Abstract
17β-Estradiol (E2) regulates estrogen receptor-α (ERα) target gene transcription through the two independent activation functions (AFs), AF1 and AF2, located in the N-terminal and ligand binding domain of ERα, respectively. We previously reported that ERα is required for the E2 atheroprotective action as well as for its accelerative action on endothelial healing, but its AF1 function is dispensable. Here, we investigated the role of ERαAF2 in these two major beneficial actions of E2 by electively targeting ERαAF2 (named ERαAF20). Our results prove four points. (i) Compared with WT ERα, the ability of ERαAF20 to stimulate the C3 complement or the estrogen response element-thymidine kinase promoter in two cell lines was dramatically decreased, confirming the importance of AF2 in the E2-induced transcriptional activity of ERα. (ii) The uterotrophic action of E2 was totally absent in ERαAF20 mice, showing the crucial role of ERαAF2 in E2-induced uterus hyperplasia. (iii) ERαAF2 was dispensable for the accelerative action of E2 on endothelial healing, underlining the functionality of ERαAF20 in vivo. (iv) Finally, the atheroprotective effect of E2 was abrogated in ERαAF20 LDL-r−/− mice. Thus, whereas ERαAF1 and ERαAF2 are both required for the uterotrophic action of E2, we show that only ERαAF2 is necessary for its atheroprotective effect.
Keywords: atherosclerosis, nuclear receptor, transactivating function
Estrogens, and particularly, 17β-estradiol (E2), play a pivotal role in sexual development and reproduction and are also implicated in a large number of physiological processes, particularly in the cardiovascular system. Although epidemiological studies (1, 2) and the Nurses’ Health Study (3) suggested and all animal models of early atheroma (4, 5) clearly showed a vasculoprotective action of both endogenous and exogenous estrogens, the Women's Health Initiative did not confirm the preventive action of estrogens against coronary heart disease (CHD) (6, 7). However, women who initiate hormone therapy closer to menopause have reduced CHD risk compared with the increase in CHD risk among women more distant from menopause (8). It is clear that these hormones have important effects on vascular physiology and pathophysiology, with potential therapeutic implications such as acceleration of endothelial healing (9) and atheroprotection (10). Interestingly, a delay between ovariectomy and E2 replacement abrogates the prevention of atheroma in the monkey (4) as well as the mouse (11), suggesting that these animal models of atheroma could mimic the problem of timing observed in women in terms of coronary artery risk (2, 4, 12).
The action of E2 is mediated by two nuclear receptors, estrogen receptor-α (ERα) and ERβ (13), encoded by two distinct genes, Esr1 and Esr2, respectively. Both ERs belong to the nuclear receptor subfamily of ligand-inducible transcription factors whose members, based on structural and functional similarities, can be subdivided into six distinct regions termed A to F (13). Ligand-induced transcription of ER involves the action of two distinct activation functions (AF), AF1 and AF2, located in the N-terminal A/B and C-terminal E domains, respectively (14). On estrogen binding, ERs undergo a conformational change that facilitates the recruitment of coactivators and the direct (or indirect) binding to cis-acting elements, thereby activating the transcription of target genes (13). These AFs exhibit distinct transactivation properties, and the full transcriptional activity of ERα is thought to proceed through a synergism between these two functions, although only AF2 activity is entirely dependent on ligand binding (15). The time lag between steroid administration and observable effects produced by newly synthesized protein is typically in the order of hours to days, eliciting cascades of gene expression changes. However, rapid effects of E2, such as vasodilation, occur within minutes of steroid administration and involve a fraction of ER localized to the plasma membrane. These fast-acting membrane-initiated steroid signaling (MISS) effects lead to the modification of existing proteins and cells, including calcium and protein kinases (such as MAPK or PI3K) modulation (reviewed in refs. 16 and 17).
Mouse models targeted for either ERα or -β allowed us and others to show that ERα is absolutely necessary to the beneficial actions of E2 in reendothelialization (18), medial hyperplasia (19), and atheroma (20, 21). Indeed, all these actions are fully abrogated in ERα−/− mice that unambiguously lack ERα (18, 19, 22, 23). In addition, ERαAF1 is dispensable for two major vasculoprotective effects of E2, namely the acceleration of the reendothelialization process and the prevention of atheroma (24). The aim of the present work was to directly evaluate the involvement of ERαAF2 in these two major vasculoprotective effects of E2. To this end, we developed a mouse model deleted of 7 amino acids of the helix 12 and thereby, deficient in ERαAF2 (named ERαAF20).
Results
Generation of ERαAF20 Mice.
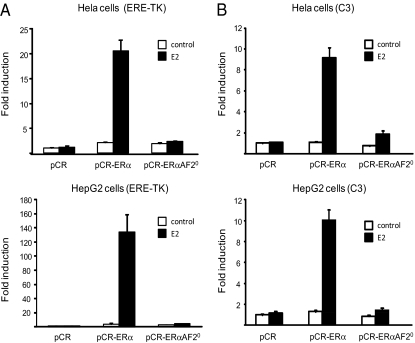
ERαAF2 activity requires a region in the C terminus of the mouse receptor at the level of the hormone binding domain between residues 539 and 554, which is conserved among many nuclear receptors (25). Different point mutagenesis or deletion of this conserved region showed that this function is essential for E2-induced transcriptional activation (25). To study the role of ERαAF2 in vivo, a mouse model was generated using a knock-in strategy in which amino acids 543–549 of ERα were deleted (Fig. 1 and Fig. S1). Beforehand, we examined the effect of deleting these residues from ERα on its ability to stimulate ERE-thymidine kinase (TK) (Fig. 2A) or C3 complement (Fig. 2B) promoters in transient transfection assays in two cell lines, HeLa and HepG2, previously characterized as devoid of endogenous ERs (26). As expected, whereas E2 induced ∼10- to 35-fold transcriptional activity using WT ERα construct, this action was dramatically reduced (less than twofold) using the ERαAF20 mutant (Fig. 2 A and B). These results showed the importance of AF2 in two cellular reporter models of the E2 transcriptional activity.
