Abstract
Aromatic amines and heterocyclic aromatic amines (HAAs) are structurally related classes of carcinogens that are formed during the combustion of tobacco or during the high-temperature cooking of meats. Both classes of procarcinogens undergo metabolic activation by N-hydroxylation of the exocyclic amine group, to produce a common proposed intermediate, the arylnitrenium ion, which is the critical metabolite implicated in toxicity and DNA damage. However, the biochemistry and chemical properties of these compounds are distinct and different biomarkers of aromatic amines and HAAs have been developed for human biomonitoring studies. Hemoglobin adducts have been extensively used as biomarkers to monitor occupational and environmental exposures to a number of aromatic amines; however, HAAs do not form hemoglobin adducts at appreciable levels and other biomarkers have been sought. A number of epidemiologic studies that have investigated dietary consumption of well-done meat in relation to various tumor sites reported a positive association between cancer risk and well-done meat consumption, although some studies have shown no associations between well-done meat and cancer risk. A major limiting factor in most epidemiological studies is the uncertainty in quantitative estimates of chronic exposure to HAAs and, thus, the association of HAAs formed in cooked meat and cancer risk has been difficult to establish. There is a critical need to establish long-term biomarkers of HAAs that can be implemented in molecular epidemioIogy studies. In this review article, we highlight and contrast the biochemistry of several prototypical carcinogenic aromatic amines and HAAs to which humans are chronically exposed. The biochemical properties and the impact of polymorphisms of the major xenobiotic-metabolizing enzymes on the biological effects of these chemicals are examined. Lastly, the analytical approaches that have been successfully employed to biomonitor aromatic amines and HAAs, and emerging biomarkers of HAAs that may be implemented in molecular epidemiology studies are discussed.
Introduction
Historically, the exposure to carcinogenic aromatic amines occurred during the production of dyes and other complex chemicals, and by their use as antioxidants in rubber-manufacturing processes (1,2). A number of aromatic amines arise during the combustion of tobacco (3,4) and occur in the emissions of cooking oils (5). Several heterocyclic aromatic amines (HAAs) are also produced during the high-temperature burning of tobacco (6,7); however, the principal source of exposure to many HAAs occurs by consumption of well-done cooked meats (8-10). HAAs are also present in pan-fried residues used for gravies (11,12), and arise in fumes of cooking oils (13) and the airborne particulates generated by the frying or grilling of meats (14). Chemicals from both classes of compounds induce tumors at multiple sites in experimental laboratory animals during long-term carcinogen bioassays (see Figure 1 for chemical structures). Certain aromatic amines are classified as human carcinogens (Group 1), and several prevalent HAAs have been listed as probable or possible human carcinogens (Group 2A and 2B), based on toxicity data reviewed by the International Agency for Research on Cancer (3,15). The Report on Carcinogens, 11th edition, of the National Toxicology Program, also concluded that prevalent HAAs are “reasonably anticipated” to be human carcinogens (16). Thus, there is much concern about the health risk associated with the exposure to these structurally related classes of chemicals.
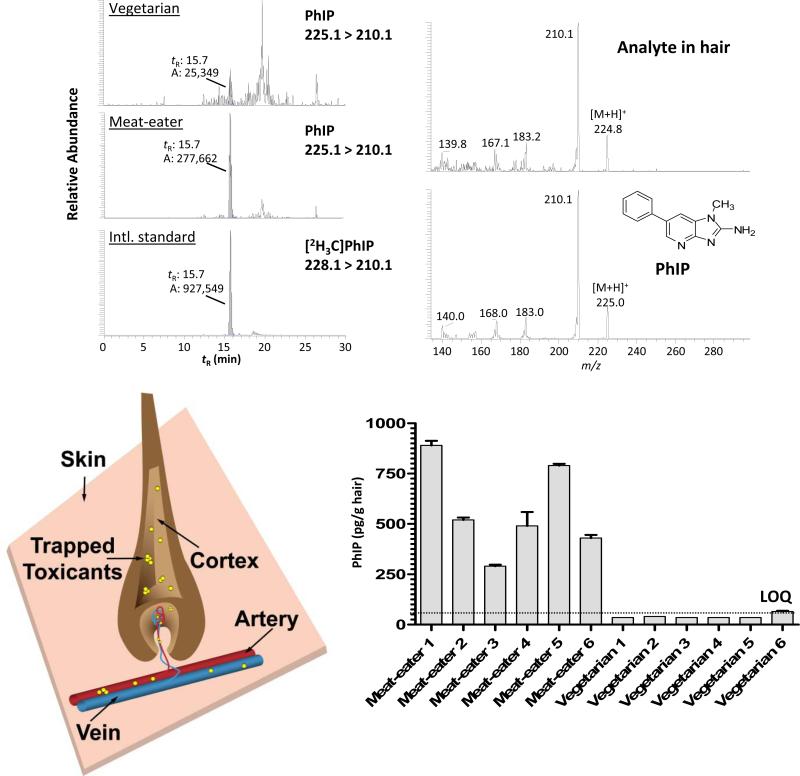
Figure 1.
Chemical structures of prevalent aromatic amines and HAAs
Aromatic amines and HAAs undergo metabolic activation by N-hydroxylation of the exocyclic amine group, to form the proposed arylnitrenium ion, which is the critical metabolite implicated in toxicity and DNA damage (17,18). However, the biochemistry and chemical properties of aromatic amines and HAAs and their metabolites are distinct and different biomarkers of these carcinogens have been employed in human biomonitoring studies. The term biomarker has varied meanings that comprise: markers of susceptibility; makers of the internal dose; markers of the biologically effective dose; markers of early biological effects; markers of altered function; and markers of clinical disease (19,20). In the context used here, the biomarkers are defined as markers of exposure and the biologically effective dose, and are representative early biomarkers of cancer risk. Some of the biomarkers include the unaltered compounds or metabolites in bodily fluids, or protein and DNA adducts derived from the genotoxic metabolites. The characterization of the urinary metabolic profiles of the genotoxicants can provide an estimate of the relative extent of bioactivation, as opposed to detoxification, undergone by the chemicals in vivo (21). These measurements can also reveal interindividual differences in metabolism due to polymorphisms that encode for enzymes involved in xenobiotic metabolism; such differences can affect the genotoxic potency of procarcinogens (22). However, urinary biomarkers of many carcinogens, including HAAs, are transient and only capture the last 24 hours of exposure. For individuals who chronically but intermittently consume grilled meats, urinary HAA biomarkers may go undetected. Longer-lived biomarkers of HAA exposure and genetic damage are required for epidemiological investigations. Certain drugs and carcinogens, including some HAAs, bind with high affinity to proteins and pigments in the hair follicle and become entrapped in the hair-shaft during hair growth (23-25). The biomonitoring of HAAs in hair may provide a more accurate estimate of chronic exposure than the inferences obtained from food frequency questionnaires that are often used in molecular epidemiology studies (19). However, the identification and measurement of chemical specific DNA adducts in the target tissue are the most relevant findings for risk assessment (20,26). Unfortunately, DNA adduct measurements in tissue are often precluded by the unavailability of biopsy samples, which restricts the usage of this biomarker in large scale human studies. Accessible biological fluids, such as blood (27), urine (21), exfoliated bladder epithelial cells in urine (28), or exfoliated mammary epithelial cells in milk of lactating women (29,30), have served as surrogate matrices in which to assess exposure to chemicals or their metabolites or the formation of protein or DNA adducts. The identification of protein or DNA carcinogen adducts clearly demonstrates exposure to the biologically active metabolite, but the adduct must correlate with cancer risk, if it is considered valid as a biomarker of health risk (31,32). The levels of macromolecular carcinogen adduct formation also should be influenced by polymorphisms in genes that encode enzymes involved in the bioactivation and/or detoxication of these chemicals (22).
2-Aminofluorene (AF) and N-acetyl-2-aminofluorene (AAF) are perhaps the most well-studied among the aromatic amines (33). AF and AAF were originally developed as pesticides but never used as intended because they were discovered to be animal carcinogens (34). The pioneering research conducted on the metabolic fate of AF, AAF, and other prototypical arylamines, and the interactions of their metabolites with nucleic acids and proteins (33,35,36) have served as a foundation of knowledge for the development of human biomarkers towards aromatic amines as well as HAAs (31,37,38). Many of the salient studies on the metabolism and biochemical toxicology of aromatic amines are summarized in review articles by Kiese (39); Irving (40), the Millers (35,36,41), Hoffmann and Fuchs (42); Neumann (43); Gorrod and Manson (44); and Kadlubar and Beland (45). The impact of occuptational and tobacco exposures to aromatic amines and cancer risk is summarized by Clayson (34), the Weisburgers (46), and reviewed in the IARC Monographs (1-3,47). The interested reader will find the historical perspectives of aromatic amine carcinogenesis and many citations of the original research in these reviews. More recent reviews on the implementation of biomarkers to monitor human exposure to aromatic amines are highlighted in articles by Neumann (38,48), Skipper and Tannenbaum (31,49), Yu and colleagues (50); Sabbioni and Jones (51), Talaska and Al-Zoughool (52), and Richter and Branner (53).
The research on HAAs commenced in 1977, when this class of genotoxicants was discovered (8). The identification of HAAs in cooked foods is highlighted by Sugimura, Nagao, Wakabayashi, and colleagues (8); Felton, Knize, and colleagues (10); and by others (54-57); mechanisms of HAA formation (58,59); metabolism and genotoxicity (60-70); genetic changes involved tumor genes of HAA carcinogenicity (9,71,72); use of transgenic and mutant animal models for investigations of HAA-induced mutagenesis and carcinogenesis (73,74); earlier reviews on aproaches for human biomonitoring of HAAs and their metabolites (24,75); and the toxicological evaluation of HAAs by IARC (15) and the National Toxicology Program (16) are also cited.
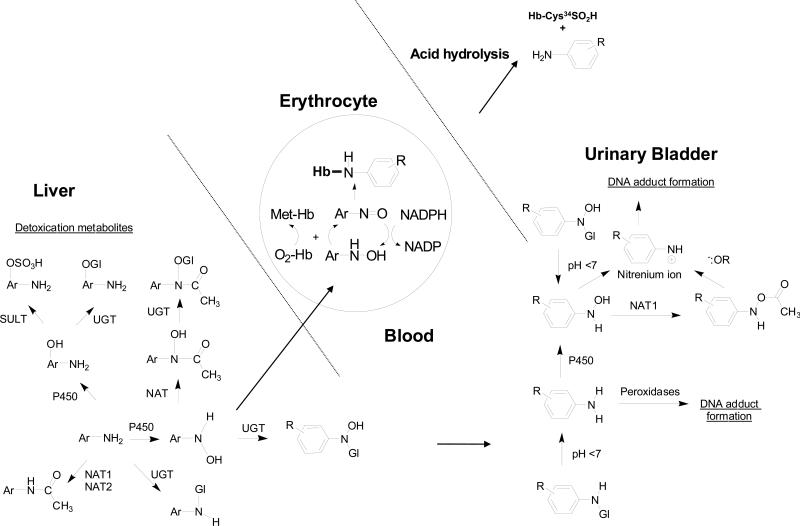
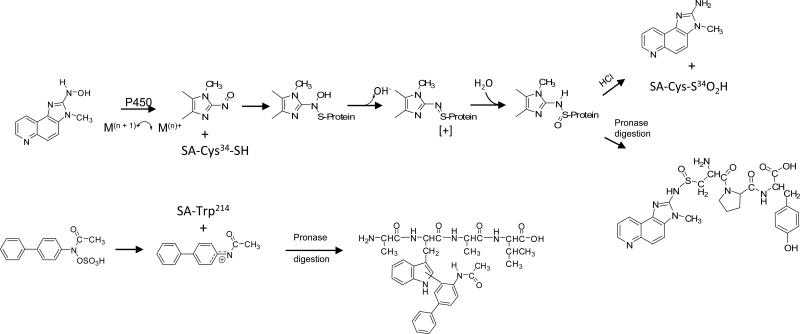
Arylamine-hemoglobin adducts have been extensively used as biomarkers to monitor occupational and environmental exposures to aromatic amines, and to assess the risk of urinary bladder cancer, a target organ of some aromatic amines (34,46,76-78). The biochemistry of arylamine-induced toxicity and methemoglobinemia are well documented (39,79). The arylhydroxylamine metabolites, produced by cytochrome P450s, can penetrate the erythrocyte and undergo a co-oxidation reaction with oxy-hemoglobin (oxy-Hb), to form the arylnitroso intermediates and methemoglobin (met-Hb). The arylnitroso compounds can undergo enzymatic redox cycling within the erythrocyte to reform the aryhydroxylamine and commence another round of co-oxidation with oxy-Hb, ultimately resulting in methemoglobinemia (Figure 2). The arylnitroso intermediate can also react with the Cys93 residue of the human β-Hb chain to form a sulfinamide adduct (79). Many aromatic amines undergo the metabolic pathway of N-oxidation and form the arylamine-Hb sulfinamide adduct (38). In the case of 4-aminobiphenyl (4-ABP), the site of adduction at the Hb-Cys93β chain was proven by xray crystallography (80,81). Arylamine-Hb sulfinamide adducts appear to be fairly stable in vivo (80), but upon acid or base treatment, the adducts undergo hydrolysis to yield the parent amine and the Hb-Cys93β sulfinic acid (31,82). The released aromatic amine can be readily measured by mass spectrometry (MS) methods (31,83). HAAs undergo metabolic activation by N-oxidation (60), but the covalent binding of the N-hydroxy-HAA metabolites to Hb in rodents (84-88) and in humans (89-92) is very low and the HAA-Hb sulfinamide adduct does not appear to be a promising biomarker to assess human exposure. Alternative biomarkers of HAAs have been sought: some of these biomarkers include urinary metabolites, DNA adducts, serum albumin (SA) adducts, and HAA residues in hair (24,63,67,93-95).
Figure 2.
Mechanisms of arylamine-induced methemoglobinemia, arylamine-Hb sulfinamide adduct formation, and arylamine-DNA adduct formation in the urinary bladder. The arylhydroxylamine metabolite can undergo oxidation to the arylnitroso intermediate within the erythrocyte and react with the Hb-Cys93β to form an arylamine-Hb sulfinamide adduct. A portion of the arylhydroxylamine is excreted in urine in the unconjugated form or as an N-glucuronide conjugate. Hydrolysis of the N-glucuronide conjugate by the mildly acidic pH conditions of urine, regenerates the arylhydroxylamine, which undergoes protonation to form the corresponding arylnitrenium ion and reacts with DNA in the urothelium.
The measurement of HAA biomarkers in humans is a difficult analytical task, because usually only ~1 μg to several micrograms of each compound is consumed per day, for individuals eating well-done cooked meat (96). This level of exposure is considerably lower than the levels of occupational exposure to many arylamines. Thus, the concentrations of HAA biomarkers in biological fluids or tissues are often below the part per billion (ppb) level. Many HAA biomarkers are polar and thermally labile molecules, which precludes the employment of gas chromatography (GC) methods for chemical analysis. During the past decade, highly sensitive electrospray ionization (ESI) techniques (97) combined with liquid chromatography (LC) have been developed to detect non-volatile and thermally labile compounds, including several different types of HAA biomarkers (98-103). The challenge remains to establish rapid and robust analytical methods that can be used to measure HAA biomarkers in large scale molecular epidemiological studies. Such biomarkers would permit an accurate measure of HAA exposure, and their inter-relationships with metabolic phenotypes/genotypes involved in HAA genotoxicity and disease risk.
Aromatic Amine and HAA Exposure and Carcinogenesis
Some aromatic amines are known human urinary bladder carcinogens (1-3,34,47). The occurrence of urinary bladder tumors among workers in dyestuff factories was first reported by Rehn in 1895 (104), who attributed these cancers to the patients’ occupation, from which evolved the term aniline cancer (46). The textile dye, chemical, and rubber-manufacturing industries were major sources of occupational exposure to AAs, such as aniline, 4-ABP, 2-naphthylamine (2-NA), benzidine (Bz), and methylenebis-2-chloroaniline (MOCA) (Figure 1), up through much of the first half of the 20th century (1). During that time, epidemiological data emerged, which demonstrated that workers occupationally exposed to these aromatic amines had elevated incidences of bladder cancer (105,106). Aniline is a key intermediate in the manufacturing of dyes. Aniline, however, was not carcinogenic in experimental animals, but 4-ABP, 2-NA, and Bz, contaminants in aniline dyes, were shown to be carcinogenic (1-3,34,46). Hueper established the first successful model for human bladder cancer by demonstrating that dogs exposed to 2-NA developed bladder tumors (107). Thereafter, Radomski and Brill showed that N-oxidation of 2-NA played a critical role in the initiation of bladder cancer in the same animal model (108). The urinary bladder, as well as the liver, intestine, and female mammary gland are among the target organs of cancer development in rodents exposed to aromatic amines (34,46,109).
