased Smad2/3 phosphorylation.
Although we do not see a role for SirT1 in the repression of FSH in LβT2 gonadotrope cells, SirT1 has been implicated in reproductive fitness. The SirT1-knockout is a perinatal lethal on an inbred 129/J background but the SirT1 knockout mice survive to adulthood if crossed to an outbred background [38]. At the reproductive level, the null mice are sterile; the males have abnormal sperm morphology and increased germ cell apoptosis, whereas the females are arrested in diestrus and have small ovaries with no corpora lutea. Superovulation of the females induces release of eggs into the oviduct, suggesting that the defect is central. Overexpression of SirT1 is also associated with impaired reproduction [39]. Given these findings, we were surprised that the repressive effect of resveratrol was independent of SirT1. It has been reported that resveratrol is not a direct activator of SirT1, however, and our study supports the notion of SirT1-independent effects of this compound [9, 10]. It is possible that another sirtuin family member mediates the effect on FSHβ. Indeed, SirT2 is induced by caloric restriction in adipocytes and represses adipogenesis by deacetylating the forkhead box protein FoxO1 [40], and SirT3 deacetylates and activates the serine/threonine kinase 11 (LKB1) to prevent cardiac hypertrophy [41]. The sirtuins SirT2, SirT3, SirT4, and SirT7 are expressed at the mRNA level in LβT2 cells (data not shown), so further studies will be needed to address whether these other sirtuins are involved in the resveratrol effects in the pituitary.
4.1 Conclusions
Our study documents that resveratrol represses basal and activin-driven FSHβ mRNA expression and FSH protein expression and secretion, which appears to be related to suppression of phosphorylation of Smad2 and 3. The activity was not dependent on SirT1 but we cannot rule out the involvement of another sirtuin. Our findings suggest that further studies will be needed to address a) whether resveratrol suppresses FSH in humans, b) how resveratrol suppresses Smad activation and FSH expression, and c) whether resveratrol supplementation is safe for women of reproductive age.
Supplementary Material
Acknowledgements
The authors would like to we would like to thank University of Virginia Center for Research in Reproduction (NICHD SCCPIR Grant U54-HD28934) for FSH and LH measurements. The authors also would like to thank Sirtris Inc (Cambridge, MA) for providing the SRT1720 compound, and Elixir Inc (Cambridge, MA) for providing SirT1 inhibitors Ex-242, Ex-243 and Ex-635.
This research was supported by the Eunice Kennedy Shriver NICHD/NIH through cooperative agreement [U54 HD012303 and HD28934] as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research, by NIH R01s HD047400 (N.J.G.W.), DK033651, DK074868, and DK063491 (J.M.O.) and HD020377 (P.L.M.), and by a VA Merit Review award (N.J.G.W).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE STATEMENT: The authors have nothing to disclose.
References
- 1.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Okawara M, Katsuki H, Kurimoto E, Shibata H, Kume T, Akaike A. Resveratrol protects dopaminergic neurons in midbrain slice culture from multiple insults. Biochem Pharmacol. 2007;73:550–560. doi: 10.1016/j.bcp.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Sharma S, Anjaneyulu M, Kulkarni SK, Chopra K. Resveratrol, a polyphenolic phytoalexin, attenuates diabetic nephropathy in rats. Pharmacology. 2006;76:69–75. doi: 10.1159/000089720. [DOI] [PubMed] [Google Scholar]
- 4.Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Penumathsa SV, Maulik N. Resveratrol: a promising agent in promoting cardioprotection against coronary heart disease. Can J Physiol Pharmacol. 2009;87:275–286. doi: 10.1139/Y09-013. [DOI] [PubMed] [Google Scholar]
- 6.Chaudhary N, Pfluger PT. Metabolic benefits from Sirt1 and Sirt1 activators. Curr Opin Clin Nutr Metab Care. 2009;12:431–437. doi: 10.1097/MCO.0b013e32832cdaae. [DOI] [PubMed] [Google Scholar]