Fig. 1.
Generation of ERαAF20 null mutant mice. Schematic representation of the ERα gene: the transcripts and proteins expressed in WT mice (Upper) and mice with specific inactivation of the activation function AF2 in ERα (ERαAF20 mutant mice; Lower).
Fig. 2.
Validation of the excision of the ERαAF2 function. HeLa (A) and HepG2 (B) cells were transiently transfected with the ERE-TK LUC or C3-LUC reporter constructs in the presence of pCR-ERα, pCR- ERαAF20, or empty pCR vectors. Cells were treated with E2 (10 nM) or vehicle (control) for 24 h. Normalized luciferase activities were expressed as fold increase above values measured with empty pCR and vehicle. Data correspond to the mean values ± SEM of at least three separate transfection experiments.
Both ERαAF1 and AF2 have been shown to exert their transcriptional activity in a cell-specific manner. Accordingly, cell contexts can be defined as ERαAF1- or AF2-permissive depending on which AF is principally involved in ERα activity. For instance, the hepatocarcinoma cell line HepG2 is frequently used to assess ERαAF1 activity (26), and we found transcriptional activity of the ERαAF1-selective agonist tamoxifen (27) on C3 complement promoter in ERα-transfected cells (Fig. S2). Interestingly, transcriptional activity of tamoxifen is totally maintained using the ERαAF-20 mutant in HepG2 cells, whereas it is totally abolished with the ERαAF-10 construct (Fig. S2), showing that AF1 of the ERαAF20 construct is functional.
ERαAF2 Is Necessary for the E2 Uterotrophic Action.
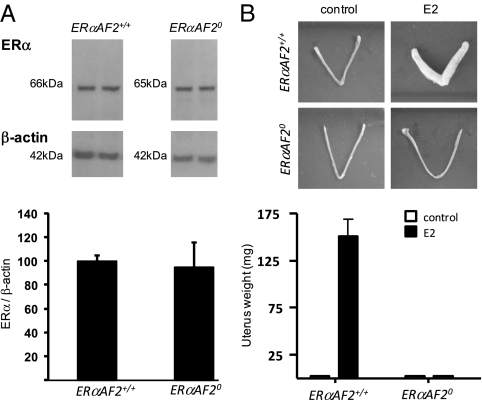
We then assessed ERα protein expression level in uteri from ERαAF2+/+ and ERαAF20 mice by Western blot. We found that the protein abundance of the ∼65-kDa ERα in the uterus from ERαAF20 mutant mice was quite similar to the 66-kDa ERα in control mice (Fig. 3A). We next explored the importance of ERαAF2 in the uterotrophic action of E2. Whereas E2 treatment elicited a major uterine hypertrophy in ERαAF2+/+ mice, this effect was completely abrogated in ERαAF20 mice (Fig. 3B), showing the crucial role of ERαAF2 in the E2-induced uterus growth.
Fig. 3.
ERαAF2 is necessary for the E2 uterotrophic action. (A) Representative Western blot of ERα and β-actin protein levels (Upper). Proteins from uteri of 10-wk-old ERαAF2+/+ and ERαAF20 nonovariectomized mice were loaded as described in Methods. Protein levels (n = 4 for each genotype) were quantified using ImageJ software (Lower). (B) Uterine weight from ERαAF2+/+ and ERαAF20 ovariectomized mice treated or not treated with E2 (80 μg/kg per d).
ERαAF2 Is Dispensable for the Effect of E2 on Reendothelialization.
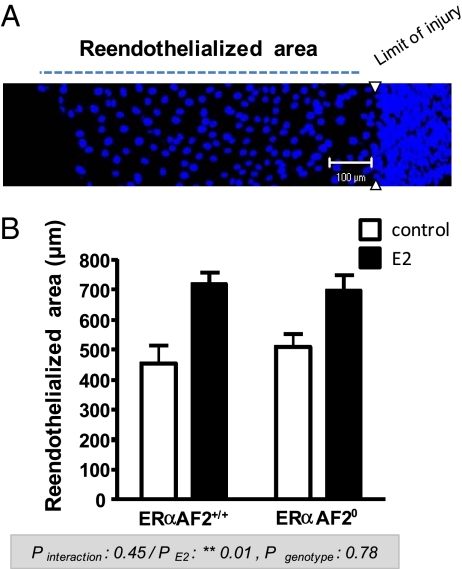
We previously reported that ERα, but not its AF1, is necessary to induce the accelerative effect of E2 in vivo (24). We then explored the role of ERαAF2 in this known vasculoprotective action of E2. As previously described (18), E2 accelerated the reendothelialization after carotid electric injury in WT mice (ERαAF2+/+). This beneficial action of E2 was similarly observed in ERαAF20 mice, showing that ERαAF2 is dispensable for the acceleration of reendothelialization in response to E2 (Fig. 4). Importantly, this result underlines the functionality of ERαAF20 protein in vivo.
Fig. 4.