Historically, the levels of industrial exposure to some aromatic amines were elevated in many manufacturing and chemical plants. In one study, the airborne concentration of Bz in a manufacturing plant, producing 3,000 pounds per shift, was reported to range from <0.007 mg/m3 to a maximum of 17.6 mg/m3, at various locations within the factory (110). This exposure resulted in levels of Bz present in urine at concentrations up to 159 μg/L, following the work shift (110). In another chemical manufacturing plant, the concentrations of MOCA in urine from post-work shift workers were detected at levels ranging from 70 - 1500 μg/L, and the urinary levels of o-toluidine reached up to 132 μg/L from workers, following the work shift in another chemical production plant (111). In the United States and many developed countries, strict federal regulations have drastically diminished the industrial usage of many carcinogenic aromatic amines. However, some aromatic amines, including 4-ABP and Bz, are still found as contaminants at the ppb concentration in color additives (112,113), paints (114), food colors (115), leather and textile dyes (116,117), fumes from heated cooking oils (5), and fuels (118). Cigarette smoking (4) is a prominent source of exposure to aromatic amines. 4-ABP and 2-NA occur in mainstream tobacco smoke at levels ranging from 0.3 - 4 and 2 – 14 ng per cigarette, respectively, whereas the amounts of o-toluidine range from 9 to 144 ng per cigarette (4,119). Another potential source of exposure to some aromatic amines is through the usage of commercial hair dyes (120,121). The exposure to a number of aromatic amines still continues via their oxidized nitroarene derivatives that are present in the atmosphere due to incomplete combustion of organic materials (51,122). There also appears to be considerable non-tobacco associated exposure to monocyclic alkylanilines; the source(s) of exposure remain to be determined (123).
Carcinogenic HAAs were discovered nearly 35 years ago, when Professor Takashi Sugimura at the National Cancer Center in Tokyo, Japan, showed that the charred parts and smoke generated from broiled fish and beef contained substances that exhibited potent activities in Salmonella typhiumurium-based mutagenicity assays (8). Since that hallmark study, more than 25 HAAs have been shown to form in meats, fish, and poultry prepared under common household cooking practices (10,57). The concentrations of HAAs can range from less than 1 ppb to greater than 500 ppb (9,10,124-126). The amounts of HAAs formed in meats are dependent upon the type of meat and the method of cooking; the HAA content generally increases as a function of temperature and the duration of cooking (125,127). There are two major classes of HAAs (Figure 1). The “pyrolytic HAAs” arise during the high-temperature pyrolysis (>250 °C) of some individual amino acids, including glutamic acid and tryptophan, or during the pyrolysis of proteins (6,9,128), but pyrolytic HAAs also can form, at the low ppb concentrations, in some cooked meats (129). HAAs of the second class, aminoimidazoarenes (AIAs), are formed in meats that are cooked at lower temperatures (150 - 250 °C) more commonly used in household kitchens. The Maillard reaction is thought to play an important role in the formation of many AIAs (10,58,130). The N-methyl-imidazole-2-yl-amine portion of the molecule is derived from creatine, and the remaining parts of the AIA skeleton are assumed to arise from Strecker degradation products (for example pyridines or pyrazines), formed in the Maillard reaction between hexoses and amino acids (58,131). An aldol condensation is thought to link the two molecules through an aldehyde or related Schiff base, to form 2-amino-3-methylimidazo[4,5-f]quinoline (IQ) and 2-amino-3-methylimidazo[4,5-f]quinoxaline (IQx)-ring-structured HAAs (132). 2-Amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) can form in a model system containing phenylalanine, creatinine, and glucose (133); however, PhIP can also form in the absence of sugar (10,132). PhIP is the most abundant of the carcinogenic AIAs formed in well-done cooked meats and poultry, where the concentration can reach up to 500 ppb (10,125-127,129,130,134).
Several of the pyrolytic HAAs also are produced during the burning of tobacco. These HAAs induce lacI transgene mutations and aberrant crypt foci in the colon of mice (135,136), and cancer of the liver and/or gastrointestinal tract of rodents (9,137-139). 2-Amino-9H-pyrido[2,3-b]indole (AαC) occurs in mainstream tobacco smoke at levels up to 258 ng/cig (140-142). The amounts of AαC formed in tobacco smoke are ~25 to 100-fold higher than those of 4-ABP (4) or benzo(a)pyrene (143), and comparable to the levels of the tobacco-specific nitrosamine 4-(methyl-nitrosamino)-1-(3-pyridyl)-1-butanone (144); these latter compounds are human carcinogens (145). Other HAAs occur at lower quantities in tobacco smoke: 2-amino-3-methyl-9H-pyrido[2,3-b]indole (MeAαC) forms at 10-fold lower amounts than AαC (6,7,140), the glutamic acid and pyrolysate mutagens, 2-amino-6-methyldiprido[1,2-a:3′,2′-d]imidazole (Glu-P-1) and 2-aminodiprido[1,2-a:3′,2′-d]imidazole (Glu-P-2), and the tryptophan pyrolysate mutagens 2-amino-1,4-dimethyl-5H-pyrido[4,3-b]indole (Trp-P-1) and 2-amino-1-methyl-5H-pyrido[4,3-b]indole (Trp-P-2) occur at <1 ng/cig (146,147). Several AIAs also arise in tobacco smoke: 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) occurs in mainstream smoke at levels up to 23 ng/cig (7,144), while IQ (148) occurs at <1 ng/cig (146). Creatine, a constituent of muscle, is thought to be an essential precursor for the formation of AIAs, on the basis of studies on AIA formation in model systems (58). For that reason, the occurrence of AIAs in tobacco smoke is surprising, although creatinine is present in the soil and in plants (149). PhIP has also been identified in incineration ash and in airborne and diesel-exhaust particles (150). The mechanisms of AIA formation during combustion remain to be determined. The possible causal role of some HAAs in tobacco-associated cancers warrants investigation.
The β-carboline compounds 9H-pyrido[3,4-b]indole (norharman) and 1-methyl-9H-pyrido[3,4-b]indole (harman) are formed at considerably higher levels in tobacco condensates and in cooked foods than are other HAAs (Figure 1) (141,151). Norharman and harman are not mutagenic in S. typhimurium in the presence or absence of liver S9 fraction mixture; however, a synergistic mutagenic effect is observed when these compounds are co-incubated with aniline or o-toluidine (152). This co-mutagenic effect is attributed to the formation of novel, mutagenic HAAs (153). The structures of the compounds formed are 9-(4′-aminophenyl)-9H-pyrido[3,4-b]indole (amino-phenylnorharman, APNH), 9-(4′-amino-3-methylphenyl)-9H-pyrido[3,4-b]indole (amino-methyl-phenylnorharman, AMPNH) and 9-(4′-aminophenyl)-1-methyl-9H-pyrido[3,4-b]indole (aminophenylharman, APH). APNH is a liver and colon carcinogen in F344 rats (154).
The HAAs studied induce tumors at multiple sites in rodents during long-term feeding studies. The target organs include the oral cavity, liver, stomach, colon, pancreas, and the prostate gland in males, and the mammary gland in females (9,155). The total dose required to induce tumor formation (TD50) varies for each HAA and is host species-dependent. The TD50 values of the individual HAAs have been reported to range from 0.1 to 64.6 mg/kg/day in rodents (9). The dose concentrations of HAAs used in these carcinogen bioassays were large: up to several hundred parts per million of HAA in the diet were given to rodents over a 2 year period (9,156). However, the carcinogenic potency of some HAAs is markedly enhanced in experimental laboratory animals exposed to tumor promoters or agents that cause cell proliferation (9,157-159). Moreover, only a fraction of the HAA doses employed during long-term feeding studies can efficiently induce aberrant colonic crypt foci, large intestinal tumors (158,160,161), or mammary gland tumors (157,162), when a diet that is high in fat is incorporated into the feeding regimen. IQ is also a powerful liver carcinogen in non-human primates, with a latent period of just 27 to 37 months, making this compound one of the most powerful carcinogens assayed in non-human primates (163). Summaries of the genetic alterations of target genes of HAAs in experimental animal carcinogenicity studies are available (9,72,73).
The average dietary HAA intake can range from less than 2 to >25 ng/kg per day (96,164). This daily intake level is about one million to 105-fold lower than the TD50 values of individual HAAs to induce tumors in rodents during long-term carcinogen bioassays with standard feeding protocols (9). Thus, the amounts of HAAs consumed may be too small to explain human carcinogenesis, assuming that the susceptibility of humans to HAAs is the same as that of rodents. However, the carcinogenic effects of chronic exposure to multiple HAAs could be additive or possibly synergistic in humans (165). A linear relationship between DNA adduct formation and the HAA dose has been demonstrated in tissues of rodents treated over a wide range with MeIQx (166), IQ (167), and PhIP (168). Moreover, several HAA-DNA adducts have been detected in human tissues (90,169-178), demonstrating that even ppb concentrations of HAAs in the diet can damage DNA. HAAs may be implicated in the development of human cancer under conditions in which many other mutagens-carcinogens, tumor promoters, and factors stimulating tumor progression exist (9,159). The colon, prostate, and female mammary gland are common sites of cancer in Western countries in which well-done cooked meats containing HAAs are frequently consumed (96,179); and the rates of cancer in these organs are increasing in Japan and other countries that are adapting western dietary habits (9). These findings have raised suspicion that HAAs may contribute to the incidences of these cancers and have led to a multitude of epidemiological studies guided by the understanding of HAA exposure and metabolism generated by the laboratory data.
Although the focal point of this review is on the metabolism and the implementation of biomarkers of HAAs for molecular epidemiology studies, the cooking of foods results in the formation of other carcinogens, which include polycyclic aromatic hydrocarbons, furan, acrylamide, among other chemicals that may be harmful to human health. The fundamental question is: do individuals who eat small quantities of any of these carcinogens over a lifetime have an increased cancer risk? There has been debate about the relative level of concern regarding exposure to HAAs as opposed to other genotoxicants in the diet, such as acrylamide, which are present at higher levels than HAAs (180). Risk assessment studies of dietary genotoxic carcinogens, including HAAs and acrylamide, have been reported (179,181-185). The risk characterisation of some genotoxic carcinogens has been conducted by the method of margin of exposure (MOE), which is defined as the ratio between a dose leading to tumor formation in experimental animals and the human intake and can be used to indicate levels of concern and also the ranking between various exposures to genotoxic carcinogens (184,186). The larger the MOE, the smaller the risk posed by exposure to the genotoxic carcinogen under consideration. The international mean intake of acrylamide, which is formed in heated starch-based foods, has been estimated to range from 0.3 to 2.0 μg/kg bw per day for the general population (187). This amount of acrylamide is at least 10-fold greater than the daily HAA exposure. The MOE value for acrylamide was determined to be ~1000-fold lower than the MOE value estimated for PhIP (184), which is the most mass-abundant HAA formed in cooked beef (10). Recent risk assessment approaches have incorporated human exposure data combined with physiologically based pharmacokinetic/pharmacodynamic (PBPK/PD) modeling, which are used to integrate rodent carcinogenicity data and reduce the uncertainty inherent in extrapolating toxicological findings across species and dose by employing common exposure biomarkers (185). In one PBPK/PD modeling study, the risk estimates of population-based lifetime excess cancer risks, based on the average acrylamide consumption in the diet range, was estimated between 1 - 4 × 10-4 (185). The human cancer risk factor estimates reported for HAAs have ranged widely (179,181-183). An upper limit was estimated as ~1 cancer case per 10,000 individuals, when considering exposure to multiple HAAs (181), and a lower limit was calculated at 50 cases per 106 individuals (182). HAA biomarkers were not employed in these risk assessment studies. The wide spread among the risk estimates can be attributed to inter-study differences in the assumptions used to calculate risk factors, including differing estimates of daily individual HAA intake, which can vary by more than 100-fold (10,125,126,134,188-190), different dose extrapolations from animal models using body weight versus surface area scalings, and the usage of TD50 values from various animal carcinogen bioassays, in which differences are seen in the HAA carcinogenic potency (9,163,191). Moreover, pro- and anticarcinogenic dietary factors can affect the metabolism and biological potency of HAAs as well as other procarcinogens (9,159,192). Biomarkers of early biological effects (i.e. macromolecular carcinogen adducts) that can be used in molecular epidemiology studies to assess the dietary exposure, absorption, as well as interspecies and interindividual differences in metabolism of procarcinogens may aid to advance our understanding of health risked posed by different environmental or dietary genotoxicants.
Enzymes of Metabolic Activation and Detoxication of Aromatic Amines and HAAs
The bioactivation of aromatic amines and HAAs, is largely carried out by cytochrome P450 (P450) enzymes (35,36,60,193). Oxidation of the exocyclic amine group produces genotoxic arylhydroxylamine and N-hydroxy-HAA metabolites, whereas oxidation of the aromatic and heterocylic aromatic ring systems produces detoxicated metabolites (34,41,44,45,194-198). There are important differences in the biotransformation pathways of arylamines and HAAs, particularly, by N-acetyltransferases (NAT1 and NAT2), which are discussed below. The conversion of 2-acetylaminofluorene to N-hydroxy-2-acetylaminofluorene in the rat was the first unequivocal proof of N-hydroxylation of an aromatic amine in vivo (199). The arylhydroxylamines, arylhydroxamic acids, and N-hydroxy-HAA metabolites are esterified by N-acetyltransferases (NATs), sulfotransferases (SULTs), L-seryl-tRNA and L-prolyl-tRNA synthetases, and other ATP-dependent enzymes (45,60,68,200-206). These esters are unstable and undergo heterolytic cleavage to produce the reactive nitrenium ion that binds to DNA (37,45,63,194) (Figure 3). In the case of monocyclic alkylanilines, oxidation of the aromatic ring produces phenols, which can can undergo spontaneous or peroxidase-catalyzed oxidation, to form the quinone imine, a highly reactive electrophile that can undergo redox cycling to produce reactive oxygen species (123). This chemical reaction pathway may contribute to the DNA damage of monocyclic alkylanilines.
Figure 3.
The metabolism of 4-ABP and MeIQx as prototypes of aromatic amines and HAAs. NAT enzymes effectively detoxicate arylamines, by N-acetylation; however, many HAAs are poor substrates for NATs. NATs also catalyze the formation of N-arylhydroxamic acids, which can undergo bioactivation by NAT1 and NAT2, or SULTs, or undergo detoxication by UGTs. NAT1 and NAT2 also serve as an N,O-acetyltransferase or O-acetyltransferase and produce reactive N-acetoxy esters of the arylhyroxyalmines and N-hydroxy-HAAs, which are formed by P450s. N-Nitroso-MeIQx formation can occur by reaction with nitric oxide under inflammatory conditions. The N-nitroso-MeIQx intermediate has been proposed to undergo metabolic activation by NAT2 to produce a reactive diazonium ion of MeIQx that may damage DNA (209).
AIAs that contain the N-methyl-imidazole-2-yl-amine moiety, such as IQ and MeIQx, can undergo nitrosation with nitric oxide, under neutral pH conditions, to form 2-nitrosoamino-3-methylimidazo[4,5-f]quinoline and 2-nitrosoamino-3,8-dimethylimidazo[4,5-f]quinoxaline. These N-nitroso-AIA compounds are converted to reactive diazonium species that may form covalent DNA adducts (207,208). A mechanism for the NAT2-catalyzed bioactivation of N-nitroso-MeIQx has been proposed (Figure 3) (209). The bioactivation of AIAs via nitrosation may be an alternative mechanism to P450-mediated N-oxidation of AIAs and contribute to their genotoxicity, under inflammatory conditions, during which elevated levels of nitric oxide can arise (209).