- 7.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 8.Allard JS, Heilbronn LK, Smith C, Hunt ND, Ingram DK, Ravussin E, et al. In vitro cellular adaptations of indicators of longevity in response to treatment with serum collected from humans on calorie restricted diets. PLoS One. 2008;3:e3211. doi: 10.1371/journal.pone.0003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pacholec M, Bleasdale JE, Chrunyk B, Cunningham D, Flynn D, Garofalo RS, et al. SRT1720, SRT2183, SRT1460, and Resveratrol Are Not Direct Activators of SIRT1. J Biol Chem. 2010;285:8340–8351. doi: 10.1074/jbc.M109.088682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beher D, Wu J, Cumine S, Kim KW, Lu SC, Atangan L, et al. Resveratrol is not a direct activator of SIRT1 enzyme activity. Chem Biol Drug Des. 2009;74:619–624. doi: 10.1111/j.1747-0285.2009.00901.x. [DOI] [PubMed] [Google Scholar]
- 11.Dasgupta B, Milbrandt J. Resveratrol stimulates AMP kinase activity in neurons. Proc Natl Acad Sci U S A. 2007;104:7217–7222. doi: 10.1073/pnas.0610068104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hardie DG. AMPK: a key regulator of energy balance in the single cell and the whole organism. Int J Obes (Lond) 2008;32 Suppl 4:S7–S12. doi: 10.1038/ijo.2008.116. [DOI] [PubMed] [Google Scholar]
- 13.Stewart JR, O'Brian CA. Resveratrol antagonizes EGFR-dependent Erk1/2 activation in human androgen-independent prostate cancer cells with associated isozyme-selective PKC alpha inhibition. Invest New Drugs. 2004;22:107–117. doi: 10.1023/B:DRUG.0000011787.75522.ec. [DOI] [PubMed] [Google Scholar]
- 14.Szewczuk LM, Forti L, Stivala LA, Penning TM. Resveratrol is a peroxidase-mediated inactivator of COX-1 but not COX-2: a mechanistic approach to the design of COX-1 selective agents. J Biol Chem. 2004;279:22727–22737. doi: 10.1074/jbc.M314302200. [DOI] [PubMed] [Google Scholar]
- 15.Kennedy A, Overman A, Lapoint K, Hopkins R, West T, Chuang CC, et al. Conjugated linoleic acid-mediated inflammation and insulin resistance in human adipocytes are attenuated by resveratrol. J Lipid Res. 2009;50:225–232. doi: 10.1194/jlr.M800258-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang OH, Jang HJ, Chae HS, Oh YC, Choi JG, Lee YS, et al. Anti-inflammatory mechanisms of resveratrol in activated HMC-1 cells: pivotal roles of NF-kappaB and MAPK. Pharmacol Res. 2009;59:330–337. doi: 10.1016/j.phrs.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 17.Aquilano K, Baldelli S, Rotilio G, Ciriolo MR. trans-Resveratrol inhibits H2O2-induced adenocarcinoma gastric cells proliferation via inactivation of MEK1/2-ERK1/2-c-Jun signalling axis. Biochem Pharmacol. 2009;77:337–347. doi: 10.1016/j.bcp.2008.10.034. [DOI] [PubMed] [Google Scholar]
- 18.Bowers JL, Tyulmenkov VV, Jernigan SC, Klinge CM. Resveratrol acts as a mixed agonist/antagonist for estrogen receptors alpha and beta. Endocrinology. 2000;141:3657–3667. doi: 10.1210/endo.141.10.7721. [DOI] [PubMed] [Google Scholar]
- 19.Gehm BD, McAndrews JM, Chien PY, Jameson JL. Resveratrol, a polyphenolic compound found in grapes and wine, is an agonist for the estrogen receptor. Proc Natl Acad Sci U S A. 1997;94:14138–14143. doi: 10.1073/pnas.94.25.14138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sevov M, Elfineh L, Cavelier LB. Resveratrol regulates the expression of LXR-alpha in human macrophages. Biochem Biophys Res Commun. 2006;348:1047–1054. doi: 10.1016/j.bbrc.2006.07.155. [DOI] [PubMed] [Google Scholar]
- 21.Solomon JM, Pasupuleti R, Xu L, McDonagh T, Curtis R, DiStefano PS, et al. Inhibition of SIRT1 catalytic activity increases p53 acetylation but does not alter cell survival following DNA damage. Mol Cell Biol. 2006;26:28–38. doi: 10.1128/MCB.26.1.28-38.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh M, Parent S, Leblanc V, Asselin E. Resveratrol Modulates the Expression of PTGS2 and Cellular Proliferation in the Normal Rat Endometrium in an AKT-Dependent Manner. Biol Reprod. doi: 10.1095/biolreprod.110.090076. [DOI] [PubMed] [Google Scholar]
- 23.Jang JY, Park D, Shin S, Jeon JH, Choi BI, Joo SS, et al. Antiteratogenic effect of resveratrol in mice exposed in utero to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Eur J Pharmacol. 2008;591:280–283. doi: 10.1016/j.ejphar.2008.05.033. [DOI] [PubMed] [Google Scholar]