ERαAF2 is dispensable for the effect of E2 on endothelial healing. (A) Representative en face confocal immunohistochemical analysis of the intima tunica of the carotid artery from an E2-treated mouse 72 h after surgery with designation of the regenerative endothelial area. Nuclei, stained with propidium iodide, appear in dark blue. (B) The effect of E2 was studied in the ERαAF2+/+ and ERαAF20 ovariectomized mice. Quantification (mean ± SEM) of the regenerative endothelial area was from an average of four mice per group. Analysis by a two-way ANOVA: effect of exogenous E2, P = 0.01; effect of genotype, not significant (P = 0.78); interaction, not significant (P = 0.45).
ERαAF2 Is Necessary for the E2 Atheroprotective Effect.
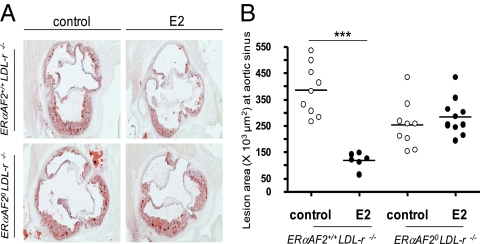
We then explored the role of ERαAF2 in a second well-known vasculoprotective action of E2 (i.e., the prevention of atherosclerosis). We previously reported that ERα, but not its AF1, is necessary to induce the atheroprotective effect of E2 in vivo (24). To evaluate the role of ERαAF2 in this process, we bred ERαAF2+/0 mice with LDL-r−/− mice to compare ERαAF20 LDL-r−/− with ERαAF2+/+ LDL-r−/− ovariectomized mice. Level of fatty streak deposit tended to be lower in ovariectomized ERαAF20 LDL-r−/− mice compared with ovariectomized ERαAF2+/+LDL-r−/− mice. Interestingly, we previously reported that ovariectomized ERα−/−LDL-r−/− also developed less lesion compared with ERα+/+LDL-r−/− mice (21) and that deletion of ERα in the endothelium (using TieCRE+ERαlox/loxLDL-r−/− mice) also had attenuated fatty streak deposit (20, 21). As expected, exogenous E2 significantly decreased fatty streak deposits at the aortic sinus in ovariectomized 18-wk-old ERαAF2+/+ LDL-r−/− mice fed with an hypercholesterolemic diet (Fig. 5 A and B and Table 1). In contrast, the atheroprotective effect of E2 was totally abrogated in ERαAF20LDL-r−/− mice. Furthermore, E2 also decreased macrophage infiltration and collagen accumulation in ERαAF2+/+LDL-r−/− but not in ERαAF20LDL-r−/− mice (Fig. S3). Altogether, these results show the crucial role of ERαAF2 in the atheroprotective effect of E2.
Fig. 5.
ERαAF2 is necessary for the effect of exogenous E2 on fatty streak deposit prevention in 18-wk-old mice. Four-week-old ovariectomized ERαAF2+/+LDL-r −/− and ERαAF20LDL-r −/− mice were given either placebo or E2 (80 μg/kg per d during 12 wk) and switched to an atherogenic diet from the age of 6–18 wk. (A) Representative micrographs of oil red-O lipid-stained cryosections of the aortic sinus. (B) Quantification of lesion area at the aortic sinus. Analysis was performed with a two-way ANOVA; because an interaction was observed between the two factors, effect of E2 treatment was studied in each genotype using a Bonferroni posttest (***P < 0.001).
Table 1.
Effect of E2 treatment on body weight, uterine weight, plasma lipid concentrations, and fatty streak lesion size on 18-wk-old AF2+/+ LDL-r−/− and AF20 LDL-r−/− mice
| AF2+/+LDL-r−/− |
AF20LDL-r−/− |
P (two-factor ANOVA) |
|||||
| Control (n = 9) | E2 (n = 6) | Control (n = 9) | E2 (n = 11) | Genotype | E2 | Interaction | |
| Body weight (g) | 22.8 ± 0.6 | 22.4 ± 0.4 | 21.6 ± 0.5 | 23.1 ± 0.5 | 0.62 | 0.37 | 0.09 |
| Uterine weight (mg) | <5 | 151 ± 18 | <5 | <5 | — | — | — |
| Total cholesterol (mg/dL) | 1,292 ± 153 | 732* ± 38 | 1,147 ± 120 | 1,167 ± 88 | 0.02 | ||
| HDL cholesterol (mg/dL) | 214 ± 19 | 143* ± 11 | 197 ± 10 | 198 ± 10 | 0.02 | ||
| Cholesterol/HDL cholesterol | 6.4 ± 11 | 5.1 ± 0.5 | 6.4 ± 0.8 | 5.9 ± 0.5 | 0.57 | 0.25 | 0.61 |
| Triglycerides (mg/dL) | 93.5 ± 11.4 | 84.6 ± 10.4 | 90.9 ± 8.4 | 93.0 ± 8.8 | 0.78 | 0.74 | 0.60 |
| Lesion (μm2) | 385,444 ± 32,280 | 118,783† ± 12,100 | 253,226 ± 29,358 | 282,321 ± 20,466 | <0.0001 | ||
Results are expressed as means ± SEM. To test the respective roles of E2 treatment and genotype, a two-way ANOVA was performed. When an interaction was observed between the two factors, the effect of E2 treatment was studied in each genotype using a Bonferroni posttest. ERα, estrogen receptor-α; AF, activation function; E2, 17β-estradiol.
*P < 0.01.
†P < 0.001.