Cytochrome P450s
The mammalian CYP1A1, CYP1A2, and CYP1B1 genes (http://drnelson.uthsc.edu/cytochromeP450.html), encoding cytochromes P450 1A1, 1A2, and 1B1, respectively, and several other xenobiotic metabolism enzyme genes, are regulated by the aromatic hydrocarbon receptor (AHR) (210,211). These P450s are responsible for the metabolic activation of many aromatic amines, HAAs, and polycyclic aromatic hydrocarbons (212-223). Cytochrome P450 1A2 accounts for approximately 15% of the P450 content in human liver (224). The P450 1A1 and 1B1 isoforms are generally not expressed in liver but are present at variable levels in a number of extrahepatic tissues (225-229). P450 1A2 catalyzes the oxidation of many clinically used drugs and alkaloids at appreciable levels including acetaminophen, imipramine, clozapine, caffeine, and theophylliine (230). The 3-N-demethylation of caffeine is catalyzed by P450 1A2, and the urinary ratios among various caffeine metabolites following ingestion of this drug have been used to estimate individual P450 1A2 activity and its inducibility in vivo (231,232). P450 1A2 catalyzes the N-oxidation of planar aromatic amines such as 4-ABP, 2-NA, AF, as well as many HAAs (193,233), while P450 3A4, which is also prominently expressed in liver, catalyzes the N-oxidation of nonplanar aromatic amines such as MOCA (234). P450 3A4 can activate other arylamines and HAAs (235), but at considerably lower rates than P450s 1A1, 1A2, or 1B1 (213,217,218). P450 2A6 was identified as the major P450 responsible for the N-oxidation of alkylanilines (236). The rates of N-oxidation of 4-ABP, MOCA, 2-NA, and HAAs are similar with human liver microsomes (212,215,216,222,233), and comparable steady-state enzyme kinetic parameters have been reported for the N-oxidation of 4-ABP and several HAAs with recombinant human P450 1A2 (220-222,237). Human bladder microsomes also catalyze the N-oxidation of 4-ABP; some of this activity may be attributed to P450 2A13 (238). In addition to N-oxidation, some P450s catalyze oxidation of the aromatic and heteroyclic aromatic ring systems (44,195,239).
The liver is the most active organ in the metabolism and bioactivation of many aromatic amines and HAAs (60,66,197). The constitutive P450 1A2 mRNA expression levels can vary by as much as 15-fold in human liver (240,241), and the expression of hepatic P450 1A2 protein ranges over 60-fold (222,242). Varying levels of CpG methylation (243) and genetic polymorphisms of the upstream 5′-regulatory region of the P450 1A2 gene (244,245) alter the levels of P450 1A2 mRNA expression. Chemicals in the environment (246), tobacco (247,248), and diet, including constituents in cruciferous vegetables (249,250) and grilled meat (251,252), and medications (248,253) bind to the AHR and increase the rate of transcription of the P450 1A2 gene, resulting in increased expression of P450 1A2 protein and other xenobiotic metabolism enzymes (210,211). The interindividual variation in P450 1A2 activity is also observed in vivo for the metabolism of caffeine, a substrate for P450 1A2 (233): more than a 70-fold range in P450 1A2 phenotype activity is observed in humans (231,248,254). The genotype(s) responsible for the large range of interindividual differences in human hepatic P450 1A2 constitutive expression is still not well understood (255). The large interindividual variation in expression of P450 1A2 may be an important determinant of individual susceptibility to aromatic amines and HAAs (22,256).
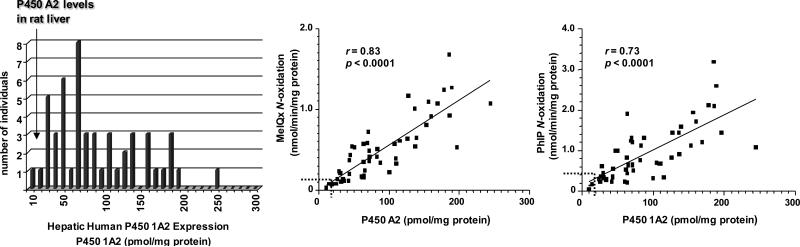
There are also large interspecies differences in the metabolism of 4-ABP and HAA by P450s among mice, rats, and humans (214,222,257-259), that are attributed to different levels of P450 expression, and differences in catalytic activities and regioselectivities of P450s towards these substrates. These interspecies distinctions in enzyme activities must be considered, when human risk assessments of genotoxicants are conducted from experimental animal toxicity data (260). An example of the range in the amount of P450 1A2 protein expressed in human liver samples is shown in Figure 4. It is noteworthy that the expression of P450 1A2 is significantly greater in humans than in rodent strains that are used for carcinogen bioassays. Forty-three out of the 51 human liver microsomal samples contain higher P450 1A2 protein levels (5–250 pmol/mg microsomal protein, median 71 pmol/mg, N = 51) than liver microsomal samples of rats, where P450 1A2 content ranged from 5 to 35 pmol/mg microsomal protein, depending upon the strain, source, and diet (222). The wide range in human P450 1A2 levels is paralleled by a large variation in the rates of N-oxidation of MeIQx and PhIP, which correlate well to the levels of P450 1A2. The rates of N-oxidation of MeIQx and PhIP are much lower in liver microsomal samples obtained from different strains of rat, which is reflective of the lower amounts of P450 1A2 protein expressed in rat liver.
Figure 4.
Levels of expression of P450 1A2 in human liver microsomes and correlation between P450 1A2 expression and rates of N-oxidation of MeIQx and PhIP (222). The checkered lines depicted in the correlation regression curves show the upper levels of P450 1A2 expression and rates of N-oxidation of MeIQx and PhIP in rat liver microsomes.
There are important differences between human and rodent P450s in terms of catalytic activity and regioselectivity of HAA oxidation; these characteristics affect the toxicological properties of the molecules (222,261). The catalytic efficiency of recombinant human P450 1A2 is superior to that of rat P450 1A2, in the N-oxidation of PhIP and MeIQx. Recombinant human P450 1A2 shows about a 1.5-fold greater kcat (nmol product/nmol P450/min) and 13-fold lower Km for PhIP N-oxidation compared to rat P450 1A2. In the case of N-oxidation of MeIQx, the Km for recombinant human P450 1A2 and rat P450 1A2-mediated N-oxidation of MeIQx are similar, but the kcat for recombinant human P450 1A2 was 16-fold greater than that of rat P450 1A2. The interspecies differences in the enzyme kinetic parameters for N-oxidation of PhIP and MeIQx have also been observed with human and rat liver microsomal samples (222). However, the enzyme kinetic parameters for the O-demethylation of methoxyresorufin are similar for human and rat P450 1A2 (222).
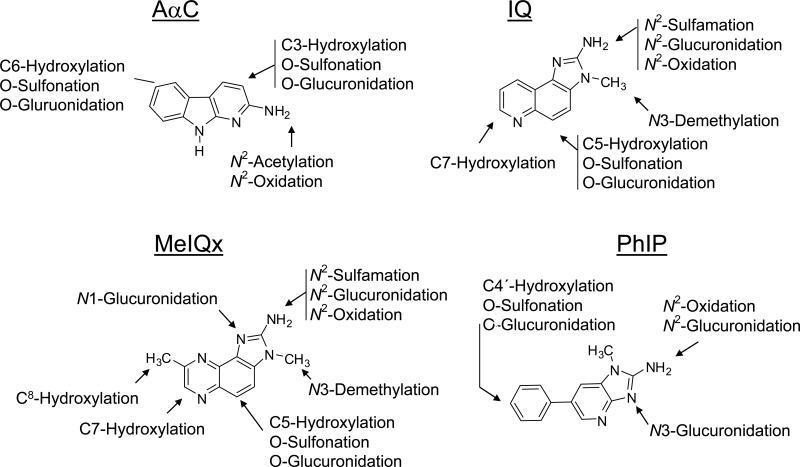
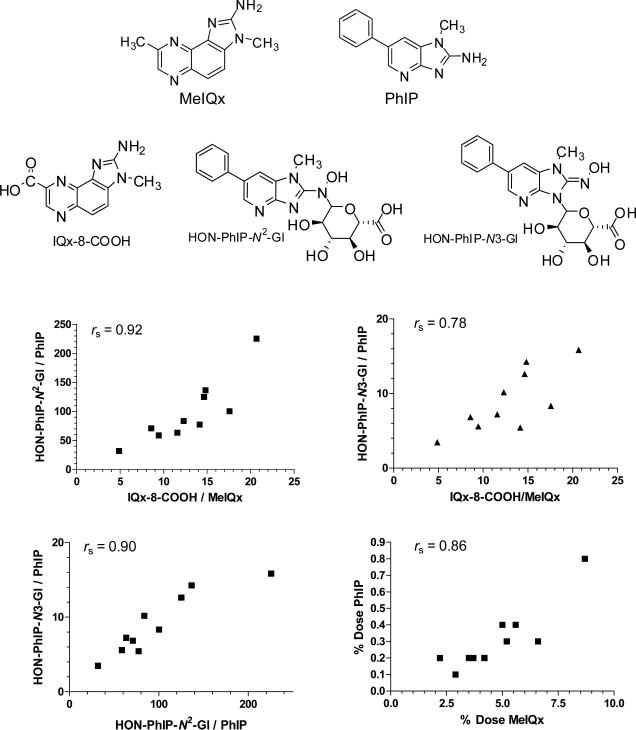
Important species differences also exist in the regioselectivity of P450 1A2-mediated oxidation of HAAs. Human P450 1A2 is regioselective for the N-oxidation (bioactivation) of HAAs, such as IQ, MeIQx, and PhIP, and this enzyme does not appreciably catalyze the ring-oxidation (detoxication) of the heteroaromatic ring systems. However, the P450 1A2 orthologues of experimental laboratory animals produce both N-oxidation and ring-oxidation products at comparable levels (103,214,222,262). Human P450 1A2 also catalyzes the oxidation of the C8-methyl group of MeIQx to form the alcohol, 2-amino-(8-hydroxymethyl)-3-methylimidazo[4,5-f]quinoxaline (8-CH2OH-IQx), which undergoes further oxidation by P450 1A2 to form the the carboxylic acid, 2-amino-3-methylimidazo[4,5-f]quinoxaline-8-carboxylic acid (IQx-8-COOH) (Figure 5) (262). IQx-8-COOH formation is the major pathway of metabolism and detoxication of MeIQx in humans (103). The rat P450 1A2 orthologue catalyzes the detoxication of MeIQx through C-5 hydroxylation, but it does not catalyze IQx-8-COOH formation (85,262-264). In the case of PhIP, human P450 1A2 is highly selective for N-oxidation, whereas rat P450 1A2 catalyzes both N-oxidation and 4′-hydroxylation of the phenyl ring of PhIP, to produce the detoxicated product, 2-amino-4′-hydroxy-1-methyl-6-phenylimdazo[4,5-b]pyridine (220-222,265).
Figure 5.
Major pathways of metabolism of AαC, IQ, MeIQx and PhIP in experimental laboratory animals and humans.
The metabolism of IQ, MeIQx, PhIP, and AαC (Figure 5) has been studied with rodent and human liver microsomes (60,197,214-216,222,257,266-273), in experimental laboratory animals (66,85,195,197,239,263,274,275,275-281), rodent hepatocytes (195,265,282,283), human hepatocytes (262,284), and HepG2 cells (283). A number of metabolites of MeIQx and PhIP have also been identified in human urine (98,101,103,176,264,285-293). P450-mediated ring-oxidation of MeIQx, IQ, and PhIP are major pathways of metabolism and detoxication in rodents (85,214,222,239,274) and in Cynomolgus monkeys (196). P450 1A2 is not expressed in liver of Cynomolgus monkeys (294) and other P450s, including P450 3A4 and/or P450 2C9/10 appear to contribute to the ring and exocyclic N-oxidation of HAAs in this species (196). These other P450s were reported to N-hydroxylate IQ to an appreciable extent, but did not catalyze the N-oxidation of MeIQx; IQ is a carcinogen in Cynomolgus monkeys, but MeIQx is not (196). The P450-mediated N-demethylation of IQ and MeIQx is another important biotransformation pathway of IQ and MeIQx in rodents and nonhuman primates (196,281). N-Demethylation of IQ is thought to be a detoxication pathway because the mutagenic potency of desmethyl-IQ is more than 60-fold weaker than IQ (295). However, the P450-mediated N-demethylation of IQ or MeIQx is negligible with human liver microsomes (215,222,233), human hepatocytes (272), or in humans (103,264). The microflora of the human colon catalyzes the oxidation of IQ and MeIQx at the C-7 atom of the heterocyclic ring (296); these oxidation metabolites are not carcinogenic in rodents (297).
Numerous studies have shown that P450 1A2 plays a major role in the metabolic activation of aromatic amines and HAAs and in the formation of DNA adducts in rodents (63,298,299) (and references cited therein). The pretreatment of human liver microsomes with various amounts of furafylline, a mechanism-based inhibitor of P450 1A2 (300), led to a concentration-dependent inhibition of HONH-MeIQx, 8-CH2OH-IQx, IQx-8-COOH, and HONH-PhIP formation by up to 95% (215,216,220,222,261), indicating the important contribution of human P450 1A2 in the metabolism of these carcinogens. The formation of 8-CH2OH-IQx and IQx-8-COOH, and the glucuronide conjugates of HONH-MeIQx and HONH-PhIP, were also inhibited to a similar degree in human hepatocytes pretreated with furafylline (262,284). In humans, the contribution of P450 1A2 to the metabolism of MeIQx and PhIP was demonstrated in a pharmacokinetic study that used furafylline (301). As much as 91% of the MeIQx and 70% of the PhIP consumed in grilled meat were estimated to undergo metabolism by P450 1A2 (301). Thus, P450 1A2 significantly contributes to the metabolism of both MeIQx and PhIP in vivo in humans, but with marked differences in substrate specificity. Human P450 1A2 primarily catalyzes the detoxification of MeIQx by oxidation of the 8-methyl group, whereas it catalyzes the bioactivation of PhIP by oxidation of the exocyclic amine group (Figure 5) (103,262). These metabolic studies support the notion that P450 1A2 is a major enzyme involved in the metabolism of MeIQx and PhIP in humans.
Conversely, the results from several studies employing transgenic rodents have led investigators to propose that alternative enzymes are involved in HAA- and arylamine-mediated toxicity and that P450 1A2 may even be protective against these carcinogens in animals (211). The levels of DNA adducts of IQ and PhIP were found to be lower in some organs of P4501A2-knockout mice than in organs of wild-type mice; however, other P450s or enzyme pathways of activation also contributed to DNA adduct formation in specific organs (302). In the neonatal mouse model, higher incidences of lymphoma and hepatocellular adenoma occurred in female P4501A2-knockout mice than in wild-type mice exposed to high doses of PhIP (11 or 22 mg/kg) (258), indicating PhIP-induced carcinogenesis is independent of P450 1A2 expression. Methemoglobin formation, a biomarker of exposure and toxicity to certain aromatic amines, was higher in P450 1A2-knockout mice than in wild-type mice exposed to 4-ABP (303). Furthermore, P450 1A2 expression in wild-type mice was not associated with 4-ABP-induced hepatic oxidative stress or with 4-ABP–DNA adduct formation (304). 4-ABP-induced hepatocarcinogenesis in P4501A2-knockout mice was also found to be independent of P450 1A2 (259). These paradoxical effects may lead us to question the importance of P450 1A2 in HAA- and 4-ABP-mediated toxicity and malignancy (211,305). We note that very high concentrations of HAAs and 4-ABP were employed in these transgenic rodent studies; the high doses may have triggered metabolic pathways that lead to formation of chemically reactive metabolites, by other P450s or Phase I enzymes, which may not arise under low-dose treatments. Indeed, liver microsomes from P450 1A2-knockout mice displayed significant N-oxidation activity of PhIP and 4-ABP (258,259). The role of P450 1A2 in the activation as opposed to the detoxication of HAAs or AAs in the intact animal is likely to depend on the extent of Phase II metabolism, the degree of coupling of N-oxidation with Phase II enzymes, and cell type- and tissue-specific context, as well as the dose and pharmacokinetics of the compound under study (211,305). Investigations in “humanized” mice containing the P450 1A2 allele in place of the orthologous mouse gene (280,306) can be used to assess the role of human P450 1A2 in the DNA damage induced by HAAs and AAs, under realistic human exposure levels.
Peroxidases
Peroxidases, including prostaglandin H synthase (PHS), an arachidonic acid-dependent peroxidase, may play a significant role in the activation of aromatic amines and HAAs in extrahepatic target tissues of experimental animals, such as urinary bladder, colorectum, and mammary gland, where the P450 content is low (307-318). Much of the data are consistent with a one-electron mechanism of arylamine or HAA oxidation by PHS, and the N-hydroxy intermediates do not appear to be involved in the metabolism by PHS (310). However, a number of the PHS oxidized products of arylamines and AIAs generate a DNA adduct profile that is similar to those generated by P450s, suggesting a common DNA-reactive species, presumably an arylnitrenium ion, produced by different pathways in these cellular and enzyme model systems (310,316,319-322).
N-Acetyltransferases
N-Acetyltransferases (NATs) are critical enzymes involved in the genotoxicity of aromatic amines and HAAs. There are two distinct N-acetyltransferase isoenzymes (designated NAT1 and NAT2, http://louisville.edu/medschool/pharmacology/consensus-human-arylamine-n-acetyltransferase-gene-nomenclature/). NAT2 is expressed primarily in the liver, whereas NAT1 appears to be more prominently expressed in extrahepatic tissues (323,324). More than 25 genetic polymorphisms have been identified for both NAT genes that can affect the catalytic activity of NATs toward aromatic amines and HAAs (323,325,326). NAT enzymes have a dual role in the metabolism of aromatic amines and HAAs: these enzymes can serve as mechanisms of bioactivation or detoxication. Some epidemiological studies suggest a role for NAT2 activity in human susceptibilities to various cancers from tobacco smoke and from consumption of well-done meats, where the exposures to aromatic amines and HAAs can be substantial (327,328).