- 24.Singh CK, Kumar A, Hitchcock DB, Fan D, Goodwin R, Lavoie HA, et al. Resveratrol prevents embryonic oxidative stress and apoptosis associated with diabetic embryopathy and improves glucose and lipid profile of diabetic dam. Mol Nutr Food Res. doi: 10.1002/mnfr.201000457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sato M, Pei RJ, Yuri T, Danbara N, Nakane Y, Tsubura A. Prepubertal resveratrol exposure accelerates N-methyl-N-nitrosourea-induced mammary carcinoma in female Sprague-Dawley rats. Cancer Lett. 2003;202:137–145. doi: 10.1016/j.canlet.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 26.Kyselova V, Peknicova J, Buckiova D, Boubelik M. Effects of p-nonylphenol and resveratrol on body and organ weight and in vivo fertility of outbred CD-1 mice. Reprod Biol Endocrinol. 2003;1:30. doi: 10.1186/1477-7827-1-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collodel G, Federico MG, Geminiani M, Martini S, Bonechi C, Rossi C, et al. Effect of trans-resveratrol on induced oxidative stress in human sperm and in rat germinal cells. Reprod Toxicol. doi: 10.1016/j.reprotox.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 28.Jiang YG, Peng T, Luo Y, Li MC, Lin YH. Resveratrol reestablishes spermatogenesis after testicular injury in rats caused by 2, 5-hexanedione. Chin Med J (Engl) 2008;121:1204–1209. [PubMed] [Google Scholar]
- 29.Henry LA, Witt DM. Effects of neonatal resveratrol exposure on adult male and female reproductive physiology and behavior. Dev Neurosci. 2006;28:186–195. doi: 10.1159/000091916. [DOI] [PubMed] [Google Scholar]
- 30.Lu M, Tang Q, Olefsky JM, Mellon PL, Webster NJ. Adiponectin activates adenosine monophosphate-activated protein kinase and decreases luteinizing hormone secretion in LbetaT2 gonadotropes. Mol Endocrinol. 2008;22:760–771. doi: 10.1210/me.2007-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharma S, Sharma PM, Mistry DS, Chang RJ, Olefsky JM, Mellon PL, et al. PPARG Regulates Gonadotropin-Releasing Hormone Signaling in LbetaT2 Cells In Vitro and Pituitary Gonadotroph Function In Vivo in Mice. Biol Reprod. doi: 10.1095/biolreprod.110.088005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suchankova G, Nelson LE, Gerhart-Hines Z, Kelly M, Gauthier MS, Saha AK, et al. Concurrent regulation of AMP-activated protein kinase and SIRT1 in mammalian cells. Biochem Biophys Res Commun. 2009;378:836–841. doi: 10.1016/j.bbrc.2008.11.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Breen DM, Sanli T, Giacca A, Tsiani E. Stimulation of muscle cell glucose uptake by resveratrol through sirtuins and AMPK. Biochem Biophys Res Commun. 2008;374:117–122. doi: 10.1016/j.bbrc.2008.06.104. [DOI] [PubMed] [Google Scholar]
- 34.Bernard DJ. Both SMAD2 and SMAD3 mediate activin-stimulated expression of the follicle-stimulating hormone beta subunit in mouse gonadotrope cells. Mol Endocrinol. 2004;18:606–623. doi: 10.1210/me.2003-0264. [DOI] [PubMed] [Google Scholar]
- 35.Nakao A, Afrakhte M, Moren A, Nakayama T, Christian JL, Heuchel R, et al. Identification of Smad7, a TGFbeta-inducible antagonist of TGF-beta signalling. Nature. 1997;389:631–635. doi: 10.1038/39369. [DOI] [PubMed] [Google Scholar]
- 36.Bilezikjian LM, Corrigan AZ, Blount AL, Chen Y, Vale WW. Regulation and actions of Smad7 in the modulation of activin, inhibin, and transforming growth factor-beta signaling in anterior pituitary cells. Endocrinology. 2001;142:1065–1072. doi: 10.1210/endo.142.3.8028. [DOI] [PubMed] [Google Scholar]
- 37.Tu AW, Luo K. Acetylation of Smad2 by the co-activator p300 regulates activin and transforming growth factor beta response. J Biol Chem. 2007;282:21187–21196. doi: 10.1074/jbc.M700085200. [DOI] [PubMed] [Google Scholar]
- 38.McBurney MW, Yang X, Jardine K, Hixon M, Boekelheide K, Webb JR, et al. The mammalian SIR2alpha protein has a role in embryogenesis and gametogenesis. Mol Cell Biol. 2003;23:38–54. doi: 10.1128/MCB.23.1.38-54.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bordone L, Cohen D, Robinson A, Motta MC, van Veen E, Czopik A, et al. SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell. 2007;6:759–767. doi: 10.1111/j.1474-9726.2007.00335.x. [DOI] [PubMed] [Google Scholar]
- 40.Wang F, Tong Q. SIRT2 suppresses adipocyte differentiation by deacetylating FOXO1 and enhancing FOXO1's repressive interaction with PPARgamma. Mol Biol Cell. 2009;20:801–808. doi: 10.1091/mbc.E08-06-0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pillai VB, Sundaresan NR, Kim G, Gupta M, Rajamohan SB, Pillai JB, et al. Exogenous NAD blocks cardiac hypertrophic response via activation of the SIRT3-LKB1-AMPK pathway. J Biol Chem. 2009 doi: 10.1074/jbc.M109.077271. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.