To explore the potential mechanisms of the atheroprotective effect of estrogens downstream ERα, we assessed the expression of several key genes at the aorta level. First, we and others reported that E2 down-regulates vascular cell adhesion molecule (VCAM)-1, a key molecular actor of monocyte recruitment in atheroma (28, 29). We now show that this regulation by E2 is completely abolished in ERαAF20LDL-r−/− mice (Fig. 6A). Second, we also show that E2 decreases intercellular adhesion molecule (ICAM)-1, another important adhesion molecule, in LDL-r−/− but not in ERαAF20LDL-r−/− mice (Fig. 6B). Finally, O'Lone et al. (30) reported that two genes [i.e., Gremlin 2 and UDP-N-acetyl-α-d-galactosamine:polypeptide N-acetylgalactosaminyltransferase-like 2 (Galntl2)] are among the most induced in the aorta from E2-treated mice. We now show that these genes are also highly induced by E2 in ERαAF2+/+LDL-r−/− control mice (Fig. 6 C and D) but not in ERαAF20LDL-r−/− mice, showing thereby that ERαAF2 is crucial for this E2-mediated transcription (Fig. 6 C and D).
Fig. 6.
ERαAF2 is necessary for the effect of exogenous E2 on aorta gene regulation in vivo. Four-week-old ovariectomized ERαAF2+/+LDL-r−/− and ERαAF20LDL-r−/− mice were given either placebo or E2 (80 μg/kg per d during 12 wk) and switched to an atherogenic diet from the age of 6–18 wk. VCAM-1 (A), ICAM-1 (B), UDP-N-acetyl-α-d-galactosamine:polypeptide N-acetylgalactosaminyltransferase-like 2 (Galntl2) (C), and Gremlin 2 (D) mRNA levels from aortas were quantified by quantitative PCR and normalized to HPRT mRNA levels. Results are expressed according to the level in aortas from the ERαAF2+/+LDL-r−/− set as one. Analysis was with a two-way ANOVA; because an interaction was observed between the two factors, effect of E2 treatment was studied in each genotype using a Bonferroni posttest (***P < 0.001).
We also assessed the role of ERαAF2 in the lipid profile (Table 1). Total plasma cholesterol and HDL cholesterol as well as cholesterol on HDL cholesterol ratio were not influenced by the genotype in the absence of E2. As previously described (21), E2 decreased both total plasma cholesterol and HDL cholesterol fraction in ERαAF2+/+LDL-r−/−, whereas this E2 effect is abolished in ERαAF20LDL-r−/− mice (Table 1). However, the total cholesterol/HDL cholesterol ratio was similar in the four groups, whatever the genotype and treatment.
Discussion
Based on structural and functional similarities, the sequences of nuclear receptors were divided into six functional domains designated A to F (31). The central well-conserved cysteine-rich C domain mediates DNA binding, and hormone lodges into a hydrophobic pocket located within the C-terminal E/F domains that constitute the ligand binding domain. Ligand-induced transcription involves the action of distinct AFs, which are located in the N-terminal A/B (AF1) and the C-terminal E/F (AF2) domains. The respective contributions that these AFs make to the activity of the full-length ERα are both promoter- and cell-specific (27, 32, 33). For instance, a maximal transcriptional activity of ERα can require both AFs in some cells but only a specific AF in others. This finding suggests that ERα does not interact with the transcriptional machinery in an identical manner in all cells. Functional and physical links between ERα and the transcriptional machinery involve the sequential recruitment by ERα of a group of proteins, called coactivators, on the target promoter, on which they build large protein complexes (34). Structurally, these recruitments occur after ligand binding and induce specific conformational changes within the protein. Two classes of nuclear receptor coactivator complexes, directly interacting with AF2 in a ligand-dependent manner, have been identified. However, several of them primarily identified as AF2-specific have now been shown to also interact with the N-terminal region of ERα and mediate AF1 activity (35–38). We recently reported the role of ERαAF1 in the E2 action on atheroma and reendothelialization (24), but the role of ERαAF2 still remained unknown.
To assess the role of ERαAF2 in the vascular effects of estrogens in vivo, we have generated a targeted deletion of amino acids 543–549 in the helix 12 using a knock-in strategy in mouse (Fig. S1). We found that, although the expression of ERαAF20 mutant is quite similar to the WT in the uterus, the uterotrophic action of E2 is abolished in ERαAF20 mice, showing the crucial role of ERαAF2 in the E2-induced uterus hypertrophy (Fig. 3). Thus, it seems that, in addition to ERαAF1, AF2 is also required for the transcriptional mechanisms leading to the uterotrophic action of E2, underlining the complementarity action of the two AFs in this classic tissue target of E2. In addition, ERαAF2 was recently shown to be necessary for the beneficial effect of estrogens on bone, whereas the role of ERαAF1 is tissue-specific, with a crucial role in trabecular but not in cortical bone (39).
We then sought to explore the role of ERαAF2 on the acceleration of endothelial healing, a recognized vasculoprotective action of E2. Indeed, E2 was found to accelerate endothelial healing after vascular injury in several animal models such as rabbit, rat and mice (9, 18). We previously showed that this E2 action is mediated through ERα (18) but that ERαAF1 is dispensable (24). Here, we report that ERαAF2 is also dispensable to mediate the E2-induced accelerative effect (Fig. 4), raising the question as to which mechanism is involved in this action of E2. First, there could be a redundancy between ERαAF1 and AF2, a mechanism that is difficult to explore in vivo using the presently available genetic tools because of the difficulty in generating both AF1 and AF2 mutations on the same chromosome. Second, in addition to the classic transcriptional actions mediated through ERαAF1 and/or AF2, E2 may also induce rapid nongenomic (MISS) actions involving the activation of a pool of ERs localized at the plasma membrane. Such nongenomic effects were essentially studied in cell culture models, such as endothelial cells, but also osteoblasts and breast cancer cell lines (reviewed in refs. 16 and 17). Chambliss et al. (40) recently provided evidence supporting the physiological relevance of this pathway in vascular pathophysiology using an estrogen-dendrimer conjugate, a selective estrogen receptor modulator that selectively activates non-nuclear ERs in vivo. Indeed, they showed that estrogen-dendrimer conjugate is able to accelerate reendothelialization using the same model of carotid electric injury as the model used in the present study. Thus, although we cannot exclude a possible redundancy between ERαAF1 and AF2, our present data may support the conclusion proposed by Chambliss et al. (40) (i.e., an important role of MISS in the E2 accelerative effect on endothelial healing).
