Abstract
We have reported that Gram negative organisms decorated with rough lipopolysaccharide (LPS) are particularly susceptible to the direct antimicrobial actions of the pulmonary collectins, surfactant proteins A (SP-A) and D (SP-D). In this study, we examined the lipid and LPS components required for the permeabilizing effects of the collectins on model bacterial membranes. Liposomes composed of 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine (POPE), with or without rough E. coli LPS (J5), smooth E. coli LPS (B5) or cholesterol, were loaded with self quenching probes and exposed to native or oxidatively modified SP-A. Fluoresence that resulted from permeabilization of liposomes and diffusion of dyes was assessed by microscopy or fluorimetry.
Human SP-A and melittin increased the permeability of J5 LPS/POPE liposomes, but not B5 LPS/POPE liposomes or control (POPE only) liposomes. At a human SP-A concentration of 100 μg/ml, the permeability of the J5 LPS/POPE membranes increased 4.4-fold (p<0.02) compared to the no added SPA control. Rat SP-A and SP-D also permeabilized the J5 containing liposomes. Incorporation of cholesterol into J5 LPS/POPE liposomes at a molar ratio of POPE:cholesterol of 1:0.15 blocked human SP-A or melittin-induced permeability (p<0.05) compared to cholesterol-free liposomes. Exposure of human SP-A to surfactant lipid peroxidation blocked the permeabilizing activity of the protein. We conclude that SP-A permeabilizes phospholipid membranes in an LPS-dependent and rough LPS-specific manner, that the effect is neither SP-A or species specific, and that oxidative damage to SP-A abolishes its membrane destabilizing properties. Incorporation of cholesterol into the membrane enhances resistance to permeabilization by SP-A, most likely by increasing packing density and membrane rigidity.
Keywords: surfactant protein, antimicrobial protein, liposomes, protein oxidation, lipid, peroxidation, free radicals, oxidative stress
The pulmonary collectins, surfactant proteins A and D, are known to opsonize a variety of microorganisms and to directly modulate the function of alveolar macrophages (1). We have recently reported that the collectins also directly inhibit the growth of Gram negative bacteria by permeabilizing the bacterial membrane (2). In general, rough strains were more readily killed than smooth strains, suggesting that LPS-saccharide chain length is an important determinant of bacterial susceptibility to the antimicrobial effects of the collectins (3). The complexity of the LPS carbohydrate structure has also been shown to play a role in bacterial killing by bacterial permeability-inducing protein, cecropin, magainin, and protamine (4). The permeabilizing activity of the alveolar lining fluid derived from wild type mice is greater than that from SP-A knockout mice (5), suggesting that SP-A plays an important role as an antibacterial protein at the air liquid interface.
The mechanism(s) of membrane permeabilization induced by the collectins is not known. Most membrane destabilizing proteins are small charged proteins that can saturate membrane surfaces or intercalate into bilayers and disrupt membrane function through charge neutralization or pore formation, respectively (6). Other proteins translocate through the cell membrane and interact with cytosolic targets (7). SP-A and SP-D are large oligomeric proteins which are unlikely to penetrate through lipid bilayers, but which may conceivably perturb membrane surfaces (8). They are known to bind to several molecular species that are prevalent on the surface of Gram negative pathogens, including lipopolysaccharides (LPS), di-mannose repeating units of some capsular polysaccharides (SP-A) (9), phospholipids such as phosphatidylcholine (SP-A) (10), phosphatidylinositol (SP-D) (11) and other membrane proteins such as P2 outer membrane protein of H. influenza (SP-A) (12). It is not clear which of these potential targets is required for transduction of the permeabilizing effect of the collectins, but LPS is a plausible possibility since it constitutes a pathogen-associated molecular pattern that is common to all Gram negative organisms (13). LPS is composed of lipid A, a core oligosaccharide and an O-antigen. Both collectins recognize LPS; SP-A preferentially binds to the lipid A moiety (14), and SP-D binds primarily to the core oligosaccharide (15).
In this study, we used fluorescent probe loaded liposomes composed of LPS containing model bacterial membranes to characterize the molecular interactions that are required for collectin-mediated membrane permeabilization.
Materials and methods
Reagents
Bovine serum albumin (BSA), E. coli Rc mutant J5 (rough) LPS, E. coli 055:B5 (smooth) LPS, melittin, Chelex-100, cholesterol, cupric sulfate, β-phenantrolinedisulfonic acid and disodium EDTA were from Sigma-Aldrich (St. Louis, MO, USA). The OxyBlot™ Protein Oxidation Detection Kit was from Chemicon (Temecula, CA, USA). The BCA Protein Assay Reagent Kit was from Pierce (Rockford, IL, USA). 1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine (POPE), 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), L-phosphatidylcholine from egg yolk (EggPC) and 1-stearoyl-2-linoleoyl-sn-glycero-3-phosphocholine (18:0-18:2PC) in chloroform were from Avanti Polar Lipids, Inc. (Alabaster, AL). The fluorescent probes, 8-aminonaphthalene-1,3,6-trisulfonic acid disodium salt (ANTS) and p-xylene-bis-pyridinium bromide (DPX), were from Molecular Probes, Inc. (Eugene, OR). All other chemicals were of analytical grade. Centrifugal filter devices YM-3 MWCO 3,000 Millipore (Bredford, MA) were used for concentration of protein. Spectra/Por cellulose membranes (MWCO 3,500) (Spectrum Laboratories, Inc., Rancho Dominguez, CA) were used for dialysis.