N-Acetylation is an important mechanism of detoxication of aromatic monoamines (324): this biotransformation pathway is catalyzed by both NAT1 and NAT2 and serves as a competing pathway of N-oxidation (203). The resulting acetamides are generally viewed as poor substrates for P450-mediated N-oxidation (Figure 3) (329). For many aromatic amines, the catalytic efficiency (kcat/Km ) of N-acetylation by recombinant NAT1 is superior to that of recombinant NAT2, but the relative affinity (Km) for each of the AA substrates investigated was higher for recombinant NAT2 (203). Bz, an aromatic diamine, is an exception. The N-acetylation of one of the amine groups of Bz appears to facilitate P450-mediated N-oxidation of the non-acetylated amine group, to form the reactive N-4-hydroxyamino-N′-acetylbenzidine (HONH-N′-acetyl-Bz) metabolite (318,330). Bz is preferentially N-acetylated by NAT1 (331,332)
N-Acetylation of the arylhydroxylamines also occurs, to form the arylhydroxamic acids, which can undergo bioactivation by N,O-acetyltransferase or sulfotransferases (SULTs) (45). Direct activation of the N-hydroxy-AAs by O-acetylation also occurs and results in formation of the reactive N-acetoxy intermediates that readily bind to DNA (60,194,333). NAT1 appears to function as an O-acetyltransferase (OAT) and as an N,O-acetyltransferase, when using acetyl coenzyme A or arylhydroxamic acids, respectively, as acetyl donors. NAT2 appears to act preferentially as an OAT and NAT (Figure 3). HAAs that contain the N-methyl-imidazo-2-yl-amine moiety (AIAs) are poor substrates for NATs and N-acetylation is not an important pathway of detoxication in rodents or humans. AαC and several other pyrolysate HAAs are substrates for rodent NATs. Nonetheless, the catalytic rates are ~1/1000 the level observed for the N-acetylation of AF (60). In contrast to the parent HAAs, the HONH-AIA and HONH-HAA metabolites do undergo O-acetylation, primarily by NAT2, to form the reactive N-acetoxy species, which bind to DNA (Figure 2) (63,334,335). N-Hydroxy-AαC is an exception and it undergoes O-acetylation by both NAT1 and NAT2 (271).
A mouse model deficient in both NAT1 and NAT2, Nat1/2(-/-), was employed to examine the pharmacokinetics of 4-ABP, AF and PhIP (336). The metabolism of AF was severely affected and the plasma clearance was increased by 4-fold in Nat1/2(-/-) mice, whereas the clearance of 4-ABP was found to be less dependent on N-acetylation, and no difference in 4-ABP plasma clearance rates was observed between wild-type and knockout animals. PhIP did not undergo N-acetylation, nor was its clearance affected by NAT genotype (336). In adult female rapid and slow acetylator rats congenic at the NAT2 locus, PhIP-DNA adduct formation was unaffected by NAT2 acetylator status in liver or any of the extrahepatic tissue examined, whereas MeIQx-DNA adducts, particularly in liver, were significantly lower in slow acetylators (337). Similar findings were observed in congenic rapid and slow acetylator Syrian hamsters, PhIP-DNA adduct formation was independent of N-acetylator activity (338). These data signify that PhIP genotoxicity in rodents is not influenced by NAT enzymes.
HONH-PhIP, like many other HONH-HAAs, undergoes activation by human NATs in subcelluar cytosolic assays (339), and by recombinant NAT2 (335), to form the reactive N-acetoxy-PhIP intermediate, which binds to DNA (340,341). However, the level of PhIP-induced mutation and DNA adduct formation in Chinese hamster ovary cell lines cotransfected with NAT2*4 (rapid acetylator) or NAT2*5B (slow acetylator) alleles with either P450 1A1 or P450 1A2 is comparable to cell lines only transfected with the P450s (342,343). A similar result was demonstrated in Salmonella typhimurium bacterial strains expressing human NAT1 or NAT2 (68,344,345), and PhIP appeared to be activated by other phase II enzymes, including SULTs (68,345,346). A much more potent effect of NAT2 phenotype was demonstrated for the induction of mutagenicity and DNA adduct formation of MeIQx (347), IQ (342), and AαC (348). The findings indicate that HONH-PhIP is a poor substrate for rodent and human NATs. Thus, metabolic data obtained with subcelluar fractions or isolated enzymes, particularly when high substrate concentrations are employed, may not be reflective of enzyme activity that occurs within cells. Therefore, the adverse biological effects of NAT2 phenotype in the gene–environmental (cooked red meat) studies may reflect exposure to other HAAs such as MeIQx and AαC more so than PhIP. The identification of exposure to specific HAAs is very important in molecular epidemiological investigations that seek to assess the significance of HAAs and NAT2 genetic polymorphism in cancer risk.
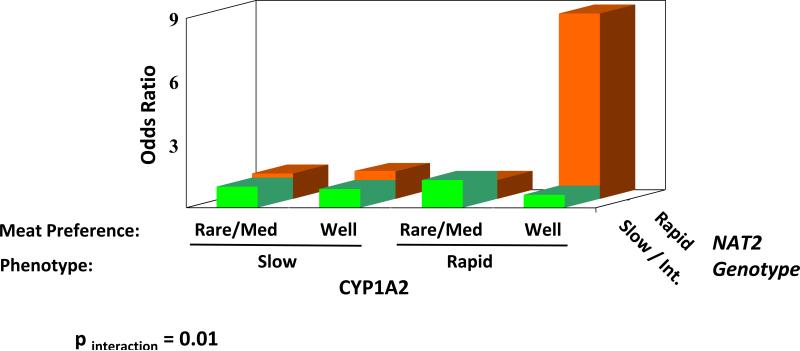
The role of NAT2 genetic polymorphism in cancer risk has been studied extensively, and the elevated risk of urinary bladder cancer in cigarette smokers who are slow N-acetylators is well documented (50,77,78,349). This increased cancer risk has been attributed to the diminished capacity of slow N-acetylator individuals to detoxicate aromatic amines present in tobacco, some of these aromatic amines are bladder carcinogens (1-3,34,326) (Figure 3). However, the role of NAT2 phenotypes in cancer risk of HAAs is unclear (204,323). NAT2 does not efficiently detoxicate most HAAs, but the N-hydroxylated HAA metabolites are substrates for O-acetylation by NAT2 and the resultant N-acetoxy intermediates readily bind to DNA (60,63,334,335,339,350,351). As a result, the increased cancer risk may be markedly elevated in individuals who are both rapid P450 1A2 N-oxidizers and rapid O-acetylators (327,328).
Sulfotransferases
The sulfotransferases (SULTs) are another Phase II enzyme involved in the metabolism of aromatic amines and HAAs. The SULTs belong to a super family of genes that are divided into two subfamilies: the phenol SULTs (SULT1), and the hydroxysteroid SULTs (SULT2) (352-354). SULT1A1, 1A3, and 1B1 are expressed in all parts of the gastrointestinal tract, often exceeding the protein levels that are expressed in the liver (355). In addition to the sulfating of phenolic xenobiotics, steroids, and estrogens, the SULT enzymes can serve to detoxicate or bioactivate HAAs or AAs (68,356). Rat SULT1A1 catalyzes the formation of sulfamates of IQ and MeIQx (357-359) as detoxication products, but the sulfamation of PhIP does not occur in rats or other experimental laboratory animals (195). The sulfamate of MeIQx is excreted in urine of humans (264,285): its formation is presumably catalyzed by SULT1A1 (359). Boyland et al. (360) demonstrated that rats dosed with aniline, 1-naphthylamine, or 2-NA excrete in the urine a very small amount of these aromatic amines as the sulfamate derivatives. The sulfamates of IQ and MeIQx are quite stable under the range of pH conditions that exist in urine (285,357,358), whereas the sulfamates of many arylamines are labile (360).
Human SULT1A1 and SULT1A2 catalyze the binding of the N-hydroxy metabolites of MOCA, AF, AAF, 4-ABP, PhIP, AαC, and MeAαC to DNA, although the N-hydroxy metabolites of MeIQx and IQ are poor substrates for both SULT isoforms (68,202,205,345,356,361-363). The SULT-mediated metabolic activation of arylhydroxylamines and N-hydroxy-HAAs has been detected in human liver, colon, prostate, and female mammary gland cytosols, but not in pancreas, larynx, or urinary bladder epithelial cytosols (361,364-366). SULT1E1, which is under hormonal regulation, catalyzes the binding of HONH-PhIP to DNA in cultured human mammary cells. Therefore, SULT1E1 was proposed to play a role in the bioactivation of PhIP in breast tissue (367). However, a recent study failed to detect the SULT1E1 protein in breast tissue and factors in the cell culture media may have induced the expression of SULT1E1 protein in cultured human mammary cells (365).
One common genetic polymorphism, an Arg213His polymorphism in the SULT1A1 gene, has a strong influence on the level of enzyme protein and phenol sulfotransferase activity in platelets, which has been used for metabolic phenotyping (205). The frequency of the variant SULT1A1*2 allele exceeds 10% in Japanese (368), African-Americans, and Caucasians (369,370). The SULT1A1*2 protein has low enzyme activity and stability compared to the wild-type SULT1A1*1 protein (361). DNA binding studies using recombinant SULT1A1*1 and SULT1A1*2 have shown that SULT1A1*1 protein catalyses HONH-4-ABP and HONH-PhIP DNA adduct formation with much greater efficiency than the SULT1A1*2 variant (205). Several molecular epidemiological studies have explored the roles of SULT1A1*1 and SULT1A1*2 genotypes and putative HAA exposure in breast (371), colorectal (370,372), and prostate cancer risk (373). The expression of the variant allele SULT1A1*2, with diminished capacity for bioactivation of some HONH-HAAs, was associated with decreased risk of breast cancer for women who often ate well-done cooked meat (371); however, this genotype was not associated with a decreased risk of colorectal (370,372) or prostate cancer (373). The frequency of consumption of grilled meats and the extent of exposure to HAAs are uncertain in these subjects. In the absence of exposure to biologically relevant levels of HAAs, a genetic polymorphism would not be expected to be manifested as a risk factor (374). Since SULTs are involved in both the metabolic activation and detoxication of HAAs and other dietary genotoxicants, as well as in maintaining hormonal homeostasis, it has been difficult to predict the impact of SULT enzymes in individual susceptibilities following exposure to cooked meat.
UDP-Glucuronosyltransferases
UDP-Glucuronosyltransferases (UGTs) catalyze the glucuronidation and elimination of numerous classes of xenobiotics, steroids, and endogenous compounds, as well as the detoxication of various carcinogens (375-377) (http://www.pharmacogenomics.pha.ulaval.ca/sgc/ugt_alleles/). The UGTs are present in the 1A, 2A, and 2B subfamilies and expressed in liver and extrahepatic tissues. Aromatic amines and HAAs undergo metabolism by UGTs. The UGT1A family contributes more to the metabolism of aromatic amines than does the UGT2B family (378,379). 4-ABP, Bz, and N-acetyl-Bz were reported to be catalyzed most efficiently by UGT1A9, followed by UGT1A4, UGT2B7, and lastly by UGT1A1 (379-382). Many of these isoforms are also involved in the N-glucuronidation of the respective arylhydroxylamines or the O-glucuronidation of N-arylhydroxamic acids (383-386). The N,O-glucuronide conjugates of N-arylhydroxamic acids are fairly stable and are viewed as detoxication products (40,383,387,388), whereas the N,O-sulfonates of N-arylhydroxamic acids are highly reactive species that bind to DNA and protein (Figure 3) (389-391).
Depending upon the structure of the HAA and the UGT isoform, glucuronidation can occur at the exocyclic amine group or the endocyclic N-imidazole atom of the AIAs and the N-hydroxy-AIAs (282,392-395). O-Glucuronide conjugates of ring-oxidized AIA metabolites are also prominent metabolites that are excreted in urine of rodents (195,239) and nonhuman primates (196), but not in urine of humans (98,103,176,264,293). The human UGT1A family of enzymes is principally involved in the N-glucuronidation of PhIP (396-398) and most likely MeIQx as well (262). On the basis of studies with recombinant enzymes, the human UGT1A1 isoform, followed by UGT1A4, UGT1A8, and UGT1A9 are the most active enzymes involved in N-glucuronidation of PhIP and HONH-PhIP (397); other studies reported that UGT1A9 (399,400) or UGT1A10 (401) were highly active isoforms in glucuronidation of HONH-PhIP. The N2 atom of HONH-PhIP is the preferred site of conjugation for all of the recombinant UGTs studied, except for UGT1A9, where the N3 imidazole atom is the preferential site of conjugation (401,402). The levels of formation of N2-(ß-1-glucosiduronyl-2-(hydroxyamino)-1-methyl-6-phenylimidazo[4,5-b]pyridine (HON-PhIP-N2-Gl), the principal metabolite of PhIP excreted in human urine (98,101,103,176), showed a high interindividual variability in formation, up to 28-fold, with human liver microsomes (398). High and variable levels of UGT-catalyzed glucuronidation of HONH-PhIP were also detected with human colon microsomes, signifying that extrahepatic UGTs, such as UGT1A10, may serve as an important enzyme of detoxication of HONH-PhIP in colon (401,402).
The differential rates of UGT isoform activities reported for aromatic amines, HAAs, and their N-hydroxylated substrates should be viewed with caution. The discrepancy in enzyme activities observed among the different UGTs may be in part due to different systems used for screening enzyme activity: UGTs are membrane-bound and recombinant UGT-over-expressing baculosomes do not necessarily mimic activities that are observed for UGT-over-expressing cell lines (397,401). Moreover, the complete activation of UGT activity in microsomal preparations requires the presence of detergents or the membrane-permeabilizing agent alamethicin (394,402) to overcome the latency associated with UGT-membrane bound enzymes; the assay conditions, buffers and cofactors were different in the studies cited above.
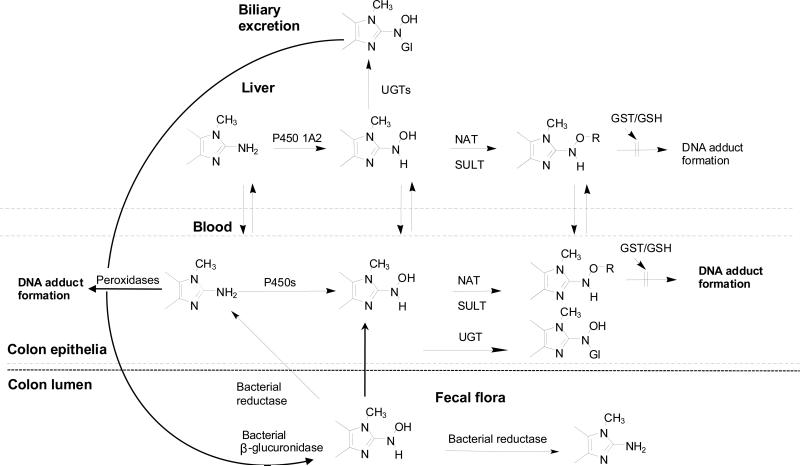
The N-glucuronidation of arylamines and arylhydroxylamines is viewed as a mechanism of transport of the carcinogenic intermediates, to the urinary bladder and colon (Figure 2 and Figure 6), and thought to contribute to the organotropism of aromatic amine carcinogenesis. The N-glucuronide conjugates of arylamines, HAAs, and their N-hydroxylated metabolites are eliminated in urine and bile of animal species and humans (66,98,101,103,196,386,393,403,404). Arylamine and arylhydroxylamine N-glucuronide conjugates can undergo hydrolysis in the range of pH conditions that exist in urine (383), whereas AIA and HONH-AIA N-glucuronide conjugates are stable (103,275,358,393,394). The half-lives of the N-glucuronides of 4-ABP and HONH-4-ABP are 10.5 and 32 min, respectively, at pH 5.5; the half-lives of N-glucuronide conjugates of Bz and the N′-glucuronide of the HONH-N′-acetyl-Bz are 7.5 min and 3.5 h at pH 5.5 (318,380,385). The regenerated arylamines can undergo bioactivation by P450s or peroxidases in the bladder epithelium (238,318). The reactivity towards DNA of many arylhydroxylamines shows strong pH dependence: the level of DNA adduct formation at pH 5.0 is 10 to 50-fold higher than the level of adduct formed at pH 7.0 (45). This enhanced reactivity at acidic pH is attributed to the formation of the nitrenium ion (76,405). Thus, arylhdroxylamines that are eliminated in urine as the unconjugated metabolites, or produced by hydrolysis of the N-glucuronide conjugates, undergo protonation in the acidic bladder lumen, to produce reactive species that readily bind to DNA of the urothelium (384,385) (Figure 3).