Ovariectomy of apoE−/− or LDL-r−/− mice is followed by an increase in fatty streak lesion area, and exogenous E2 prevents the fatty streak deposit in both models (10). The atheroprotective effect of E2 is abolished in ERα−/− mice in these two main models of atheroma (20, 21). Moreover, we previously showed that ERαAF1 is dispensable to mediate the atheroprotective effect of estrogens (24). We now show that the atheroprotective action of E2 is abolished in ERαAF20 LDL-r−/− female mice (Fig. 5 and Table 1), showing the absolute requirement of ERαAF2 for this action.
Although these two major vasculoprotective effects of E2, namely atheroprotection and acceleration of endothelial healing, are clearly both mediated by ERα, our results show that they involve profoundly different cellular and molecular mechanisms. Indeed, we previously reported that ERα expression in both endothelial and hematopoietic cells is required for the accelerative effect of E2 on reendothelialization (41), whereas ERα expression in the endothelial compartment is necessary to mediate the atheroprotective effect of E2 (21). We also previously published that FGF2 (42) and osteopontin (43) are absolutely necessary for the accelerative effect of E2 on endothelial healing. We found that these two cytokines are dispensable for the E2 atheroprotective effect (Table S1). Finally, this mechanistic divergence was also previously underlined at the level of the endothelial NO synthase, which was found to be required for the E2 action on endothelial healing (44) but not for atheroprotection (45).
Because ERαAF1 (24, 39) and ERαAF2 (39) are both required for the E2 uterotrophic action, we hypothesize that a SERM stimulating ERαAF2 with minimal activation of ERαAF1 would not stimulate uterus growth. Such a molecule should, however, retain protective effects on arteries and cortical bone (39). Altogether, these results could help to pave the way for the development of new SERMs that are urgently needed in regard to the lack of optimal treatment of menopause (46).
Methods
Mice.
All experimental procedures involving animals were performed in accordance with the principles and guidelines established by the National Institute of Medical Research (INSERM) and were approved by the local Animal Care and Use Committee. Mice (C57Bl6/J strain) were housed in cages in groups of five and kept in a temperature-controlled facility on a 12-h light to dark cycle. ERαAF2-deficient (ERαAF20) mice were generated as described and depicted in Fig. S1. WT littermates (ERαAF2+/+) of the same age as the homozygous mutants were used as control. To generate the double-deficient mice, LDL-r−/− mice, purchased from Charles River, were crossed with ERαAF2+/−. Heterozygous LDL-r+/−ERαAF2+/− mice were used to generate LDL-r−/−ERαAF2+/− mice, which were used as genitors.
Ovariectomy was performed at 4 wk of age, and concomitantly (for carotid artery injury), the mice received s.c. pellets releasing either placebo or E2 [0.1 mg, 60 d release (i.e., 80 μg/kg per d); Innovative Research of America]. We systematically checked that placebo-treated ovariectomized mice had an atrophied uterus (<10 mg), nondetectable circulating levels of E2 (<5 pg/mL or <20 pM), and for those mice implanted with an E2-releasing pellet, a significant increase in uterine weight and serum E2 concentrations (100–150 pg/mL), irrespective of the genotype. In atherosclerosis experiments, mice were implanted with the first pellet at week 6, and then, a second pellet was implanted at week 14. At 6 wk of age, the mice were switched to an hypercholesterolemic diet (1.25% cholesterol, 6% fat, no cholate, TD96335; Harlan Teklad) until 18 wk.
Transfection Assays.
HeLa and HepG2 cells were maintained in DMEM (Sigma-Aldrich) supplemented with 10% FCS (Biowest) and antibiotics (Sigma-Aldrich) at 37 °C in 5% CO2. Transfections were carried out using jetPEI reagent according to manufacturer's instructions (Polyplus). One day before transfection, cells were plated in 24-well plates at 50% confluence. One hour before transfection, the medium was replaced with phenol red-free DMEM (Sigma-Aldrich) containing 2.5% charcoal-stripped FCS (Biowest). Transfection was carried out with 100 ng C3-LUC or ERE-TK reporter genes, 100 ng CMV-βGal internal control, and 50 ng pCR, pCR-ERα, or pC-ERαAF20 expression vectors. After an overnight incubation, cells were treated for 24 h with E2 (10 nM) or ethanol (vehicle control). Cells were then harvested, and luciferase and β-galactosidase assays were performed as previously described (47).
Western Blot Analysis.