SP-A purification
Human SP-A was isolated from patients with pulmonary alveolar proteinosis, a lung disease associated with the accumulation of surfactant lipids and proteins in airspaces (16). Briefly, SP-A was purified by the method of Suwabe (17) from the cell-free surfactant pellet of bronchoalveolar lavage by serial sedimentation and resuspension in buffer containing 5 mM Tris, 150 mM NaCl, and 1 mM CaCl2, release by incubation with 2 mM EDTA, and adsorption of the recalcified supernatant to mannose-Sepharose affinity columns. SP-A was eluted from the carbohydrate affinity column using 2 mM EDTA. The purified proteins were dialyzed for two days against daily changes of 2,000 volumes of 5 mM Tris (pH 7.4), 150 mM NaCl and for one day against 2,000 volumes of 5 mM Tris (pH 7.4), and stored at -20 °C. The EDTA content of all protein samples used was measured by colorimetric method as previously reported (18). The average final EDTA concentration was 5 μM, and was less than 25 μM in all SP-A reagents used.
Membrane vesicle preparation
POPE and LPS were used to produce liposomes composed of model bacterial membranes for assays of collectin-mediated permeabilization. Multilamellar liposomes were prepared by the method of Gregoriadis (19). In brief, POPE or POPE with LPS (85:15; w/w) was dissolved in CHCl3-methanol. The organic solvent was evaporated to dryness in a rotary evaporator under an N2 atmosphere at 20 °C. The lipid film was hydrated in 5 mM Tris, 150 mM NaCl, 1 mM EDTA, 12.5 mM ANTS, 45 mM DPX and multilamellar liposomes were generated by vigorous vortexing for 5 min. Liposomes were separated from unincorporated fluorescent label by several rounds of centrifugation at 2000 g for 5 min. and resuspension in 5 mM Tris, 150 mM NaCl, and 1 mM EDTA.
Liposomes composed of saturated and unsaturated lipids were generated for use as substrates in the lipid peroxidation experiments. A lipid mixture composed of DPPC, cholesterol, egg PC, 18:0-18:2PC (1:1:0.15:0.15 w/w/w/w) in CHCl3 was evaporated to dryness under N2 and then hydrated in 5 mM Tris, 150 mM NaCl and 3% Chelex100-treated buffer. Multilamellar liposomes were generated by vigorous vortexing for 10 min.
Assays of protein oxidation
Human SP-A was oxidized by exposing the protein to copper-induced peroxidation of model surfactant lipids. Stock solutions of 10 mM CuSO4 were freshly prepared daily. Reaction mixtures composed of 1 mg/ml DPPC/PC/Chol liposomes, 10 μM CuSO4, and proteins or controls were prepared in 3% Chelex treated saline (150 mM NaCl) or phosphate-buffered saline (PBS). The mixtures were incubated at 37 °C in a shaking water bath for 24 h (18). Control reactions that included SP-A only, lipids only or CuSO4 only were also performed.
Analysis of liposomal permeability
An assay was developed to assess the effect of SP-A on the permeability of model bacterial membranes. Liposomes composed of POPE, LPS and various amounts of cholesterol were loaded with self quenching dyes, ANTS and DPX, as above, and exposed to SP-A or melittin (positive control) at various concentrations. Fluorescence that resulted from antimicrobial peptide-induced leak and diffusion of ANTS and DPX was measured by fluorescent microscopy or fluorimetry at excitation and emission wavelengths of 353 nm and 520 nm, respectively. For fluorescent microscopy, samples were loaded together on the same slide and photographed at the same gain, so that direct comparisons of fluorescence intensity could be made.
Assays of protein-associated carbonyls
Oxidative modification of SP-A was determined by Western blot analysis (Oxyblot) using an antibody to 2,4-dinitrophenylhydrazine (DNP)-derivatized carbonyl groups. SP-A was incubated with DNP, which forms adducts with protein-associated carbonyls. After size fractionation by 8–16% SDS-polyacrylamide gel electrophoresis under reducing conditions, protein species were electrophoretically transferred to nitrocellulose membranes. The membranes were sequentially incubated with a rabbit anti-DNP IgG and horseradish peroxidase-conjugated goat anti-rabbit IgG. Blots were developed by horseradish peroxidase-dependent oxidation of the chemiluminescent substrate, SuperSignal® (Pierce, Rockford, IL, USA), and visualized using autoradiography.
Total protein assays
Total protein concentrations were analyzed using the BCA Protein Assay Reagent Kit (Pierce, Rockford, IL, USA). Absorbance was read at 570 nm.
Statistical analysis
The Student's t-test was used for comparisons between experimental groups and p<0.05 was considered to indicate statistical significance. All data are presented as mean ± S.E unless otherwise noted.
Results
LPS dependent permeabilizing activity of SP-A on model bacterial membranes
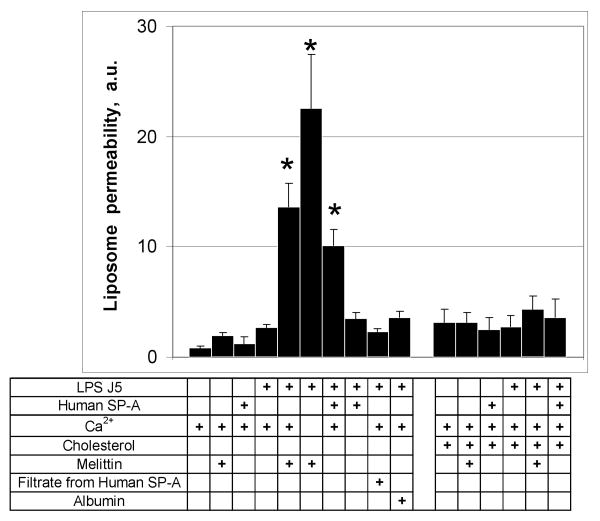
Experiments were performed to characterize the direct permeabilizing effects of SP-A on liposomal membranes composed of bacterial phospholipids and LPS. Multilamellar vesicles were loaded with a set of self quenching probes which fluoresce as they diffuse apart. Incubation of 100 μg/ml of human SP-A with liposomes composed of POPE alone resulted in no changes from the background level of fluoresence (Fig. 1 a). Similarly, the well characterized permeabilizing peptide, melittin, had no effect on the permeability of POPE liposomes at a concentration that has been shown to be effective at killing bacteria (15 μg/ml) (Fig. 1 b). Incorporation of J5 LPS into the membrane of POPE liposomes (Fig. 1 c), rendered them susceptible to permeabilization by 15 μg/ml melittin (fig. 1 e) or 100 μg/ml human SP-A (Fig. 1 f), but not 100 μg/ml albumin (used as a negative control) (Fig. 1 d). These data indicate that rough LPS confers susceptibility to collectin-mediated permeabilization of POPE liposomes.