Figure 6.
Metabolism of aromatic amines and HAAs by UGTs, and the role of UGTs in transport of the genotoxic arylhydroxylamine and N-hydroxy-HAA metabolites to the colon, to form DNA adducts.
The pH of urine has also been reported to have a strong influence on the levels of urinary Bz and its urothelial DNA adducts formed in humans: A high urine pH was inversely correlated with the proportions of free Bz, N-acetyl-Bz in urine of post-shift factory workers, and the average of each subject's urine pH was negatively associated with the urothelial adduct N-(deoxyguanosin-8-yl)-N′-acetylbenzidine (332,406). When internal dose was controlled, subjects with a urine pH < 6 had 10-fold higher DNA adduct levels than subjects with a urine pH > 7 (406). A more recent study has reported that urine pH is a risk factor for bladder cancer and a dose-response relationship in bladder cancer risk was observed with increasing urinary acidity among current smokers (407). These findings are consistent with the biochemical properties of aryalmines and support a causal role of arylamines in bladder cancer. The glucuronide conjugates of HAAs, formed at either exocyclic or endocyclic nitrogen atoms of the AIA and HONH-AIA imidazole moieties are stable in weak acid (103,275,358,393,394), and the reactivity of N-hydroxy-AIAs with DNA is not appreciably enhanced by weak acid (339,408). These chemical properties may help to explain why AIAs are not bladder carcinogens in experimental laboratory animals and possibly in humans (9).
The UGT metabolism of arylamines is also thought to contribute to the organotropism of aromatic amine-mediated large intestinal carcinogenesis. Studies on aromatic amines in rodents with surgically performed colostomies showed that tumors exclusively appeared proximal to the colostomy, where the intestinal segments were in actual contact with the fecal stream (403,409-411). These experiments provided strong evidence that the induction of tumors in the intestine was related to the transport of some form of the carcinogen via the bile into the intestines rather than by the blood stream. The N-glucuronide conjugates of arylhydroxylamines undergo hydrolysis by bacterial β-glucuronidases within the intestines, to release the arylhydroxylamine species (66,412), which are bioactvated by NATs or SULTs expressed in the intestines, to form DNA adducts (Figure 6) (22,66,339,361,413).
The N2- and N3-glucuronide conjugates of HONH-PhIP are substrates for β-glucuronidases of E. coli. from the fecal flora of rodents and humans (393). The liberated HONH-PhIP would be expected to form DNA adducts in colorectal tissue. However, the same level of PhIP-DNA adducts were reported to form in colon and other extrahepatic tissues of sham- and bile duct-ligated rats (404), implying that the N-glucuronide conjugates of HONH-PhIP eliminated in bile or the blood stream are not involved in PhIP-DNA adduct formation in the colon or other extrahepatic tissues. Intestinal bacteria of rodents and humans have been reported to catalyze the reduction of HONH-PhIP back to PhIP (393). Perhaps, this enzymatic reduction occurs before the HONH-PhIP (or other HONH-AIAs) in the fecal stream can reach the colonic crypt and damage DNA (Figure 6). In the rat, the bioactivated PhIP metabolites appear to be either transported from the liver through the blood circulation to extrahepatic tissues or the bioactivation of PhIP occurs directly within extrahepatic tissues (404). The N2-glucuronide conjugates of IQ, MeIQx and their N-hydroxylated metabolites are resistant towards the hydrolytic action of β-glucuronidases (103,275,358,394). The N3-methyl group of these AIAs appears to sterically hinder the enzyme since the N2-glucuronide conjugate of N-desmethyl-IQ is a substrate for bacterial β-glucuronidase (275). Since significant interindividual variation in the N-glucuronidation of HAAs and HONH-HAA occurs in vitro (398,402) and in vivo (98,101,103,176,264,288,289), it is of interest to further examine the interrelationship amongst genetic polymorphisms in UGT1A isoforms, HAA exposure, and cancer risk (377,401,414-416).
Glutathione S-transferases and Glutathione Conjugates
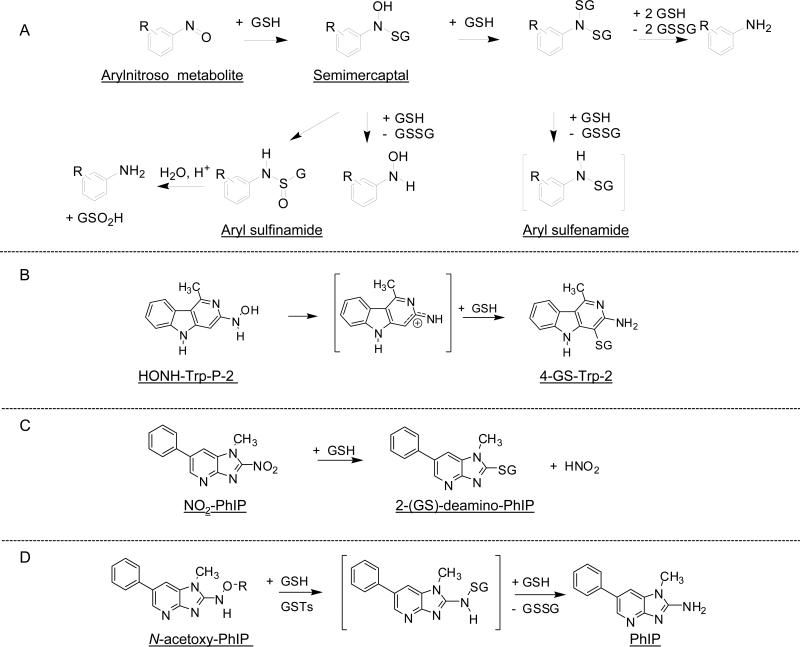
The glutathione S-transferases (GSTs) are another important class of enzymes involved in the detoxication of many endogenous electrophiles and classes of xenobiotics, including aromatic amines and HAAs (417). In humans, these enzymes are classified as Alpha, Mu, Omega, Pi, Sigma, Theta, and Zeta (418). The enzymes occur as dimeric protein structures and named according to their subunit composition, for example GST A1–2 is the enzyme composed of subunits 1 and 2 in the Alpha class. The non-enzymatic reactions of GSH or other thiols also can occur with arylhydroxylamines, N-hydroxy-HAAs, their esterfied products, the oxidized nitroso derivatives, and in some cases, oxidized nitro-AIAs. The interaction of GSH or other thiols with arylnitroso compounds has been extensively examined. The reactions are complex and product formation is dependent on thiol concentration, pH, and substituent effects (419-421). The initial product formed between the arylnitroso derivatives and GSH is a labile semimercaptal. However, the products formed by the reaction of 3-nitrosonitrobenzene and 4-nitrosonitrobenzene with GSH were sufficiently stable and characterized by NMR spectroscopy and mass spectrometry (422). The short-lived semimercaptals can react in several ways as depicted in Figure 7A.
Figure 7.
Reaction pathways of nitrosoarenes, nitroso-HAAs, or nitro-HAA intermediates with GSH and GSTs (393,419,420,429,430).
High exposures to 4-ABP result in depletion of glutathione (GSH) in liver of mice (303). The depletion of GSH in primary hepatocytes, by L-buthione sulfoximine, resulted in a 15-fold increase in the formation of PhIP-DNA adducts (423), and GSH depletion in vivo in rats resulted in a 5-fold increase in hepatic PhIP-DNA adducts (404). An increase in the level of IQ bound to DNA also occurs in primary cultures of rat hepatocytes, following depletion of cellular GSH (424). These findings show that GSH is protective against the genotoxicity of some AAs and HAAs.
The peroxidatic activity of met-Hb and H2O2 catalyzed the oxidation of N-acetyl-Bz, presumably to the reactive nitroso intermediate, which was trapped with GSH to form a stable sufinamide adduct. The GSH conjugate was characterized by electrospray ionization/mass spectrometry as N-(glutathion-S-yl)-N′-acetylbenzidine S-oxide (425). The non-enzymatic reaction of GSH with the nitroso and N-hydroxy metabolites of AF produced the sulfinamide, N-(glutathione-S-yl)-2-aminofluorene S-oxide, and the sulfenamide, N-(glutathione-S-yl)-2-aminofluorene; analogous GSH conjugates were formed with the nitroso and N-hydroxy metabolites of 1-naphthylamine and 2-NA (426). In rats treated with N-hydroxy-2-acetylaminoflourene, the two biliary conjugates were identified as 1- and 3-(glutathion-S-yl)-N-acetyl-2-aminofluorene: no S-N linked conjugates were reported (427).
Sulfinamide and sulfonamide adducts were produced from the non-enzymatic in vitro reaction of the nitroso metabolite of Glu-P-1 with GSH (428). Enzymatic reaction of GSTs from rat liver with the N-hydroxylated metabolite of Trp-P-2 produced three GSH conjugates (429). One of the conjugates was found to be a more potent bacterial mutagen than HONH-Trp-P-2: the structure may have been the semimercaptal conjugate, on the basis of mass spectral data (429). The structures of the two detoxicated products appear to be respectively, a sulfinamide adduct, and a stable S-C adduct that may have formed at the C-4 atom of Trp-P-2 (Figure 7B) (429). GSH reaction products with the oxidized nitro derivatives of MeIQx and PhIP have been reported to form in rodent hepatocytes (282,393). In these reactions, the thiol group of GSH displaced the nitro moieties, by direct nucleophilic substitution, to form 2-(glutathion-S-yl)-3,8-dimethylimidazo[4,5-flquinoxaline (282) and 2-(glutathion-S-yl)-1-methyl-6-phenylimidazo[4,5-b]pyridine (393); the GSH conjugate of NO2-PhIP was also detected in rat bile and suggests that NO2-PhIP formation occurs in vivo (393). The S-C linked GSH reaction products with NO2-MeIQx and NO2-PhIP can form non-enzymatically (Figure 7C).
The effects of GSH and of purified human and rat GSTs on the covalent DNA binding of the reactive N-acetoxy derivatives of PhIP, IQ, and MeIQx, were studied in vitro. GSH alone slightly inhibited (10%) the binding of N-acetoxy-PhIP to DNA, but the binding was strongly inhibited in the presence of both GSH and GSTs. Among human GSTs, the isozyme A1-1 was most effective (90% inhibition), followed by A1-2 (40% inhibition), and P1-1 (30% inhibition); other GSTs studied appeared to have little to no activity towards N-acetoxy-PhIP (430,431).
Analysis of the incubation mixture containing N-acetoxy-PhIP, GSH and GST A1-1 revealed the presence of oxidized GSH (GSSG) and reduced PhIP (Figure 7D), but no GSH adducts were detected, suggesting a redox mechanism is involved in the deactivation of N-acetoxy-PhIP. A short-lived GSH sulfenamide conjugate of PhIP may have formed and undergone an ensuing reaction with GSH to produce PhIP and GSSG (430). GST P1-1 showed even higher substrate specificity for the inhibition of DNA binding of ATP-dependent metabolite(s) of HONH-PhIP than for N-acetoxy-PhIP (432). The binding of N-acetoxy-IQ or N-acetoxy-MeIQx to DNA was unaffected by human or rat GSTs; however, GSH alone significantly inhibited (40%) their binding to DNA (430).
The GST-dependent detoxication pathway may be an important determinant for the organ specificity of PhIP-carcinogenesis in rodents and possibly humans (430,431). Human liver cytosol, which contains high levels of GST A1-1, catalyzes the GST-mediated detoxication of N-acetoxy-PhIP (430), whereas the cytosol of colon, which contains about 100-fold lower levels of the GST A1-1 subunit than the liver (433), does not display GST-mediated inhibition of N-acetoxy-PhIP binding to DNA (430). The high levels of hepatic GST A1-1 activity may help to explain the lower levels of PhIP-DNA adduct formation in liver in comparison to pancreas or colorectal tissue of rats (340,404). In humans, a polymorphism in the 5′-regulatory region of GSTA1 gene results in the diminished expression of the GSTA1 and GSTA2 subunits (434). In two case control studies, individuals who possess the homozygous single nucleotide polymorphisms hGSTA1*B (*B/*B) genotype and who would be predicted to have the lowest levels of GSTA1 expression in liver, were at a greater risk for developing colorectal cancer, especially among consumers of well-done cooked meat, than subjects with the homozygous hGSTA1*A (*A/*A) genotype and express high levels of GSTA1 (431,435). Individuals who are homozygous GSTA1*B, could be at risk of developing colorectal cancer, possibly as a result of inefficient hepatic detoxication of N-oxidized derivatives of PhIP (431). However, a third case control study failed to detect an elevated risk for colorectal cancer in subjects harboring the (*B/*B) genotype (436). Urinary mercapturic acid conjugates of PhIP, if formed, could serve as biomarkers to assess the efficacy of detoxication of PhIP by GSTs. Thus far, mercapturic acid conjugates of PhIP or other HAAs have not been identified in urine of experimental laboratory animals or humans.
Biomonitoring Aromatic Amines, HAAs, and their Metabolites in Human Urine
There are only a few reports on the direct chemical analyses of carcinogenic arylamine metabolites in human urine (406,437-440). On the basis of metabolism studies in experimental laboratory animals (44,441), in vitro with human liver slices (318), and in vivo in humans (406,437-440), arylamine metabolites can be grouped according to: (a) a substitution on the amino group by acylation (acetylation or formylation) or by conjugation with sulfate or glucuronic acid, (b) N-oxidation, (c) ring oxidation, followed by sulfation or glucuronidation, and (d) in some instances, mercapturic acid formation. The analysis of carcinogenic aromatic amines in human urine has been done primarily by gas chromatography with electron capture detection or negative ion chemical ionization mass spectrometry (GC-NICI-MS), following chemical derivatization (442-444). The procedures employed for the isolation of aromatic amines from urine generally include acid or base hydrolysis, followed by organic solvent extraction and/or solid phase extraction. Thus, the amount of aromatic amine measured represents the unmetabolized compound plus the Phase II conjugates. In one study, smokers were reported to have 1.5 to 2-fold higher levels of 2-NA, 4-ABP and o-toluidine in their urine than non-smokers: up to 204 ng o-toluidine, 21 ng of 2-NA and 15 ng of 4-ABP present in urine of smokers collected over 24 h (445).
There is one report on the detection of of N-acetyl-4-ABP and the N-glucuronide of 4-ABP in urine of smokers, by liquid chromatography-electrospray ionization/tandem mass spectrometry (LC-ESI/MS/MS) methods (440). In that study, the geometric mean (95% CI) of the total 4-ABP concentration was 1.64 pg/mg creatinine (1.30–2.07) in nonsmokers (N = 41), and significantly greater, at 8.69 pg/mg creatinine (7.43–10.16) in smokers (N = 89) (p < 0.001). Other studies reported no major differences in the excreted levels of 2-ABP and 4-ABP in urine between smokers, passive smokers and nonsmokers (444), or in the levels of aniline and o-toluidine in smokers as opposed to non-smokers urine (442). Significantly higher concentrations of aniline, o-toluidine, m-toluidine, 2-NA, and 4-methyl-1,3-phenylenediamine were detected in the urine of factory workers who smoked than in urine of nonsmoking factory workers, and there was a significant increase in the renal excretion of unaltered 4-chloroaniline and m-toluidine in slow N-acetylators as opposed to rapid N-acetylators among the smoking workers, indicating NAT enzymes are involved in the detoxication of these chemicals (446). Aniline, p-toluidine, 2-NA, and 4-chloro-o-toluidine were also detected in the urine of nonsmoking subjects who were not occupationally exposed to aromatic amines (446). Another study reported up to 50-fold higher levels or o-toluidine in occupationally exposed individuals than in non-occupationally exposed subjects (111). Certain aromatic amines have been observed to undergo decomposition in urine within a few hours and may explain why some arylamines have been difficult to detect in urine (444). In contrast to some arylamines, MeIQx, PhIP, and AαC are stable in the urine matrix (447).
Various analytical approaches have been devised to isolate HAAs from human urine: such techniques have included solvent extraction (24,301), solid-phase enrichment (SPE) (447), treatment of urinary HAAs with blue cotton and ion exchange chromatography (448), the use of molecularly imprinted polymers (292), and immunoaffinity methods (286), followed by quantification by GC-NICI-MS (24,301,449), or LC-ESI/MS/MS (292,447), or alternatively, followed by HPLC with UV or fluorescence detection (291,448). [14C]-MeIQx, [14C]-PhIP, and their [14C]-radiolabeled metabolites have also been measured in human urine by accelerator mass spectrometry (AMS) (176,264,287). Urinary metabolites have also been detected by LC-ESI/MS/MS (98,101,103,293), or indirectly, after chemical reduction or acid hydrolysis of HONH-PhIP conjugates, with detection by LC-ESI/MS/MS or GC-NICI-MS (289,450).