Dissected uteri were homogenized using a Qiagen Tissue Lyser (Qiagen) in lysis buffer [20 mM Tris⋅HCl, pH 8, 100 mM NaCl, 1% Triton X-100, 10 mM MgCl2, 5 mM EDTA, pH 8, 20 mM NaF, proteinase inhibitors (complete EDTA-free; Roche), 1 mM PMSF, 2 mM orthovanadate], sonicated, and centrifuged at 13,000 × g for 10 min at 4 °C; 20 μg protein of the supernatant were separated by SDS/PAGE (10%) and transferred onto a nitrocellulose membrane. Blocking (1 h at room temperature) and incubation with primary rabbit anti-mouse ERα antibody (o/n, 4 °C, 04-820; Millipore) and secondary antibody (1 h at room temperature, goat HRP-conjugated anti-rabbit IgG; Cell Signaling Technology) were done in Tris-Buffered Saline Tween 20 containing 3% dry milk. ECL West Pico (Pierce) was used to reveal signals.
Morphometric and Immununohistochemical Analyses of Fatty Streak Lesions.
Fatty streak lesion size was estimated at the aortic sinus as previously described (10). Counter coloration was carried out using Mayer's hemalun. At least six sections per animal were analyzed for each staining.
Determination of Serum Lipids.
Overnight fasted mice were anesthetized, and samples were collected from the retro-orbital venous plexus. Total plasma cholesterol was assayed using the CHOD-PAD kit (Horiba). The HDL fraction was isolated from 10 μL serum and assayed using the C-HDL + third generation kit (Roche).
Mouse Carotid Injury and Quantification of Reendothelialization.
The carotid electric injury was performed as previously described (18). Briefly, surgery was carried out with a stereomicroscope (SMZ800; Nikon), and the left common carotid artery was exposed through an anterior incision in the neck. The electric injury was applied to the distal part (4 mm precisely) of the common carotid artery with a bipolar microregulator. Three days postinjury, carotid arteries were stained with propidium iodide, and preparations were mounted with Kaiser's glycerol gelatin (Merck). Microscopy imagery was performed on a ZEISS LSM 510 confocal microscope, and quantification was performed with ZEISS LSM 510 software. The reendothelialized area was scanned with special emphasis on endothelial cells (z stacks of about 1.5 μm). The lengths of the reendothelialized area are means of at least 10 measures of endothelial cells that have maximal migration from the line of injury spanning the carotid obtained with the software ZEISS LSM image Borswer v.3.1 (48).
Analysis of mRNA Levels by Quantitative RT-PCR.
Dissected aortas were homogenized using a Precellys tissue homogenizer (Bertin Technology), and total RNA from tissues was prepared using TRIzol reagent (Invitrogen). One microgram was reverse-transcribed for 10 min at 25 °C and 2 h at 37 °C in a 20 μL final volume using the High-Capacity cDNA Reverse Transcriptase Kit (Applied Biosystems). Real-time quantitative PCRs were performed on stepone (Applied Biosystem). Primers were validated by testing PCR efficiency using standard curves (95% ≤ efficiency ≤ 105%). Gene expression was quantified using the comparative Ct (threshold cycle) method, and hypoxanthinephosphoribosyltransferase was used as reference.
Statistical Analyses.
Results are expressed as means ± SEM. To test the respective roles of E2 treatment and genotype (ERαAF2 deficiency), a two-way ANOVA was performed. When an interaction was observed between the two factors, the effect of E2 treatment was studied in each genotype using a Bonferroni posttest. A value of P < 0.05 was considered as statistically significant.
Supplementary Material
Acknowledgments
The staffs of the animal facilities (C. Evra) and the Plateforme d'experimentation fonctionnelle (A. Desquesnes) are acknowledged for skillful technical assistance. We also thank Mrs. M. J. Fouque and Mr. J. C. Albouys for technical support. This work was supported by the Institut National de la Santé et de la Recherche Médicale U1048 by Institut National de la Santé et de la Recherche Médicale, Université de Toulouse III and Faculté de Médecine Toulouse-Rangueil, the Agence Nationale de la Recherche (Endoth-ER-E2), the Fondation de France, the Conseil Régional Midi-Pyrénées, the Société and the Fondation Française de Cardiologie and the Fondation Coeur et Artère, and the Institut de Génétique et de Biologie Moléculaire et Cellulaire by the European project EWA (Estrogen in Women Aging Contract LSHM-CT-2005-518245). A.A. was supported by a grant from the Groupe de Réflexion sur la Recherche Cardiovasculaire.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1105632108/-/DCSupplemental.