Figure 1.
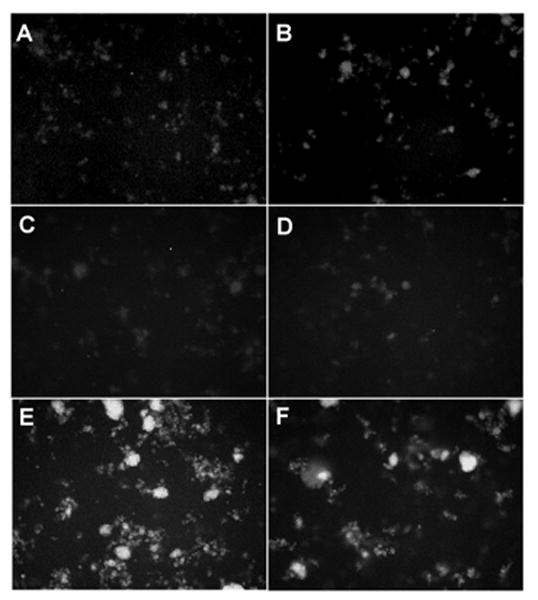
Incorporation of rough LPS-J5 into POPE liposomes confers susceptibility to permeabilization by human SP-A.
Fluorescence microscopy of ANTS/DPX probe loaded POPE liposomes upon exposure to (A) 100 μg/ml of human SP-A; (B) 15 μg/ml melittin (positive control); or POPE:LPS-J5 liposomes alone (C) upon exposure to (D) 100 μg/ml albumin; (E) 15 μg/ml melittin; (F) 100 μg/ml of human SP-A. Experiments were performed in the presence of 1 mM CaCl2. All photograph were made at 100× magnification.
Calcium dependence of the membrane permeabilizing effects of SP-A
Most of the host defense properties of SP-A, including opsonization (20), aggregation (21) and direct bacterial killing, are calcium dependent. We used fluorimetery to assess the calcium requirements for permeabilization and release of labeled probes into the extraliposomal space (Fig. 2). Incubation of POPE liposomes with CaCl2 and 100 μg/ml of human SP-A or 15 μg/ml of melittin did not result in the release of label. Melittin but not SP-A increased the permeability of J5 LPS/POPE membranes in the absence of calcium. When 100 μg/ml SP-A was incubated with J5/POPE liposomes in the presence of 5 mM calcium, however, permeability increased 4.4-fold (p<0.02) compared to the no added SP-A control. In contrast, addition of calcium diminished the permeabilizing activity of 15 μg/ml melittin by over 40 % as has been reported by others (22). Neither the protein-free filtrate (3,000 MWCO) prepared from the SP-A reagent or 100 μg/ml albumin affected J5 LPS/POPE liposome permeability. We conclude that the membrane destabilizing effect of SP-A on J5/POPE liposomes is calcium dependent and that the mechanism of permeabilization is distinct from that of melittin.
Figure 2.
SP-A-induced leakage of POPE containing liposomes is calcium dependent.
Incubation of fluorescent probe loaded POPE:LPS-J5 liposomes with 100 μg/ml of human SPA or 15 μg/ml melittin in the presence of calcium resulted in the release of fluorescent label into the extraliposomal space. In contrast, neither 100 μg/ml albumin, or the filtrate from the human SP-A reagent (3000 MWCO), had an effect on permeability of the POPE:LPS-J5 liposomes. Incorporation of cholesterol (25%) into the liposomal membrane blocked the susceptibility of POPE:LPS-J5 liposomes to permeabilization. Leakage of ANTS/DPX into the extraliposomal space was measured by fluorimetry as described under Materials and Methods. Data are expressed as means ± S.E., n=5. *, p<0.05.
Effect of cholesterol on the SP-A-induced permeability of POPE liposomes
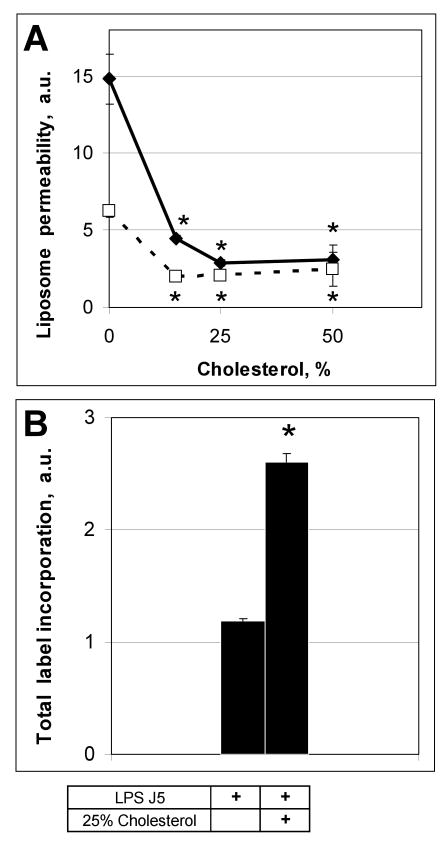
Cholesterol increases the stability and packing density of membranes (23) and decreases the permeability of phosphatidylcholine liposomes (24). Liposomes composed of POPE/J5 (Fig. 3a) or POPE/J5/cholesterol (Fig. 3b) exhibited only minimal background fluorescence. Addition of cholesterol to J5 LPS/POPE liposomes appeared to decrease their size but enhanced resistance to permeabilization by 100 μg/ml of human SP-A or 15 μg/ml melittin (Fig. 3 c, d), as assessed by both fluorimetry (Fig. 2) and fluorescence microscopy (Fig. 3 e, f). Incorporation of increasing amounts of cholesterol (up to 25% w:w cholesterol:POPE) into J5 POPE liposomes produced a dose-dependent reduction in permeabilization by human SP-A or melittin (Fig. 4 a). Cholesterol containing liposomes incorporated more of the fluorescent probes ANTS and DPX than cholesterol free vesicles (Fig. 4 b), so diminished fluorescence from the cholesterol liposomes was not a consequence of less efficient vesicle loading. These data indicate that the rigidity of the membrane plays an important role in the susceptibility of the model bacterial membranes to antimicrobial peptides.