Most of the studies that have examined HAA biomarkers were conducted with subjects on a controlled diet, eating well-done cooked meat (252,285,291,451-454). However, there are reports on the identification of HAAs, including MeIQx, PhIP, APNH, AαC, in urine of subjects on a free-choice diet (448,453,455-457). In the case of AαC, urinary levels of this carcinogen were associated with tobacco usage and not meat consumption (456). Many of the early biomonitoring studies focused on MeIQx and PhIP because they are the two most mass-abundant HAAs formed in cooked meat (10). The metabolism pathways of pyrolytic HAAs in humans are unknown (458). The plasma half-life of MeIQx was estimated at 3.4 h and the plasma half-life of PhIP was estimated at 4.6 h in humans (301). These short half-lives are consistent with the rapid elimination of MeIQx and PhIP in urine after consumption of cooked meat (285,286,291,301,452,454,459). The metabolism of both HAAs is extensive. The amounts of non-metabolized MeIQx ranges from about 1 – 6% of the dose, whereas the amount of unaltered PhIP in 0 – 24 h post-meal urine ranges from about 0.5 to 2% of the dose (93,285,291,452,454,459). In one study in Japan, MeIQx, PhIP, and the tryptophan pyrolysate mutagens Trp-P-1 and Trp-P-2 were detected in the urine of healthy volunteers on a normal diet, but they were not found in urine of hospitalized patients receiving parenteral alimentation (448). This finding shows that the exposure to HAAs occurs from food and that these compounds are not formed endogenously. However, APNH, the reaction product formed from norharman and aniline in the presence of P450 3A4 or 1A2 (460), was detected in 24-h urine samples at levels ranging from 21 to 594 pg in subjects on a non-restricted diet; similar levels were measured in urine from inpatients receiving parenteral alimentation (457). These results suggest that APNH is a novel endogenous mutagen/carcinogen; the biological significance of this rodent carcinogen for human cancer development requires further study.
Widely ranging concentrations of HAAs have been detected in urine of individuals on unrestricted diets evaluated world-wide; such differences are probably attributable to variability in the concentrations of HAAs in the diet (448,453,455,456). Some biomonitoring studies have examined the amount of MeIQx and PhIP recovered in urine following acid hydrolysis. The hydrolysis of urine provides an estimate of the contribution of phase II conjugation to the metabolism of these AIAs (103,285,455,461). Acid treatment (1 N HCl, 80 °C for 8 h) increased the levels of MeIQx in urine by as much as 18-fold (103,285,453,454), while the amounts of PhIP generally increased by only several fold or less (103,285). The increase in MeIQx content is attributed to the acid-labile MeIQx-N2-SO3H and MeIQx-N2-Gl conjugates present in urine (285), whereas the acid-labile N2- and N3-glucucuronide conjugates of PhIP make up only a very minor percentage of the urinary metabolites of PhIP and explains the modest increase in the amounts of PhIP in urine, following acid treatment (98,103,176).
Interindividual differences in the urinary excretion of MeIQx and PhIP have been reported in subjects on controlled diets (452). Metabolic phenotypes may be expected to influence the levels of HAAs excreted in urine. For example, higher P450 1A2 activity was associated with significantly lower levels of unmetabolized MeIQx in the urine of omnivores (P = 0.008), when adjusted for the amount of meat eaten (459). However, the levels of PhIP in urine were not associated with P450 1A2 activity (286), a finding that is surprising since the contribution of P450 1A2 to the clearance of PhIP was estimated to account for 70% of the elimination of PhIP in a pharmacokinetic study (301). The contribution of P450 1A2 to the metabolism of PhIP may have been obscured since GSTs reduce N-oxidized metabolites of PhIP back to the parent amine (430,434).
N-Oxidation is an important biotransformation pathway of MeIQx and PhIP in humans. The levels of the urinary N2-glucuronide conjugate of HONH-MeIQx were reported to range from 2 – 17% of the ingested dose of MeIQx (288), but the major urinary metabolite of MeIQx, the carboxylic acid, IQ-8-COOH, which is also produced by P450 1A2 (261,262), ranged from 32 to 65% of the ingested dose (103). The N2- and N3-glucuronide metabolites of HONH-PhIP account for up to ~24 – 54% of the ingested dose of PhIP in urine within 24 h (176,287,289). The large variation in the urinary levels of IQx-8-COOH and HONH-MeIQx and HONH-PhIP N-glucuronide conjugates is likely due to the wide range of P450 1A2 content expressed in liver (222,242), combined with varying levels of UGT activity, and other competing pathways of metabolism (264,287-289,402).
The urinary level of the N2-glucuronide conjugate of HONH-MeIQx did not correlate to P450 1A2 activity (N = 66 subjects), whereas the level of the N2-glucuronide conjugate of HONH-PhIP did correlate to P450 1A2 acitivity, when caffeine was employed as the metabolic probe for P450 1A2 phenotyping (288,289). The pathway of IQx-8-COOH formation (261,262,264), which was discovered after these metabolism studies were completed (288,289), is a competing reaction pathway of MeIQx-N-oxidation and may have obscured the relationship between HON-MeIQx-N2-Gl and P450 1A2 activity. The interindividual variability in enzymatic reduction of the N-hydroxy-HAAs (430,462,463) is likely to contribute to the variability of urinary excretion of N-glucuronide conjugates of HONH-MeIQx and HOHN-PhIP and weaken the association between these HAA urinary biomarkers and P450 1A2 activity. There was no evidence for an inverse association between NAT2 phenotype activity and the amounts of HON-MeIQx-N2-Gl or HON-PhIP-N2-Gl excreted in urine (288,289); a finding that is consistent with the poor rates of N-acetylation of MeIQx and PhIP by NAT2 (335,339). In a pilot study, individuals with a rapid P450 1A2 phenotype and who excreted high levels of HONH-PhIP-N2-Gl urine had the lowest level of colon PhIP-DNA adducts (176). Thes data indicate that N-glucuronidation plays an important role in the detoxication of HONH-PhIP (176,400,402,464). Definitive conclusions from a small data set (N = 10 subjects) are tenuous and further investigations using a much larger study group should be pursued to confirm the protective role of UGT enzymes and the usefulness of the HONH-PhIP-N2-Gl urinary biomarker in HAA carcinogenesis studies.
The concurrent analysis of MeIQx and PhIP is important since the urinary excretion levels of either HAA by themselves can serve only as an approximate measure for the other, in assessing exposures in humans consuming unrestricted diets (455). The interrelationship between the oxidative metabolism of MeIQx and that of PhIP in urine samples from 10 volunteers on a controlled diet was examined by calculation of the urinary metabolic ratio (MR) (% dose of urinary metabolite/% dose of unmetabolized urinary HAA) values for several of their P450 1A2-catalyzed oxidation products (103). The employment of MR values to assess enzyme metabolizing activity must be done with caution because MR values can be influenced by changes in urine flow rate (465). The extent of MeIQx and PhIP metabolism and the MR for IQx-8-COOH and HON-PhIP-N2-Gl and HON-PhIP-N3-Gl, the major P450 1A2 oxidative urinary metabolites of MeIQx and PhIP, were significantly correlated for a given subject (Figure 8). The MR values were independent of urine flow rate and support the notion that P450 1A2 is an important enzyme in the metabolism of both procarcinogens in vivo (103). A study on a larger number of subjects will be required to firmly establish the MR values and the inter-relationship between P450 1A2-mediated metabolism of MeIQx and that of PhIP.
Figure 8.
Scatter plots relating the percentage of the unmetabolized dose of MeIQx and PhIP, and relating the MR of P450 1A2-oxidized MeIQx and PhIP metabolites (% of dose of metabolite/% of dose of unmetabolized MeIQx or PhIP) eliminated in urine collected for 10 h after meat consumption (103).
Aromatic Amine and HAA DNA Adducts
Some of the early structural characterization of arylhydroxylamine DNA adducts were reported by Kriek (466) and by King and Phillips (467), who proposed that covalent linkage should occur between C8 atom of dG and the arylamine nitrogen of N-hydroxyaminofluorene. The structural assignments were later confirmed through the use of nuclear magnetic resonance spectroscopic techniques (468). The characterization of many other arylamine DNA adducts followed these studies: both acetylated and non-acetylated adducts were identified in vitro and in vivo (37,76,469,470).
Synthesis and Characterization of DNA Adducts
The structures of a number of the arylamine DNA adducts were originally obtained by reacting the synthetic arylhydroxylamine derivatives with DNA, followed by enzymatic digestion and spectroscopic characterizations (37,76,469,470). The reactivity of many arylhydroxylamines towards DNA is enhanced under slightly acidic pH as opposed to neutral pH; this increase in reactivity has been ascribed to the formation of the aryl nitrenium ion (76,405). In contrast to arylhydroxylamines, the chemical reactivity of a number of N-hydroxy-HAAs with DNA is only modestly enhanced under acidic pH conditions and alternative reaction conditions were employed to produce the presumed nitrenium ion (339,408,471). Many arylhydroxylamines and N-hydroxy-HAAs primarily bind to dG at the C8 atom of guanine. However, this site is only weakly nucleophilic, and the dG-C8 adducts (and presumably dA-C8 adducts) have been proposed to be rearrangement products that are preceded by electrophilic substitution at the nucleophilic N7 atom of dG. This scheme has been postulated to be a general reaction for activated aromatic amines and HAAs (472,473). Minor reaction products of arylhydroxylamines and N-hydroxy-HAAs are also formed at the N2 atom of dG and the C8 and N6 atoms of dA (37,474-476). The structures of prominent arylamine and HAA adducts are shown in Figure 9.
Figure 9.
Structures of DNA adducts of aromatic amines and HAAs.
DNA adducts of HAAs have been synthesized by biomimetic reactions of the N-hydroxy-HAA intermediateswith deoxynucleosides or DNA, in the presence of ketene gas, or acetic anhydride, so as to produce the reactive N-acetoxy intermediate and facilitate the formation of the nitrenium ion (63,339-341,408,471,474,475,477,478). N-Acetoxy-IQ and N-acetoxy-MeIQx (339,408) have lifetimes of seconds or less, but N-acetoxy-PhIP has been isolated and characterized by mass spectrometry (341). The imidazo moiety of AIAs may facilitate the formation of the oxime tautomer and influence the chemical reactivity of N-hydroxy-AIAs with DNA. The oxime structure favors O-acetylation of the N-hydroxy-AIAs by acetic anhydride, to produce the N-acetoxy intermediates, instead of N-acetylation to form the hydroxamic acids. The dG-C8 adducts of IQ (408,474,479), MeIQ (170), MeIQx (474), 2-amino-3,4,8-trimethylimidazo[4,5-f]quinoxaline (4,8-DiMeIQx) (477), PhIP (340,341,480), AαC and 2-amino-3-methyl-9H-pyrido[2,3-b]indole (MeAαC) (478,481), and the glutamic acid and tryptophan pyrolysate mutagens (471,482) are formed by biomimetic reactions; these DNA adducts also occur in tissues of experimental laboratory animals (see above citations, and (63,100), and references therein) (Figure 9).
Isomeric dG-N2-adducts of IQ and MeIQx are also formed in vitro and in vivo; adduct formation occurs at the C-5 atoms of the heteronuclei of these HAAs (474,483-485). Recently, an hydrazine-linked N7-dG adduct with IQ, and a dA adduct of IQ formed in vitro by reaction of dG or dA with N-acetoxy-IQ was reported (475), and a dA adduct of MeIQx was also detected in liver of rats (476). Bond formation within these dA adducts is believed to occur between the N6 atom of adenine and the C-5 atom of the IQ or MeIQx heteronucleus to form 5-(deoxyadenosin-N6-yl)-IQ (dA-N6-IQ) or 5-(deoxyadenosin-N6-yl)-MeIQx (dA-N6-MeIQx). The formation of dG-N2 and dA-N6 adducts of IQ and MeIQx, indicate that both nitrenium and carbenium ion resonance forms exist for these activated HAAs (474,475).
The overall yield of DNA adducts formation with deoxynucleosides or DNA with arylhdroxylamines or N-hydroxy-HAAs are generally several percent or lower. More recently, non-biomimetic approaches have been developed to synthesize aromatic amine- and HAA-DNA adducts in high yields, using the Buchwald–Hartwig reaction of cross-coupling of primary and secondary amines with aryl halides. High-yield synthesis of dG and dA adducts of arylamines (486), dG-C8 and dG-N2 adducts of IQ, and the dG-C8 adduct of PhIP (dG-C8-PhIP) (487-490) have been achieved with this chemistry. The phosphoramidites of these adducts have been site-specifically incorporated into oligonucleotides to explore the effect of adducts in perturbations of DNA structure, and the fidelity of polymerases during translesional synthesis (42,491-500). The results demonstrated that each arylamine-DNA or HAA-DNA adduct structure and the location of the adduct within the sequence context of the oligonucleotide affected the solution structure of the DNA and the fidelity and the catalytic efficiency of the polymerases in a unique manner.
Aromatic amine and HAA DNA Adduct Formation in vitro and in Experimental Animal Models
The early investigations on the measurements of arylamine-DNA adducts in experimental animals employed tritium-labeled carcinogens. Adduct identification was achieved by HPLC with radiometric detection and by co-chromatography with unlabeled DNA adducts, which served as UV standards (37,76,469). More recent methods to detect and quantitate arylamine- and HAA-DNA adducts include: 32P-postlabeling (501,502); immunohistochemistry (IHC) (503,504); GC-NICI-MS of alkaline-treated DNA, a technique that cleaves the bond between the guanyl C8 atom and the amino group of aromatic amines or HAAs (169,505); accelerator mass spectrometry (AMS) for the detection of tritiated or 14C-labelled adducts (89,166,506), and LC-ESI-MS/MS methods (100,480,507-513).
Five DNA adducts of 4-ABP are formed, when HONH-ABP is reacted with calf thymus DNA at pH 5.0 (76) (Figure 9). N-(Deoxyguanosin-8-yl)-4-ABP (dG-C8-4-ABP) is the principal adduct and accounts for 80% of the total adducts formed, followed by dA-C8-ABP (15% of total adducts) and then N-(deoxyguanosin-N2-yl)-4-ABP (dG-N2-N4-4-ABP) (~5% of the total adducts). Two other minor dG adducts have been identified: 3-(deoxyguanosin-N2-yl)-4ABP (dG-N2-4-ABP) and N-(deoxyguanosin-N2-yl)-4-azobiphenyl (514,515). In urothelial cells of male Beagle dogs (37), dG-C8-4-ABP accounted for 76% of the total binding, followed by dG-N2-N4-4-ABP (15%), and then followed by dA-C8-4-ABP (9%), 2 days after the oral administration of [3H]-4-ABP (37). DNA adducts of 4-ABP were quantified, by 32P-postlabeling and immunohistochemistry (IHC), in liver and bladder of male and female BALB/c mice, following treatment with 4-ABP at a range of concentrations (from 0, 7 up to 220 ppm) in the drinking water for 28 days (516). The principal adduct in both tissues, for both sexes, was dG-C8-4-ABP. The level of adduct formation increased as a function of dose and correlated with the incidence of liver tumors, in female mice. However, the relationship between adducts and tumorigenesis was distinctly nonlinear in the bladders of male mice, and tumor incidence rose rapidly at doses above the 50 ppm dose 4-ABP. Toxicity and cell proliferation may have increased the tumor incidence in the bladder.
The reaction of N-hydroxy-2-aminonaphthalene (HONH-2-NA) with DNA in vitro, at pH 5.0, results in formation of three DNA adducts (Figure 9) (76). The major adduct is an imidazole ring-opened derivative of N-(deoxyguanosin-8-yl)-2-NA (dG-C8-NA, 50% of the total adducts), followed by lower levels of 1-(deoxyguanosin-N2-yl)-2-NA (dG-N2-NA, 30% of total adducts), and 1-(deoxyadenosin-N6-yl)-2-NA (dA-N6-NA, 15% of total adducts). These same three DNA adducts were formed in target (urothelium) and nontarget (liver) tissues of dogs 2 days after oral administration of [3H]-2-NA (37). A 4-fold higher binding level of 2-NA was found in the urothelial DNA than formed in liver DNA. The major adduct in both tissues was the ring-opened dG-C8-NA, followed by lower levels of dA-N6-NA and dG-N2-NA. The dG-N2 adduct persisted in the liver, and this adduct and the ring-opened dG-C8-NA adduct persisted in the bladder. The differential loss of adducts indicates that active repair processes are present in both tissues. The relative persistence of the ring-opened dG-C8-NA adduct in the target but not in the nontarget tissue suggests that this adduct is a critical lesion for the initiation of urinary bladder cancer.