References
- 1.Kalin MF, Zumoff B. Sex hormones and coronary disease: A review of the clinical studies. Steroids. 1990;55:330–352. doi: 10.1016/0039-128x(90)90058-j. [DOI] [PubMed] [Google Scholar]
- 2.Dubey RK, Imthurn B, Barton M, Jackson EK. Vascular consequences of menopause and hormone therapy: Importance of timing of treatment and type of estrogen. Cardiovasc Res. 2005;66:295–306. doi: 10.1016/j.cardiores.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 3.Grodstein F, et al. Postmenopausal estrogen and progestin use and the risk of cardiovascular disease. N Engl J Med. 1996;335:453–461. doi: 10.1056/NEJM199608153350701. [DOI] [PubMed] [Google Scholar]
- 4.Mikkola TS, Clarkson TB. Estrogen replacement therapy, atherosclerosis, and vascular function. Cardiovasc Res. 2002;53:605–619. doi: 10.1016/s0008-6363(01)00466-7. [DOI] [PubMed] [Google Scholar]
- 5.Arnal JF, et al. Estrogen receptors and endothelium. Arterioscler Thromb Vasc Biol. 2010;30:1506–1512. doi: 10.1161/ATVBAHA.109.191221. [DOI] [PubMed] [Google Scholar]
- 6.Rossouw JE, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 7.Anderson GL, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: The Women's Health Initiative randomized controlled trial. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 8.Rossouw JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297:1465–1477. doi: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]
- 9.Krasinski K, et al. Estradiol accelerates functional endothelial recovery after arterial injury. Circulation. 1997;95:1768–1772. doi: 10.1161/01.cir.95.7.1768. [DOI] [PubMed] [Google Scholar]
- 10.Elhage R, et al. 17 beta-estradiol prevents fatty streak formation in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 1997;17:2679–2684. doi: 10.1161/01.atv.17.11.2679. [DOI] [PubMed] [Google Scholar]
- 11.Rosenfeld ME, et al. Estrogen inhibits the initiation of fatty streaks throughout the vasculature but does not inhibit intra-plaque hemorrhage and the progression of established lesions in apolipoprotein E deficient mice. Atherosclerosis. 2002;164:251–259. doi: 10.1016/s0021-9150(02)00178-8. [DOI] [PubMed] [Google Scholar]
- 12.Stevenson JC. Type and route of estrogen administration. Climacteric. 2009;12(Suppl 1):86–90. doi: 10.1080/13697130903007389. [DOI] [PubMed] [Google Scholar]
- 13.Ascenzi P, Bocedi A, Marino M. Structure-function relationship of estrogen receptor alpha and beta: Impact on human health. Mol Aspects Med. 2006;27:299–402. doi: 10.1016/j.mam.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Tora L, et al. The human estrogen receptor has two independent nonacidic transcriptional activation functions. Cell. 1989;59:477–487. doi: 10.1016/0092-8674(89)90031-7. [DOI] [PubMed] [Google Scholar]
- 15.Métivier R, Penot G, Flouriot G, Pakdel F. Synergism between ERalpha transactivation function 1 (AF-1) and AF-2 mediated by steroid receptor coactivator protein-1: Requirement for the AF-1 alpha-helical core and for a direct interaction between the N- and C-terminal domains. Mol Endocrinol. 2001;15:1953–1970. doi: 10.1210/mend.15.11.0727. [DOI] [PubMed] [Google Scholar]
- 16.Levin ER. Integration of the extranuclear and nuclear actions of estrogen. Mol Endocrinol. 2005;19:1951–1959. doi: 10.1210/me.2004-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim KH, Bender JR. Membrane-initiated actions of estrogen on the endothelium. Mol Cell Endocrinol. 2009;308:3–8. doi: 10.1016/j.mce.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brouchet L, et al. Estradiol accelerates reendothelialization in mouse carotid artery through estrogen receptor-alpha but not estrogen receptor-beta. Circulation. 2001;103:423–428. doi: 10.1161/01.cir.103.3.423. [DOI] [PubMed] [Google Scholar]
- 19.Pare G, et al. Estrogen receptor-alpha mediates the protective effects of estrogen against vascular injury. Circ Res. 2002;90:1087–1092. doi: 10.1161/01.res.0000021114.92282.fa. [DOI] [PubMed] [Google Scholar]
- 20.Hodgin JB, et al. Estrogen receptor alpha is a major mediator of 17beta-estradiol's atheroprotective effects on lesion size in Apoe-/- mice. J Clin Invest. 2001;107:333–340. doi: 10.1172/JCI11320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Billon-Galés A, et al. Endothelial estrogen receptor-alpha plays a crucial role in the atheroprotective action of 17beta-estradiol in low-density lipoprotein receptor-deficient mice. Circulation. 2009;120:2567–2576. doi: 10.1161/CIRCULATIONAHA.109.898445. [DOI] [PubMed] [Google Scholar]
- 22.Dupont S, et al. Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development. 2000;127:4277–4291. doi: 10.1242/dev.127.19.4277. [DOI] [PubMed] [Google Scholar]
- 23.Pendaries C, et al. The AF-1 activation-function of ERalpha may be dispensable to mediate the effect of estradiol on endothelial NO production in mice. Proc Natl Acad Sci USA. 2002;99:2205–2210. doi: 10.1073/pnas.042688499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Billon-Galés A, et al. The transactivating function 1 of estrogen receptor alpha is dispensable for the vasculoprotective actions of 17beta-estradiol. Proc Natl Acad Sci USA. 2009;106:2053–2058. doi: 10.1073/pnas.0808742106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Danielian PS, White R, Lees JA, Parker MG. Identification of a conserved region required for hormone dependent transcriptional activation by steroid hormone receptors. EMBO J. 1992;11:1025–1033. doi: 10.1002/j.1460-2075.1992.tb05141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mérot Y, et al. The relative contribution exerted by AF-1 and AF-2 transactivation functions in estrogen receptor alpha transcriptional activity depends upon the differentiation stage of the cell. J Biol Chem. 2004;279:26184–26191. doi: 10.1074/jbc.M402148200. [DOI] [PubMed] [Google Scholar]