Figure 3.
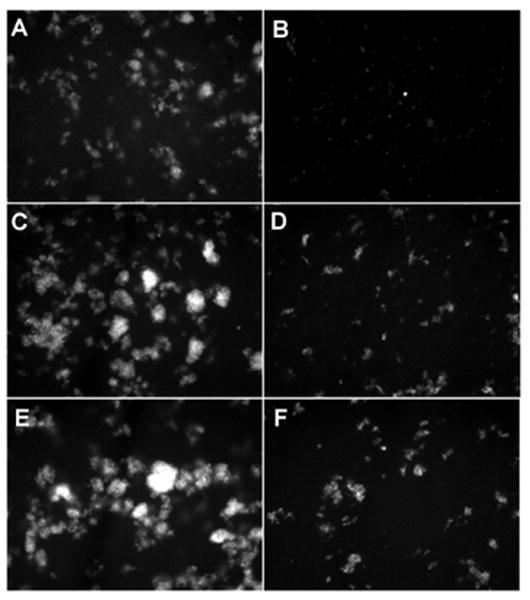
Incorporation of cholesterol into rough LPS-J5 containing liposomes blocks SP-A-induced permeabilization.
Fluorescent microscopy of (A) POPE:LPS-J5 liposomes alone; (B) POPE:LPS-J5:cholesterol liposomes alone; (C) POPE:LPS-J5 liposomes upon exposure to 15 μg/ml melittin; (D) POPE:LPS-J5:cholesterol liposomes upon exposure to 15 μg/ml melittin; (E) POPE:LPS-J5 liposomes upon exposure to 100 μg/ml SP-A; (F) POPE:LPS-J5:cholesterol liposomes upon exposure to 100 μg/ml SP-A are shown. Experiments were performed in the presence of 1 mM CaCl2. 100× magnification was used.
Figure 4.
Cholesterol blocks the SP-A-mediated permeabilization of POPE:LPS-J5 liposomes in a concentration-dependent manner.
Permeabilization of POPE:LPS-J5 liposomes containing various amounts of cholesterol in the presence of 100 μg/ml human SP-A (dotted line) or 15 μg/ml melittin (solid line) are shown in panel A. Loading of ANTS/DPX label into the cholesterol containing liposomes was more effective than for the cholesterol-free liposomes (panel B). Leakage of ANTS/DPX into the extraliposomal space was measured by fluorimetry as described under Materials and Methods. Data are means ± S.E., n=5. *, p<0.05. Experiments were performed in the presence of 1 mM CaCl2.
Activity of SP-D and collectins from other species on membrane permeability
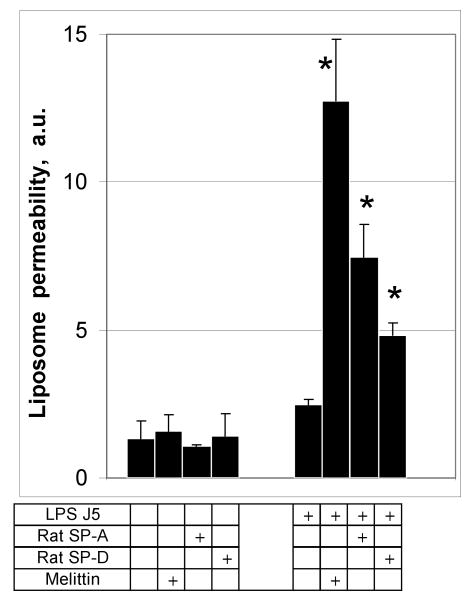
To determine if LPS-dependent collectin mediated permeabilization was species dependent, we tested rat pulmonary collectins as permeabilizing factors for J5/POPE liposomes (Fig. 5). Similar to human SP-A, rat SP-A at a concentration of 100 μg/ml increased the leak of ANTS and DPX from J5 LPS/POPE liposomes 3-fold (p<0.05) compared to the no protein added control. Rat SP-D at 10 μg/ml was also effective. Neither of the rat collectins increased the permeability of POPE only liposomes. These data indicate that the permeabilizing effect of the pulmonary collectins on model bacterial membranes is not restricted to the human protein or to SP-A.
Figure 5.
Permeabilization of rough LPS-J5 containing POPE liposomes is not species or collectin-type specific.
Incubation of POPE:LPS-J5 liposomes with 100 μg/ml of rat SP-A or 10 μg/ml of rat SP-D resulted in leakage of fluorescent label from the liposome. Permeabilization of ANTS/DPX liposomes was measured by fluorimetry as described under Materials and Methods. Data are means ± S.E., n=5. *, p<0.05. Experiments were performed in the presence of 1 mM CaCl2.