Peroxidative enzymes, such as PHS, catalyze both the N-oxidation and ring-oxidation of 2-NA; 2-amino-1-naphthol is a major ring-oxidation product (319). PHS catalyze the binding of 2-NA to DNA and produced the same three adducts arising from N-hydroxy-2-NA (vide supra). Three other adducts were also formed from 2-imino-1-naphthoquinone, the oxidative product of 2-amino-1-naphthol. The major adduct is N4-(deoxyguanosin-N2-yl)-2-amino-1,4-naphthoquinoneimine (dG-N2-NAQI) (Figure 9) (37,319). This adduct and two other minor adducts, accounted for approximately 60% of the total DNA binding that was obtained by incubation of 2-NA with PHS in vitro. The DNA adducts derived from 2-imino-1-naphthoquinone were reported to account for approximately 20% of the 2-NA bound to urothelial DNA in dogs, but these peroxidative DNA adducts were not detected in liver DNA (319). The remaining adduction products in urothelium were derived from HONH-2-NA. PHS expressed in the bladder could play a significant role in bioactivation of 2-NA directly in the bladder, and could contribute to carcinogenesis of 2-NA and other arylamines that serve as substrates of PHS.
The reaction of calf thymus DNA with HONH-N′-acetylBz at pH 5 gives rise to N-(deoxyguanosin-8-yl)-N′-acetylbenzidine (Figure 9) (517,518). The structural isomer, N-(deoxyguanosin-8-yl)-N-acetylbenzidine and the nonacetylated derivative, N-(deoxyguanosin-8-yl)-benzidine, have not been identified in rat or mouse liver DNA (518). However, benzidine diimine, a reactive intermediate formed during the enzymatic peroxidation of Bz, can undergo deprotonation of the cationic diimine to form its nitrenium ion, which reacts with dG to form N-(deoxyguanosin-8-yl)-benzidine. This adduct was detected in vivo in the dog urothelium (519).
The reaction of HONH-MOCA in vitro, at pH 5 or 7, with DNA produces N-(deoxadenosin-8-yl)-4-amino-3-chlorobenzyl alcohol and N-(deoxadenosin-8-yl)-4-amino-3-chlorotoluene as the major adducts (Figure 9); these lesions are also formed in rat liver (520) and in dog urinary bladder epithelium (521), following treatment with MOCA. The preferred reactivity of MOCA with dA is atypical of most AAs and HAAs, which primarily react with dG (37,63,522). The chemistry of MOCA-DNA adduct formation is also unusual: the adducts contain only a single ring derived from MOCA. The incipient DNA adduct formed appears to undergo fission at the methylene bridge of MOCA to form N-(deoxadenosin-8-yl)-4-amino-3-chlorobenzyl alcohol or N-(deoxadenosin-8-yl)-4-amino-3-chlorotoluene (520) (Figure 9).
dG-C8 adducts of monocyclic alkylanilines as well as 2- and 4-chloroaniline are also formed by reaction of the corresponding N-(acyloxy)arylamines with dG and DNA (523,524). A study with 3,5-DMA revealed that this arylamine forms a C8 adduct with dG but also forms adducts at the N6 and C8 atoms of dA, and a unique adduct with dC (525,526). The formation of these DNA adducts are consistent with the involvement of nitrenium ion chemistry. It is noteworthy that several monocyclic arylamines have been reported to form sulfinamide adducts with Hb in rodents, but dG-C8 or other DNA adducts attributed to the nitrenium ion were not detected in liver or extrahepatic tissues (524,526). Therefore, biologically available N-hydroxy-AAs that form Hb adducts do not necessarily produce DNA adducts (524). The quinone imine or quinone methide electrophiles may contribute to the DNA damage and adduct formation of monocyclic alkylanilines (123).
The doses of HAAs employed in many experimental laboratory animal studies exceeded daily human exposures by more than a million-fold; many of these studies are reviewed in (63,100). In early studies, several variants of the 32P-postlabeling method (501,527), followed by DNA adduct separation by 2-dimensional thin layer chromatography or HPLC (63,479,528,529), were used to discern HAA-DNA adducts. A myriad of lesions were detected in a number of these DNA binding studies; many of the adduction products were subsequently shown to be incompletely digested oligomers of the dG-C8-HAA adducts (167,479,530). For many HAAs, DNA adduct formation is greatest in the liver of rodents, a result perhaps attributable to the high levels of P450 1A2 expression (63). However, DNA adducts are formed in all tissues investigated, even in tissues that do not develop cancer, indicating other factors, such as tumor promotion, are involved in tumorigenesis (9,159).
DNA adduct formation of MeIQx (166) and IQ (167) was found to occur in a near linear dose-response relationship in liver of rodents over a wide range of doses. DNA adducts were formed at dose levels approaching human exposures for both of these AIAs, as well as for PhIP (531). In contrast to many other HAAs, PhIP shows levels of adduct formation in rodents that are lower in liver than in extrahepatic tissues; adduct levels are particularly elevated in colon and pancreas (340,404), and in prostate of male rats (155), and mammary glands of female rats (532). Both GSTs and UGTs, which are expressed at high levels in the liver, catalyze the detoxication of reactive PhIP metabolites (397,402,430,464), thus accounting for the relatively lower level of PhIP–DNA adduct formation in the liver.
The isomeric dG-N2 adducts of IQ and MeIQx are minor adducts formed in vitro with the N-acetoxy-IQ and N-acetoxy-MeIQx (<10% of total adducts) (474), but their contribution to the total amount of DNA adducts formed in rodents is greater (479,483,485,533). The dG-N2-IQ adduct became the prominent lesion in slowly dividing tissues of non-human primates that underwent chronic treatment with IQ (483), suggesting preferential repair of the dG-C8-IQ adduct. The contribution of dG-N2-MeIQx to the total adducts in rats was significantly more important than that observed in vitro when calf thymus DNA was reacted with N-acetoxy-MeIQx (485). dG-N2-MeIQx was the major adduct detected in liver of rats 24 h after gavage with a 0.5 mg/kg dose. Thus, isomeric dG-N2-AIA adducts are prominent lesions formed in slowly dividing tissues rodents and non-human primates, particularly during chronic exposure.
DNA Adduct Formation of Aromatic Amines and HAAs in Human Tissues
The analyses of DNA adducts from human tissues have often been conducted on biopsy samples of patients that were obtained during clinical diagnosis of cancer (27,534). The DNA adducts formed are likely attributed to recent exposures; however, the most relevant time to measure DNA adduct formation is when tumor initiation is in progress and not many years later when the cancer has been diagnosed (27,32). Hence, the assumption made is that current adduct levels are reflective of the levels that existed during the time of cancer initiation. This assumption may be valid for inviduals subjected to long-term habitual exposure to genotoxic agents, such as those exposures that occur through smoking or by frequently consuming well-done cooked meats; however, only few studies have investigated the variations in DNA adduct levels in individuals over time (27,535).
dG-C8-4-ABP was first detected by 32P-postlabeling in human urinary bladder tissue biopsy samples and exfoliated uorothelial cells (28,536). Subsequently, the adduct was detected by GC-NICI-MS methods in lung and urinary bladder mucosa; dG-C8-4-ABP was found at levels ranging from <0.32 to 49.5 adducts per 108 nucleotides in the lung, and from <0.32 to 3.94 adducts per 108 nucleotides in bladder samples (505). dG-C8-4-ABP has also been detected, by IHC, 32P-postlabeling or GC-NICI-MS methods in bladder and lung tissues from smokers and ex-smokers (502), and by IHC in the liver of Taiwanese subjects with hepatocelluar carcinoma (537). In pancreas tissue, a major adduct was observed that was chromatographically identical to dG-C8-4-ABP in eight of 29 organ donors, at levels ranging from 0.2 to 1.1 adducts per 108 nucleotides. Pancreas tissue displays low enzyme activities for P450-mediated N-oxidation and prostaglandin hydroperoxidation of 4-ABP, but high levels of 4-nitrobiphenyl reductase and NAT1-mediated O-acetyltransferase activity are present (538). The dG-C8-4-ABP adduct levels in pancreas did not correlate with the number of cigarettes smoked per day or the length of smoking history; other sources of environmental exposure to 4-ABP or the exposure to 4-nitrobiphenyl or other nitroarenes, produced during combustion (122), may contribute to arylamine-DNA adduct formation in pancreas.
The DNA present from the induced sputum of smokers, representing DNA of the lower respiratory tract, was shown to possess significantly higher levels of 4-ABP-DNA adducts than were found in the sputum of non-smokers, when assessed by IHC (539). 4-ABP-DNA adducts were also detected in female breast tissue biopsy samples, when visualized by IHC (504); the levels of 4-ABP-DNA in tumor-adjacent normal tissues, but not in tumorigenic tissue, were correlated to the frequency of women's smoking. 4-ABP-DNA adducts were also detected in laryngeal biopsies by IHC (540), and adduct levels were significantly higher in smokers than were the levels measured in tissue from non-smokers.
The putative dG-C8 adduct of 4-ABP, measured as 4-ABP after acid treatment of DNA, was detected in biopsy samples from 37 out of 75 bladder cancer patients, corresponding to 86 ± 22 adducts per 108 nucleotides (Mean ± SE). The amount of 4-ABP-DNA adducts in the bladder of current smokers was elevated in subjects with more aggressive grade levels of bladder tumors (541). In another study, the putative dG-C8 adducts of 4-ABP and o-toluidine were measured as the parent amines, after acid treatment of DNA from epithelial and submucosal bladder tissue of bladder cancer patients: 4 and 11 of 12 tumour samples contained adducts of 4-ABP (1.9 ± 4.1 adducts per 108 nucleotides) and o-toluidine (2.9 ± 1.5 adducts per 106), respectively (542). The levels of the putative dG-C8 adduct o-toluidine, but not 4-ABP, were significantly higher in epithelium of smokers than in non-smokers. The detection of high levels o-toluidine-releasing DNA adducts is suggestive of a causal role of o-toluidine in the human bladder cancer.
LC-ESI-MS/MS methods have been employed to directly quantitate dG-C8-4-ABP in human tissues. dG-C8-4-ABP was detected in urinary bladder epithelium in 12 out of 27 subjects in DNA extracted from tumor tissue or non-tumor surrounding tissue (543). The levels of adducts ranged from 5 to 80 adducts per 109 bases, but a correlation was not observed between tobacco smoking and adduct levels (543). In another pilot study, dG-4-C8-ABP was identified, by LC-ESI-MS/MS, in six of 12 human pancreas samples (512). The levels ranged anywhere from 1 to 60 adduct per 108 nucleotides; again, there was no correlation observed between the level of adducts, and smoking preference, age, or gender. The prediction of the relationship between 4-ABP exposure from tobacco smoke and adduct levels is not straightforward, being confounded by environmental exposure to 4-nitrobiphenyl and a variable persistence of dG-C8-4-ABP in the tissues. Because the activation or detoxification processes of 4-ABP metabolism, as well as DNA repair mechanisms, can be tissue-specific, a correlation between tobacco usage and DNA adducts in different tissues may not exist.
Another environmental source of exposure to 4-ABP is hair dyes (120,121). The relationship between 4-ABP-DNA adduct levels and hair-dye usage has only been examined in one study, which determined the levels of the putative 4-ABP-DNA adduct, by 32P-postlabeling, in exfoliated breast epithelial cells in milk from lactating mothers (544). The adduct levels were associated with the use of hair coloring products (odds ratio 11.2), but not with tobacco usage, in a statistically significant manner. Some commercial permanent hair dyes are known to contain 4-ABP (120,121).
The presumed N-(deoxyguanosin-8-yl)-N′-acetylbenzidine adduct was identified by 32P-postlabeling of DNA from exfoliated urothelial cells of workers in factories manufacturing Bz in India (332). This finding supports the hypothesis that N-acetylation and the ensuing formation of HONH-N′-acetyl-Bz is an important bioactivation pathway for at least one Bz-related adduct in humans (318); this bioactivation pathway is analogous to the pathway proposed for Bz activation in rodents (518). Moreover, the same Bz adduct was identified in white blood cells of exposed workers, and there was a significant correlation between WBC and exfoliated urothelial cell Bz adduct levels (545). This was the first study in humans to show a relationship for a specific carcinogen adduct in a surrogate tissue and in urothelial cells, the target for urinary bladder cancer of Bz.
The major DNA adduct isolated from exfoliated urothelial cells collected from urine of a subject after an accidental acute exposure to MOCA, was detected as N-(deoxadenosin-8-yl)-4-amino-3-chlorobenzyl alcohol, by the 32P-postlabeling method (546). The adduct was found in cell samples obtained between 4 and 98 h after initial exposure but not in samples collected at later times. The level of DNA adducts 4 h after exposure was determined to be 516 adducts/108 nucleotides.
The putative dG-C8-MeIQx adduct was detected in the colon and kidney of some individuals at levels of several adducts per 109 DNA bases, when assayed by 32P-postlabeling (547). A GC/MS assay, following hydrolysis of putative dG-C8-HAA adducts, was employed to measure the levels of HAA adducts in DNA of the colorectal mucosa (169) and lymphocytes from colorectal cancer subjects (175); the levels of the putative dG-C8-PhIP adduct were found to be in the range of several adducts per 108 DNA bases. In the latter study, the adduct was found in lymphocytes of about 30% of the subjects, and the adduct levels varied by a factor of 10-fold between the lowest and the highest level. The level of lymphocyte PhIP DNA adducts was not significantly higher in smokers or high meat consumers than individuals who ate meat less frequently (175). A subset of younger individuals carrying two mutated GSTA1 alleles had higher adduct levels than homozygous wild-type and heterozygous subjects. This finding is consistent with the reported activity of the GSTA1 protein in the detoxication of N-oxidized metabolites of PhIP (430,548). In another study, a PhIP-related DNA adduct (the presumed dG-C8-PhIP adduct) was detected by the 32P-postlabeling method in 106 of the 150 colorectal tissues analyzed: similar levels of adducts were detected in tissues from controls, polyp patients, or cancer patients (549).
Adducts attributed to dG-C8-PhIP were frequently detected, by the 32P-postlabeling method, in exfoliated breast epithelial cells in milk of lactating mothers (30). Thirty samples from the 64 subjects contained the presumed PhIP-DNA adduct, and the mean level of adduct formation was 4.7 adducts/107 nucleotides. In an ensuing study, PhIP-DNA adducts were detected, by IHC, in mammary tissue of 82% of the women with breast cancer (N = 106) and also found in 71% of the tissue samples of the healthy control patients (N= 49) (173). An interactive effect was observed among well-done meat consumption and NAT2 genotype and the level of PhIP-DNA adducts. This observed interactive effect is surprising since HONH-PhIP does not appear to be bioactivated by human NAT2 at appreciable levels (342,343). A very high percentage of pancreas and prostate tissue biospecimens were also positive for PhIP-DNA adducts, when assayed by IHC (174,177). The mean level of the PhIP-DNA adducts was ≥ 2.7 adducts/107 nucleotides, by IHC, in both patients and the healthy control populations (173,174,177). The levels of PhIP-DNA adducts reported in breast, pancreas and prostate tissues of humans are comparable to the adduct levels observed in these corresponding tissues of rodents treated with either a single acute dose or chronic carcinogenic doses of PhIP (10 - 50 mg /kg bw) (155,404,550). These findings imply that PhIP-DNA adduct formation occurs with far greater efficiency in humans exposed to ppb levels of dietary PhIP than the adducts which occur in the rodents given high, carcinogenic doses of PhIP.
PhIP-DNA adducts were also measured, by AMS, in breast tissue of female cancer patients who had received a dose of [14C]PhIP (20 μg PhIP/70 kg body weight) via oral administration prior to surgery (172). The estimates of PhIP-DNA adducts obtained by AMS ranged from 26 to 480 adducts/1012 nucleotides (172), or nearly 1,000 to 10,000-fold lower than the levels of adducts reported by the IHC or 32P-postlabeling techniques cited above. The large discrepancy in estimates of PhIP adducts in breast tissue obtained by IHC (173) and 32P-postlabeling (30), as opposed to the precise AMS method (172), suggests that these biochemical assays are detecting a variety of lesions in addition to or other than dG-C8-PhIP. Moreover, the dG-C8-PhIP adduct is underestimated, when determined by 32P-postlabeling in comparison to LC/MS methods (551,552); dG-C8-PhIP adduct measurements should be conducted by quantitative LC/MS techniques.