- 27.Berry M, Metzger D, Chambon P. Role of the two activating domains of the oestrogen receptor in the cell-type and promoter-context dependent agonistic activity of the anti-oestrogen 4-hydroxytamoxifen. EMBO J. 1990;9:2811–2818. doi: 10.1002/j.1460-2075.1990.tb07469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gourdy P, et al. The atheroprotective effect of 17 beta-estradiol is not altered in P-selectin- or ICAM-1-deficient hypercholesterolemic mice. Atherosclerosis. 2003;166:41–48. doi: 10.1016/s0021-9150(02)00322-2. [DOI] [PubMed] [Google Scholar]
- 29.Nathan L, Pervin S, Singh R, Rosenfeld M, Chaudhuri G. Estradiol inhibits leukocyte adhesion and transendothelial migration in rabbits in vivo : Possible mechanisms for gender differences in atherosclerosis. Circ Res. 1999;85:377–385. doi: 10.1161/01.res.85.4.377. [DOI] [PubMed] [Google Scholar]
- 30.O'Lone R, et al. Estrogen receptors alpha and beta mediate distinct pathways of vascular gene expression, including genes involved in mitochondrial electron transport and generation of reactive oxygen species. Mol Endocrinol. 2007;21:1281–1296. doi: 10.1210/me.2006-0497. [DOI] [PubMed] [Google Scholar]
- 31.Green S, et al. Human oestrogen receptor cDNA: Sequence, expression and homology to v-erb-A. Nature. 1986;320:134–139. doi: 10.1038/320134a0. [DOI] [PubMed] [Google Scholar]
- 32.Tzukerman MT, et al. Human estrogen receptor transactivational capacity is determined by both cellular and promoter context and mediated by two functionally distinct intramolecular regions. Mol Endocrinol. 1994;8:21–30. doi: 10.1210/mend.8.1.8152428. [DOI] [PubMed] [Google Scholar]
- 33.Metzger D, Ali S, Bornert JM, Chambon P. Characterization of the amino-terminal transcriptional activation function of the human estrogen receptor in animal and yeast cells. J Biol Chem. 1995;270:9535–9542. doi: 10.1074/jbc.270.16.9535. [DOI] [PubMed] [Google Scholar]
- 34.McKenna NJ, O'Malley BW. Combinatorial control of gene expression by nuclear receptors and coregulators. Cell. 2002;108:465–474. doi: 10.1016/s0092-8674(02)00641-4. [DOI] [PubMed] [Google Scholar]
- 35.Webb P, et al. Estrogen receptor activation function 1 works by binding p160 coactivator proteins. Mol Endocrinol. 1998;12:1605–1618. doi: 10.1210/mend.12.10.0185. [DOI] [PubMed] [Google Scholar]
- 36.Onate SA, et al. The steroid receptor coactivator-1 contains multiple receptor interacting and activation domains that cooperatively enhance the activation function 1 (AF1) and AF2 domains of steroid receptors. J Biol Chem. 1998;273:12101–12108. doi: 10.1074/jbc.273.20.12101. [DOI] [PubMed] [Google Scholar]
- 37.Benecke A, Chambon P, Gronemeyer H. Synergy between estrogen receptor alpha activation functions AF1 and AF2 mediated by transcription intermediary factor TIF2. EMBO Rep. 2000;1:151–157. doi: 10.1093/embo-reports/kvd028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kobayashi Y, et al. p300 mediates functional synergism between AF-1 and AF-2 of estrogen receptor alpha and beta by interacting directly with the N-terminal A/B domains. J Biol Chem. 2000;275:15645–15651. doi: 10.1074/jbc.M000042200. [DOI] [PubMed] [Google Scholar]
- 39.Borjesson AE, et al. Roles of transactivating functions 1 and 2 of estrogen receptor-alpha in bone. Proc Natl Acad Sci U S A. 2011;108:6288–6293. doi: 10.1073/pnas.1100454108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chambliss KL, et al. Non-nuclear estrogen receptor alpha signaling promotes cardiovascular protection but not uterine or breast cancer growth in mice. J Clin Invest. 2010;120:2319–2330. doi: 10.1172/JCI38291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Toutain CE, et al. Estrogen receptor alpha expression in both endothelium and hematopoietic cells is required for the accelerative effect of estradiol on reendothelialization. Arterioscler Thromb Vasc Biol. 2009;29:1543–1550. doi: 10.1161/ATVBAHA.109.192849. [DOI] [PubMed] [Google Scholar]
- 42.Fontaine V, et al. Essential role of bone marrow fibroblast growth factor-2 in the effect of estradiol on reendothelialization and endothelial progenitor cell mobilization. Am J Pathol. 2006;169:1855–1862. doi: 10.2353/ajpath.2006.060260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leen LL, et al. Estrogen-stimulated endothelial repair requires osteopontin. Arterioscler Thromb Vasc Biol. 2008;28:2131–2136. doi: 10.1161/ATVBAHA.108.167965. [DOI] [PubMed] [Google Scholar]
- 44.Billon A, et al. The estrogen effects on endothelial repair and mitogen-activated protein kinase activation are abolished in endothelial nitric-oxide (NO) synthase knockout mice, but not by NO synthase inhibition by N-nitro-L-arginine methyl ester. Am J Pathol. 2008;172:830–838. doi: 10.2353/ajpath.2008.070439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hodgin JB, Knowles JW, Kim HS, Smithies O, Maeda N. Interactions between endothelial nitric oxide synthase and sex hormones in vascular protection in mice. J Clin Invest. 2002;109:541–548. doi: 10.1172/JCI14066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lenfant F, Trémollières F, Gourdy P, Arnal JF. Timing of the vascular actions of estrogens in experimental and human studies: Why protective early, and not when delayed? Maturitas. 2011;68:165–173. doi: 10.1016/j.maturitas.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 47.Penot G, et al. The human estrogen receptor-alpha isoform hERalpha46 antagonizes the proliferative influence of hERalpha66 in MCF7 breast cancer cells. Endocrinology. 2005;146:5474–5484. doi: 10.1210/en.2005-0866. [DOI] [PubMed] [Google Scholar]
- 48.Filipe C, et al. Estradiol accelerates endothelial healing through the retrograde commitment of uninjured endothelium. Am J Physiol Heart Circ Physiol. 2008;294:H2822–H2830. doi: 10.1152/ajpheart.00129.2008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.