Effect of O-antigen chain length on SP-A permeabilizing activity of model bacterial liposomes
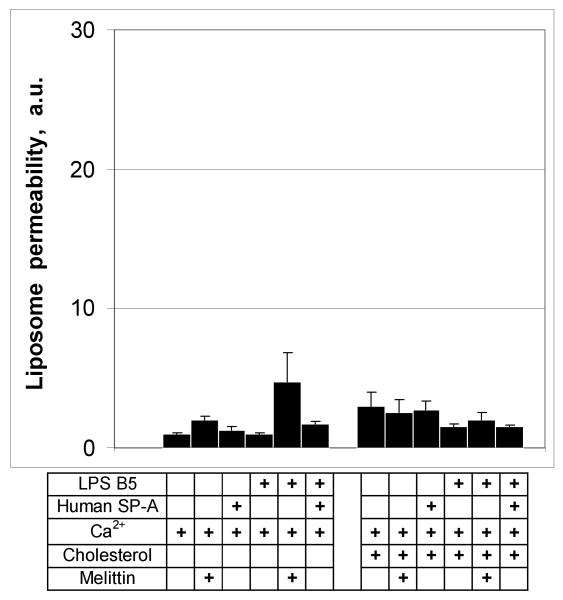
SP-A binds preferentially to rough LPS. To determine if smooth E. coli LPS (B5) also confers susceptibility to permeabilization by SP-A, we prepared fluorescent probe loaded POPE liposomes with or without LPS B5 and exposed them to 100 μg/ml of SP-A or 15 μg/ml melittin (as a positive control). Neither SP-A or melittin had significant permeabilizing activity on B5/POPE liposomes (Fig. 6) and incorporation of cholesterol into the membrane had no effect. These data indicate that smooth LPS fails to confer susceptibility to collectin-mediated permeability on POPE liposomes.
Figure 6.
Smooth LPS does not confer susceptibility of POPE liposomes to permeabilization by human SP-A.
Incubation of cholesterol-free or cholesterol-containing POPE:LPS-B5 liposomes with 100 μg/ml of human SP-A or 15 μg/ml melittin did not result in the release of fluorescent label from liposomes. Incorporation of the cholesterol into the POPE liposomes at a w:w ratio of 25% had no effect on label release. Fluorimetry was performed as described under Materials and Methods. Data are means ± S.E., n=5. *, p<0.05. Experiments were performed in the presence of 1 mM CaCl2.
Effect of lipid peroxidation on the membrane destabilizing activity of SP-A
SP-A is intimately associated with saturated and unsaturated phospholipids in the alveolar space, and is exposed to oxygen tensions which vary from atmospheric levels to 100% in patients with hypoxic respiratory failure on supplemental oxygen therapy. To determine whether clinically relevant oxidative damage to SP-A affects the permeabilizing activity of the protein on model bacterial liposomes, we exposed SP-A to air or copper-induced surfactant lipid peroxidation and then to fluorescent probe loaded J5/POPE liposomes. Oxidative damage of the collectins was first confirmed by a Western blotting technique which detects carbonyl adducts (Fig. 7, a). Both copper-induced oxidation, as well as air- and copper- induced lipid peroxidation, caused a significant increase in SP-A-associated carbonyls. Oxidation of SP-A by any of these methods clearly blocked the permeabilizing activity of the protein, whereas simple mixing of SP-A with model surfactant lipids immediately prior to incubation with the liposomes had no effect on this function (Fig. 7, b). Taken together, these data suggest that oxidative damage to SP-A blocks permeabilizing activity of the collectin on LPS-containing POPE liposomes, as it does for whole organisms (5).
Figure 7.
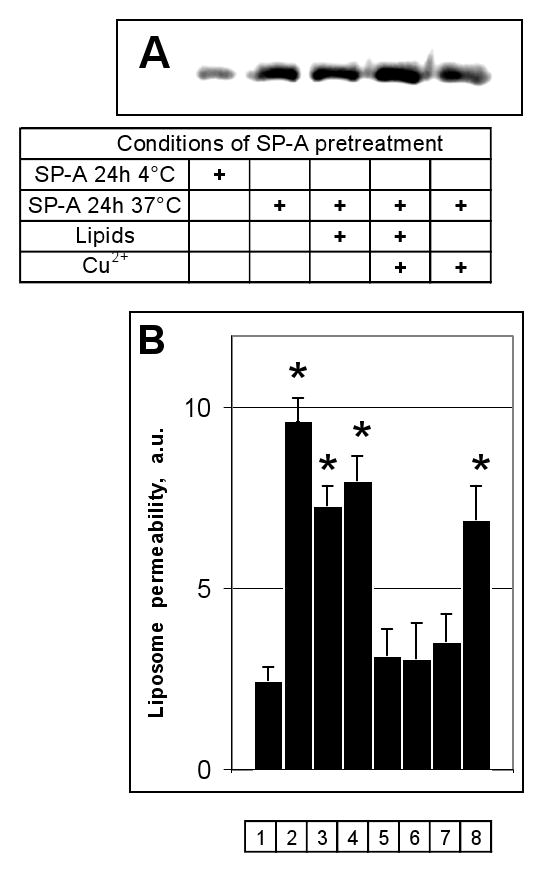
Reactive oxygen species produced by copper-initiated lipid peroxidation of surfactant phospholipids blocked the permeabilizing activity of human SP-A on POPE:LPS-J5 liposomes.
Panel A) Oxidative modification of SP-A that occurred without an exposure and during 24 h exposure at 4° C or 37° C was determined by Western analysis using an antibody to DNP-derivatized carbonyl moieties. Panel B) The fluorescence which resulted upon leakage of ANTS/DPX from POPE:LPS-J5 liposomes was measured in the absence of any addition (1); or upon exposure to 15 μg/ml melittin (2); SP-A preincubated at 4 °C for 24h (3); SP-A preincubated at 37 °C for 24h (4); SP-A preincubated with surfactant phospholipids at 37 °C for 24h (5); SP-A preincubated with surfactant phospholipids and 10 μM CuSO4 at 37 °C for 24h (6); SP-A preincubated with 10 μM CuSO4 at 37 °C for 24h (7); SP-A and surfactant phospholipids without preincubation at 37 °C for 24h (8). Data are means ± S.E., n=5. *, p<0.05. Experiments were performed in the presence of 1 mM CaCl2.
Discussion
We have previously reported that surfactant proteins A and D cause bacterial aggregation and permeabilization (3;25). Rough Gram negative bacteria containing truncated LPS domains are most susceptible to these activities. In this study, we used model bacterial liposomes containing phospholipids and lipopolysaccharides to characterize collectin-mediated membrane destabilization. The major findings here are that the collectin antimicrobial activity is calcium and LPS dependent, that rough but not smooth LPS can confer susceptibility to permeabilization, and that oxidative damage to SP-A blocks the permeabilizing function of the protein.
SP-A and SP-D inhibit the incorporation of metabolic precursors into RNA and protein of Gram negative organisms, produce zonal growth inhibition of E. coli in agar plates, and increase access of the impermeant probes, propidium iodide and phosphatase substrate, ELF 97, to nuclear and periplasmic targets, respectively (3;5). Rough E. coli laboratory strains are more susceptible than smooth strains to collectin-mediated permeabilization, and the growth of some but not all clinical isolates of E. coli, Klebsiella, Enterobacter are inhibited (3;26). Bordetella pertussis and Bordetella bronchiseptica are Gram negative organisms with very simple LPS structures characterized by a branching core and a nonrepeating terminal trisaccharide, which are resistant to the collectins (25). Deletion of the terminal sugar renders the organisms susceptible to aggregation and permeabilization by SP-A and SP-D. Collectively, these data suggest that bacterial permeabilization and killing require an interaction between collectins and LPS, most likely by binding of the proteins to the proximal core and/or lipid A moieties of the glycolipids. Liposomes composed of POPE were used for the permeabilization studies because POPE is a major lipid component of Gram negative bacterial membranes (27) and because neither SP-A or SP-D interacts with POPE (28). Fluorescent probe loaded POPE liposomes were permeabilized by the collectins only if rough LPS was incorporated into the membranes; smooth LPS had no effect. These data are consistent with the observed preferential permeabilization of rough E. coli strains by SP-A and SP-D (3). Permeabilization by SP-A only occurred in the presence of calcium. The majority of collectin binding interactions with microbial ligands are mediated by the carbohydrate recognition domain CRD and require calcium (29). Solution of the crystal structure of the trimeric C-terminal domains of rat SP-A suggests possible mechanisms for interaction of SP-A with LPS (30). Calcium binding sites in the CRD determine the positioning of a flexible loop involved in ligand binding interactions. In close proximity, a hydrophobic cleft lined by tyrosines 161, 164, 192 and 208, forms a putative binding pocket for acyl side chains. Polar moieties of LPS may interact with a cluster of basic residues (Arg216, Arg222 and Gln 220) which was found to be tightly associated with 2-(N-morpholino)ethanesulfonic acid (MES), a small molecule which is structurally analogous to phospocholine. Garcia-Verdugo et. al. recently reported that the aggregation of rough LPS micelles but not the binding of SP-A to Re-LPS is calcium dependent, most likely by a mechanism that includes calcium-induced self aggregation of SP-A (30). How calcium participates in the mechanism of permeabilization is unclear, but may include a cation requirement for binding of SP-A to LPS or to itself (self aggregation), a calcium-induced conformational shift in the CRD, or a calcium-dependent intramolecular relationship between protein domains.
We found that incorporation of cholesterol into POPE/J5 LPS liposomal membrane produced a dose-dependent resistance to permeabilization by the collectins. Cholesterol is known to reduce membrane permeability by ordering acyl chains and promoting the straighter ‘trans’ configuration of double bonds; effects that stabilize and thicken phospholipid bilayers in vitro (23;31) and in vivo (32). Cholesterol decreased melittin-induced membrane leakage (24), presumably by enhancing membrane packing and stability. The cholesterol content of mammalian membranes may constitute a protective mechanism against antimicrobial peptides. Although bacterial membranes generally contain little or no cholesterol, some species are able to modulate their cholesterol content in response to environmental influences (33).
Melittin binds to electrically neutral and negatively charged lipid bilayers, and forms pores in bilayer vesicles (34;35). We found that the requirements for melittin-induced membrane disruption were in some respects similar to the collectins, in that cholesterol reduced leakage from liposomes and rough but not smooth LPS conferred susceptibility to permeabilization. However, in contrast to the collectins, the membrane disruptive effects of melittin were calcium-independent, and in fact were partially blocked by calcium addition, as also reported by others (22). These data suggest that melittin and collectins permeabilize membranes by different mechanisms.
We found that exposure of SP-A to lipid peroxidation results in loss of membrane permeabilizing activity. These results are consistent with the previously reported effects of oxidizing stimuli, including the neutrophil oxidative burst and hyperoxia, on the antimicrobial activity of SP-A (5). Oxidative modification of tyrosine residues, which form part of the putative LPS interacting domain of SP-A, has been implicated in the loss of phospholipid binding activity (36). Additional studies will be required to determine the oxidative protein modifications which result in loss of permeabilizing activity.
In conclusion, we find that LPS can confer susceptibility of model bacterial membranes to collectin-mediated permeabilization. Cholesterol can enhance the ordering and integrity of the membrane and increase resistance of POPE membranes to destabilizing collectin effects. The LPS structural requirements for collectin permeabilization and the susceptibility of this collectin activity to oxidative damage are similar to those reported for Gram negative bacteria (3). We postulate that LPS constitutes a ‘handle’ that provides the collectins with a mechanism to distort or perturb membrane structure, and create defects that allow small hydrophilic molecules such as water to enter and traverse the bilayer. The model used in this study may be useful for the testings of truncated collectin molecules or peptides based on collectin sequences that have potential therapeutic value.
Supplementary Material
Acknowledgments
This research was supported by a Merit Grant from the Department of Veteran's Affairs and NIH Grant HL-68861 (both to FXM).
Abbreviation
- LPS
lipopolysaccharide
- SP-A
surfactant protein A
- SP-D
surfactant protein D
- POPE
1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine
- DPPC
1,2-dipalmitoyl-sn-glycero-3-phosphocholine
- EggPC
L-phosphatidylcholine from egg yolk
- 18:0-18:2PC
1-stearoyl-2-linoleoyl-sn-glycero-3-phosphocholine
- ANTS
8-aminonaphthalene-1,3,6-trisulfonic acid disodium salt
- DPX
p-xylene-bis-pyridinium bromide
- PBS
phosphate-buffered saline
- MES
2-(N-morpholino)ethanesulfonic acid
- DNP
2,4-dinitrophenylhydrazine
References
- 1.McCormack FX, Whitsett JA. The pulmonary collectins, SP-A and SP-D, orchestrate innate immunity in the lung. J Clin Invest. 2002;109:707–712. doi: 10.1172/JCI15293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCormack FX, Gibbons R, Ward SR, Kuzmenko A, Wu H, Deepe GS., Jr Macrophage-independent fungicidal action of the pulmonary collectins. J Biol Chem. 2003;278:36250–36256. doi: 10.1074/jbc.M303086200. [DOI] [PubMed] [Google Scholar]
- 3.Wu H, Kuzmenko A, Wan S, Schaffer L, Weiss A, Fisher JH, Kim KS, McCormack FX. Surfactant proteins A and D inhibit the growth of Gram-negative bacteria by increasing membrane permeability. J Clin Invest. 2003;111:1589–1602. doi: 10.1172/JCI16889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weiss J, Beckerdite-Quagliata S, Elsbach P. Resistance of gram-negative bacteria to purified bactericidal leukocyte proteins: relation to binding and bacterial lipopolysaccharide structure. J Clin Invest. 1980;65:619–628. doi: 10.1172/JCI109707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuzmenko AI, Wu H, Wan S, McCormack FX. Surfactant protein A is a principal and oxidation-sensitive microbial permeabilizing factor in the alveolar lining fluid. J Biol Chem. 2005;280:25913–25919. doi: 10.1074/jbc.M411344200. [DOI] [PubMed] [Google Scholar]
- 6.Yeaman MR, Yount NY. Mechanisms of antimicrobial peptide action and resistance. Pharmacol Rev. 2003;55:27–55. doi: 10.1124/pr.55.1.2. [DOI] [PubMed] [Google Scholar]
- 7.Lohner K, Blondelle SE. Molecular mechanisms of membrane perturbation by antimicrobial peptides and the use of biophysical studies in the design of novel peptide antibiotics. Comb Chem High Throughput Screen. 2005;8:241–256. doi: 10.2174/1386207053764576. [DOI] [PubMed] [Google Scholar]
- 8.Palaniyar N, Ridsdale RA, Holterman CE, Inchley K, Possmayer F, Harauz G. Structural changes of surfactant protein A induced by cations reorient the protein on lipid bilayers. J Struct Biol. 1998;122:297–310. doi: 10.1006/jsbi.1998.4004. [DOI] [PubMed] [Google Scholar]
- 9.Kabha K, Schmegner J, Keisari Y, Parolis H, Schlepper-Schaeffer J, Ofek I. SP-A enhances phagocytosis of Klebsiella by interaction with capsular polysaccharides and alveolar macrophages. Am J Physiol. 1997;272:L344–L352. doi: 10.1152/ajplung.1997.272.2.L344. [DOI] [PubMed] [Google Scholar]
- 10.Kuroki Y, Akino T. Pulmonary surfactant protein A (SP-A) specifically binds dipalmitoylphosphatidylcholine. J Biol Chem. 1991;266:3068–3073. [PubMed] [Google Scholar]
- 11.Persson AV, Gibbons BJ, Shoemaker JD, Moxley MA, Longmore WJ. The major glycolipid recognized by SP-D in surfactant is phosphatidylinositol. Biochemistry. 1992;31:12183–12189. doi: 10.1021/bi00163a030. [DOI] [PubMed] [Google Scholar]
- 12.McNeely TB, Coonrod JD. Aggregation and opsonization of type A but not type B Hemophilus influenzae by surfactant protein A. Am J Respir Cell Mol Biol. 1994;11:114–122. doi: 10.1165/ajrcmb.11.1.8018334. [DOI] [PubMed] [Google Scholar]
- 13.Watson RW, Redmond HP, Bouchier-Hayes D. Role of endotoxin in mononuclear phagocyte-mediated inflammatory responses. J Leukoc Biol. 1994;56:95–103. doi: 10.1002/jlb.56.1.95. [DOI] [PubMed] [Google Scholar]
- 14.van Iwaarden JF, Pikaar JC, Storm J, Brouwer E, Verhoef J, Oosting RS, van Golde LM, Van Strijp JA. Binding of surfactant protein A to the lipid A moiety of bacterial lipopolysaccharides. Biochem J. 1994;303(Pt 2):407–411. doi: 10.1042/bj3030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuan SF, Rust K, Crouch E. Interactions of surfactant protein D with bacterial lipopolysaccharides. Surfactant protein D is an Escherichia coli-binding protein in bronchoalveolar lavage. J Clin Invest. 1992;90:97–106. doi: 10.1172/JCI115861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hattori A, Kuroki Y, Katoh T, Takahashi H, Shen HQ, Suzuki Y, Akino T. Surfactant protein A accumulating in the alveoli of patients with pulmonary alveolar proteinosis: oligomeric structure and interaction with lipids. Am J Respir Cell Mol Biol. 1996;14:608–619. doi: 10.1165/ajrcmb.14.6.8652189. [DOI] [PubMed] [Google Scholar]
- 17.Suwabe A, Mason RJ, Voelker DR. Temporal segregation of surfactant secretion and lamellar body biogenesis in primary cultures of rat alveolar type II cells. Am J Respir Cell Mol Biol. 1991;5:80–86. doi: 10.1165/ajrcmb/5.1.80. [DOI] [PubMed] [Google Scholar]
- 18.Kuzmenko AI, Wu H, Bridges JP, McCormack FX. Surfactant lipid peroxidation damages surfactant protein A and inhibits interactions with phospholipid vesicles. J Lipid Res. 2004;45:1061–1068. doi: 10.1194/jlr.M300360-JLR200. [DOI] [PubMed] [Google Scholar]
- 19.Gregoriadis G, Ryman BE. Lysosomal localization of β-fructofuranosidase-containing liposomes injected into rats. Biochem J. 1972;129:123–133. doi: 10.1042/bj1290123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ofek I, Mesika A, Kalina M, Keisari Y, Podschun R, Sahly H, Chang D, McGregor D, Crouch E. Surfactant protein D enhances phagocytosis and killing of unencapsulated phase variants of Klebsiella pneumoniae. Infect Immun. 2001;69:24–33. doi: 10.1128/IAI.69.1.24-33.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yong SJ, Vuk-Pavlovic Z, Standing JE, Crouch EC, Limper AH. Surfactant protein D-mediated aggregation of Pneumocystis carinii impairs phagocytosis by alveolar macrophages. Infect Immun. 2003;71:1662–1671. doi: 10.1128/IAI.71.4.1662-1671.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Portlock SH, Clague MJ, Cherry RJ. Leakage of internal markers from erythrocytes and lipid vesicles induced by melittin, gramicidin S and alamethicin: a comparative study. Biochim Biophys Acta. 1990;1030:1–10. doi: 10.1016/0005-2736(90)90231-c. [DOI] [PubMed] [Google Scholar]
- 23.Fatouros DG, Bouropoulos N, Ioannou PV, Antimisiaris SG. Stability and aggregation studies of non-sonicated arsonolipid-containing vesicles. Cell Mol Biol Lett. 2005;10:173–183. [PubMed] [Google Scholar]
- 24.McIntosh TJ. The 2004 Biophysical Society-Avanti Award in Lipids address: roles of bilayer structure and elastic properties in peptide localization in membranes. Chem Phys Lipids. 2004;130:83–98. doi: 10.1016/j.chemphyslip.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 25.Schaeffer LM, McCormack FX, Wu H, Weiss AA. Interactions of pulmonary collectins with Bordetella bronchiseptica and Bordetella pertussis lipopolysaccharide elucidate the structural basis of their antimicrobial activities. Infect Immun. 2004;72:7124–7130. doi: 10.1128/IAI.72.12.7124-7130.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sahly H, Ofek I, Podschun R, Brade H, He Y, Ullmann U, Crouch E. Surfactant protein D binds selectively to Klebsiella pneumoniae lipopolysaccharides containing mannose-rich O-antigens. J Immunol. 2002;169:3267–3274. doi: 10.4049/jimmunol.169.6.3267. [DOI] [PubMed] [Google Scholar]
- 27.Dowhan W. Molecular basis for membrane phospholipid diversity: why are there so many lipids? Annu Rev Biochem. 1997;66:199–232. doi: 10.1146/annurev.biochem.66.1.199. [DOI] [PubMed] [Google Scholar]
- 28.Meyboom A, Maretzki D, Stevens PA, Hofmann KP. Interaction of pulmonary surfactant protein A with phospholipid liposomes: a kinetic study on head group and fatty acid specificity. Biochim Biophys Acta. 1999;1441:23–35. doi: 10.1016/s1388-1981(99)00142-0. [DOI] [PubMed] [Google Scholar]
- 29.Kishore U, Greenhough TJ, Waters P, Shrive AK, Ghai R, Kamran MF, Bernal AL, Reid KB, Madan T, Chakraborty T. Surfactant proteins SP-A and SP-D: Structure, function and receptors. Mol Immunol. 2005 doi: 10.1016/j.molimm.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 30.Head JF, Mealy TR, McCormack FX, Seaton BA. Crystal structure of trimeric carbohydrate recognition and neck domains of surfactant protein A. J Biol Chem. 2003;278:43254–43260. doi: 10.1074/jbc.M305628200. [DOI] [PubMed] [Google Scholar]
- 31.Kokkona M, Kallinteri P, Fatouros D, Antimisiaris SG. Stability of SUV liposomes in the presence of cholate salts and pancreatic lipases: effect of lipid composition. Eur J Pharm Sci. 2000;9:245–252. doi: 10.1016/s0928-0987(99)00064-0. [DOI] [PubMed] [Google Scholar]
- 32.Wieslander A, Christiansson A, Rilfors L, Khan A, Johansson LB, Lindblom G. Lipid phase structure in the regulation of lipid composition in Acholeplasma laidlawii membranes. Rev Infect Dis. 1982;4 Suppl:S43–S49. doi: 10.1093/clinids/4.supplement_1.s43. [DOI] [PubMed] [Google Scholar]
- 33.Banemann A, Deppisch H, Gross R. The lipopolysaccharide of Bordetella bronchiseptica acts as a protective shield against antimicrobial peptides. Infect Immun. 1998;66:5607–5612. doi: 10.1128/iai.66.12.5607-5612.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwarz G, Zong RT, Popescu T. Kinetics of melittin induced pore formation in the membrane of lipid vesicles. Biochim Biophys Acta. 1992;1110:97–104. doi: 10.1016/0005-2736(92)90299-2. [DOI] [PubMed] [Google Scholar]
- 35.Smith R, Separovic F, Milne TJ, Whittaker A, Bennett FM, Cornell BA, Makriyannis A. Structure and orientation of the pore-forming peptide, melittin, in lipid bilayers. J Mol Biol. 1994;241:456–466. doi: 10.1006/jmbi.1994.1520. [DOI] [PubMed] [Google Scholar]
- 36.Davis IC, Zhu S, Sampson JB, Crow JP, Matalon S. Inhibition of human surfactant protein A function by oxidation intermediates of nitrite. Free Radic Biol Med. 2002;33:1703–1713. doi: 10.1016/s0891-5849(02)01170-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.