DNA Adducts of Aromatic Amines and HAAs in the Oral Cavity
The oral cavity is the portal of entry for carcinogens that are ingested in the diet or inhaled through smoking. The bioactivation of aromatic amines and HAAs can occur directly by P450s 1A1, 1A2 or 1B1 that are expressed in buccal cells of the oral cavity (553,554), or by P450 1A2 expressed in salivary glands (555). The N-hydroxy metabolites also can form by the action of P450 1A2 in the liver (233,339) and reach the oral cavity through systemic circulation (404), followed by phase II activation in cells of the oral cavity. Peroxidases in saliva (556) may also catalyze the bioactivation of HAAs and arylamines (308,312,314,316,322,519). The potential of the oral microflora to contribute to metabolism and DNA adduct formation of HAAs or arylamines has not been investigated.
Both 32P-postlabeling (557-560) and IHC techniques (561-563) were employed to screen for DNA adducts in cells of the oral cavity. Several of the studies reported differences in total DNA adduct levels between smokers and non-smokers; however, the complexity of the adduct profiles and the inability to identify specific DNA adducts precluded any interpretation on the principal DNA damaging agents and their significance in the risk of development of oral cancer or cancers of other organs (557-563). Some of the lesions detected were believed to be derived from polycyclic aromatic hydrocarbons or aromatic amines (557,561-563). As an extension of those studies, a selective LC/MS method was recently employed to detect DNA adducts in saliva derived from carcinogens formed in tobacco smoke and cooked meats. The dG-C8 adducts of 4-ABP, PhIP, AαC, and MeIQx were identified in saliva samples from volunteers on unrestricted diets, by LC-ESI/MS/MSn, at the MS3 scan stage mode with a linear quadrupole ion trap MS (178). DNA adducts of PhIP were found most frequently: dG-C8-PhIP was identified in saliva samples from 13 of 29 ever-smokers and in saliva samples from 2 of 8 never-smokers. dG-C8-AαC and dG-C8-MeIQx were identified solely in saliva samples of 3 current smokers, and dG-C8-4-ABP was detected in saliva from 2 current-smokers. The levels of these different adducts ranged from 1 to 9 adducts per 108 nucleotides. Moreover, the employment of the linear quadrupole ion trap MS permitted the acquisition of product ion spectra of the aglycone adducts [BH2]+, at the MS3 scan stage, for unambiguous identification of the carcinogen-DNA adducts (Figure 10) (476,552,564). Some HAAs induce oral cancer in rodents during long-term feeding studies (9). Moreover, an appreciable level of metabolic activation of Trp-P-2 was observed in rat tongue (565), and elevated levels of salivary DNA adducts were reported in rats fed with MeAαC, resulting in severe atrophy of the salivary glands (566). Thus, saliva may be a promising non-invasive fluid to monitor exposure and DNA damage of some HAAs and aromatic amines.
Figure 10.
LC/ESI/MS/MS3 traces of dG-C8-4-ABP and dG-C8-PhIP adducts in saliva DNA, acquired with a linear ion trap MS (178): (A) non-smoker and (B) current smoker. The MS3 product ion spectra of the aglycone [BH2]+ and the [13C10]-dG-labeled internal standards, added to DNA at a level of 1 adduct per 107 DNA bases, are presented in the lower panel (adapted from (178)).
Exfoliated epithelial buccal cells and leukocytes are the principal mammalian cells present in saliva (567,568). The time frame from new cell production to exfoliation of the buccal cell from the mucosal surface is estimated to be between 5 and 12 days (569), and the leukocytes, which originate mainly from the gingival crevice, and then migrate into the oral cavity, are predominantly short-lived neutrophils and other granulocytes (568,570). Given the short life spans of both buccal and leukocyte cell types, the DNA adducts present in saliva are likely to occur from recent exposures to carcinogens. It is not known whether adducts are formed in both cell-types and whether they preferentially form in one type. Studies that examine kinetics of PhIP-DNA adduct formation in cells of the oral cavity of humans exposed to defined amounts of PhIP, combined with studies that can unravel the myriad of plausible enzymes that contribute to PhIP adduct formation in oral cells are required. Moreover, the level of adduct formation in the oral cavity must be shown to correlate to target tissues of cancer, for the validation of this biomarker.
Aromatic Amine and HAA Protein Adducts
Carcinogen blood protein adducts have been used as an alternative to DNA adducts for human biomarkers of several different classes of carcinogens, including aromatic amines, polycyclic aromatic hydrocarbons, and aflatoxin B1 (AFB1) (31,49,571-574). The research on protein adducts originates from the pioneering studies of the Millers and is based upon the paradigm of chemical carcinogenesis in which electrophilic species or electrophilic metabolites of carcinogenic compounds react with nucleophilic centers on proteins as well as DNA (35,36,575,576). The use of Hb as a dosimeter for alkylation agents was introduced by Ehrenberg and his collaborators (577,578). The biomonitoring of carcinogen adducts with serum albumin (SA) also has been examined for several different classes of carcinogens (31,49). The biomonitoring blood protein carcinogen adducts is advantageous, because up to several hundred mg of Hb or SA, as opposed to ~100 μg DNA, can be obtained from a 10 mL blood sample. Moreover, stable carcinogen protein adducts are expected to accumulate and follow the kinetics of the lifetime of Hb or half-life of SA, during chronic exposure. In humans, the lifetime of Hb is 120 days and the half-life of SA is between 20-25 days (31). Thus, the steady state levels of Hb and SA carcinogen adducts would be, respectively, about 60 and 29-fold higher after chronic exposure than after a single dose (578,579). Carcinogen protein adducts of aromatic amines and HAAs are formed through their N-oxidized metabolites and represent a measure of the biologically effective dose (31,38). However, there are caveats in the application of blood protein adducts for human risk assessment. The adduction of carcinogens to blood proteins does not represent genetic damage, and adduct formation with Hb occurs in the erythrocyte, which may not reflect the genetic damage that occurs in the target organ. In the case of SA adducts, the adduct can form in the hepatocyte, the cell where SA is biosynthesized (580) and where metabolic activation of arylamines and HAAs occurs (60).
Hemoglobin Adducts
A number of aromatic amines form adducts with Hb, via a sulfinamide linkage (Figure 2), in experimental laboratory animals (38,80,524). The existence of AA-Hb sulfinamide adducts for many arylamines demonstrates that the N-oxidation is a common metabolic pathway for this class of genotoxicants. The percent of the arylamine dose bound to Hb as a sulfinamide linkage ranges over 100-fold in rodents, depending upon the structure of the chemical (38,48,524). The highest levels of arylamine-Hb adducts were reported for 4-ABP, where over 5% of the dose is bound to Hb in the form of the sulfinamide adduct (80). In a hallmark study, Hb sulfinamide adducts of 15 aromatic amines were determined in nonsmokers and smokers, and significant differences between smokers and nonsmokers were observed for Hb adducts of 4-ABP, 3-aminobiphenyl, 2-NA, o- and p-toluidine, 2,4-dimethylaniline, and 2-ethylaniline; some of these arylamines are human bladder carcinogens (581). In a study among factory workers to exposed to Bz and Bz-based dyes, the Hb-adduct levels of acetylBz, Bz and 4-ABP correlated strongly with each other (111). The levels of N-acetyl-Bz adducts were 20-fold higher than the levels of Bz-sulfinamide adducts and the results are consistent with P450 activation of N-acetyl-Bz to form HONH-N′-acetylBz as a major reactive metabolite in vivo (318,582).
The validation of a protein carcinogen adduct as a biomarker requires that the biomarker correlates to exposure, DNA damage, and cancer risk. The Hb-sulfinamide adduct of 4-ABP fulfills these requirements. The levels of 4-ABP-Hb sulfinamide adduct formation were shown to correlate with the number of cigarettes smoked per day (581,583,584). Subjects with rapid N-acetylator phenotypes have decreased levels of the 4-ABP-Hb adduct in comparison to slow N-acetylator phenotypes (583,584). Thus, N-acetylation, a detoxication pathway and a competing metabolic fate to N-hydroxylation, results in a decreased level of the biologically effective dose of 4-ABP in rapid N-acetylator phenotypes. The levels of 4-ABP-Hb adducts were also shown to correlate with the amount of dG-C8-4-ABP adduct present in exfoliated urothelial cells (536). Elevated levels of 4-ABP-Hb and other arylamine-Hb sulfinamide adduct levels are associated with increased bladder cancer risk, both in smokers as well as non-smokers (50,77,78,584,585).
HAAs also react with Hb and SA. However, the levels of IQ, MeIQx, and PhIP bound to Hb are low in experimental laboratory animals (~0.01% of the dose), and the levels of HAA-SA adducts are only several fold higher (49,88,90,263,586-588). The low level of HAA-Hb sulfinamide formation does not seem to be attributed to poor reactivity of the N-hydroxy-HAA metabolites with Hb. In the case of IQ, a metabolic study conducted in vitro showed that the N-hydroxy-IQ metabolite penetrates the human erythrocyte and induces methemoglobinemia, and that a portion of the IQ bound to Hb (~10%) was released by acid and recovered as IQ (586). Thus, N-hydroxy-IQ does appear to form a sulfinamide adduct with Hb. The N-hydroxy and nitroso derivatives of Glu-P-1 were also shown to modify the thiol groups of Hb (589). The low levels of HAA-Hb sulfinamide formation with HAAs in rodents suggest that there is little free N-hydroxy-HAA present in the blood that is available to react with Hb. The inefficient binding of HAAs to Hb will probably preclude the development of HAA-Hb adducts as biomarkers in humans (88,90).
Serum Albumin Adducts
Aromatic amines also react with SA. Human SA is of 585 amino acids in length and is the most abundant protein in plasma (~45 mg/mL). Its roles include maintenance of osmotic pressure and transport of endogenous (i.e., fatty acids, bilirubin, steroids) and exogenous (drugs) chemicals (590). The single tryptophan residue at position 214 of rat SA is a selective site of binding for several activated arylamines (391). This amino acid reacts with N-sulfonyloxy ester of N-acetyl-4-aminobiphenyl, to form an adduct with a stable 4-ABP-tryptophan-linkage (Figure 11) (390). The same adduction product was shown to form by reaction of synthetic sulfate ester of N-hydroxy-N-acetyl-4-aminobiphenyl, and the sulfate esters of N-hydroxy-N-acetyl-2-aminofluorene and HONH-N,N′-diacetylbenzidine with human SA in vitro (F. F. Kadlubar, personal communication) (391). SULT enzymes play a critical role in the formation of these adducts in hepatocytes (391). To our knowledge, an analytical method has not been established to biomonitor this arylamine-tryptophan SA adduct in humans.
Figure 11.
Mechanism of formation of protein adducts of 4-ABP and IQ with rat SA. The of N-sulfonyloxy ester of N-acetyl-4-aminobiphenyl reacts with the sole tryptophan residue of rat SA (390). In the case of IQ, the HONH-IQ metabolite undergoes further oxidation, by either transition metals or P450 1A2, to form the nitroso metabolite (602). Nitroso-IQ reacts with SA-Cys34 residue to form the semimercaptal, which undergoes rearrangement to the sulfinamide structure. A tetrapeptide containing N-acetyl-4-ABP adducted to ala-trp-alaval and a tripeptide containing the IQ adduct at cys-pro-tyr are recovered upon digestion of SA with pronase. In analogy to arylamine-Hb sulfinamide adduct chemistry (see Figure 2), acid treatment of the IQ-SA sulfinamide adduct results in hydrolysis and the generation of IQ and the SA-Cys34 sulfinic acid (587).
Some of the adduct(s) of IQ, MeIQx and PhIP formed with SA in rodents or produced in vitro with rat or human SA are acid-labile (88,586,587,591,592). An adduct formed between IQ and rat SA adduct was characterized by MS, 1H NMR, and amino acid analysis and shown to contain a sulfinamide linkage formed through the SA-Cys34: this adduct accounted for about 10% of the total SA adducts formed in rats (Figure 11) (586). The Cys34 is one of 35 conserved cysteine residues in SA across species (590). Thirty-four of these cysteines are involved in 17 disulfide bonds. The single unpaired Cys34 is present either as a free thiol or in an oxidized form: this residue is present partially as disulfide linkages with low molecular-weight thiols (593). The SA-Cys34 is thought to be responsible for many of the antioxidant properties of SA and accounts for ~80% of the net free thiols in plasma (594,595). The scavenging properties of this Cys34 residue to reactive carcinogenic and toxic electrophiles are well documented (596). Adducts at the Cys34 have been identified with reactive metabolites of various toxicants in rodent or human SA, including: MeIQx (587), PhIP (592,597), acrylamide (598), sulfur mustard (599), benzene (600), and acetaminophen (601), in addition to IQ (586). Acid-labile adducts of MeIQx and PhIP have been reported to form with SA; these adducts may be sulfinamide linkages at the Cys34 residue (587,591). A plausible scheme for HAA-SA sulfinamide adduct formation, based upon studies with IQ (586), is depicted in Figure 11. The N-hydroxy-HAA metabolites can undergo further oxidation by P450 1A2 or by transition metals, to form the nitroso-HAA intermediates (602), which can react with the SA-Cys34, to form HAA-SA-Cys34 sulfinamide adducts. There is one report in the literature on the measurement of putative acid-labile MeIQx-SA-Cys34 sulfinamide adducts in a pilot human study. The level of this adduct, if present, was reported to be below the LOD of the GC/MS assay (29 attomol MeIQx/mg SA) (587). It is unlikely that the sulfinamide adduct of MeIQx with human SA can be used as a dosimeter for human AIA exposure.
The binding of 14C-PhIP to SA was shown to be much greater than the binding of 14C-MeIQx to SA in humans, by AMS measurements (92,588). Moreover, PhIP-SA adduct formation was up to 40-fold greater in humans than in rats, given comparable doses of chemical (92). The levels of PhIP adducts formed with human SA (90) may be sufficient to establish MS methods of biomonitoring towards this adduct(s). An acid-labile PhIP-SA adducts(s) was (were) detected in human subjects on a non-controlled diet; levels were 10-fold higher in meat-eaters than vegetarians (6.7 ± 1.6 vs. 0.7 ± 0.3 fmol PhIP/mg protein; mean ± SE) (591). The structure(s) of the adduct attributed to the acid-labile lesion has not been determined. It seems likely that some portion of the acid-labile PhIP adduction products were formed at the Cys34 residue in human SA (592,597). The chemical stability of the adduct(s) is (are) unknown. The same study revealed the presence of acid-labile PhIP-Hb adducts in human subjects; the adduct levels were about 2-fold lower than the levels of the acid-labile PhIP-SA adducts (591). These findings suggest that HONH-PhIP forms sulfinamide adducts with SA and Hb in humans. The results contradict the data reported on PhIP blood protein adduct formation in humans by AMS measurements, where the levels of PhIP-Hb adducts were about 40 to 50-fold lower than PhIP-SA adducts (90). However, the PhIP blood protein adduct data was obtained following a single dose, in the AMS study, whereas the adduct levels in the population-based study represent an integral value of chronic exposure over the lifespan of the blood proteins (591). Further investigations on the implementation of PhIP blood protein adducts in human population studies are warranted
Biomonitoring of HAAs in Hair
Human hair and animal fur have served as matrices for biomonitoring of chemicals such as nicotine, other drugs and narcotics, and hormones (23,25,603). Studies with experimental laboratory animals have shown that 3H-labeled PhIP accumulates in melanin-rich tissues, including including fur (24). The radioactivity cleared from the body within several days, but stayed in the hair, and was present in the cortex of the distal hair shafts 4 weeks after the exposure. Following digestion of the hair matrix, chemical analyses showed that the radioactivity represented unmetabolized PhIP (24). However, the exposures in the animal studies occurred at levels exceeding the levels of PhIP or other HAAs in the human diet by at least 4 orders of magnitude. Nevertheless, Alexander and co-workers established a method to quantitate PhIP in mouse fur and then applied the technique to measure PhIP in human hair by GC/NCI/MS (24,604). Thereafter, Kobayashi and collaborators established a method to quantitate PhIP in human hair by LC-ESI/MS, employing the selected ion monitoring mode (605,606). Both methods require up to several hundred mg of hair and entail lengthy extraction procedures for chemical analysis. The pre-rinsing of the hair shaft prior to digestion of the hair matrix is required in order to remove HAAs that may have been deposited on the external surface of the cuticle by exposures from the fumes of cooking oils or airborne particulates generated by the frying or grilling of meats (13,14). The pre-rinsing procedure is essential to distinguish dietary intake of PhIP from airborne exposure.
A more recent study reported a simplified method for the extraction and analysis of HAAs in hair, by employing base hydrolysis to digest hair, followed by tandem solvent/solid phase extraction for a clean-up method (607). The quantification of PhIP was performed by LC-ESI/MS/MS in the selected reaction monitoring mode: the LOQ value was 50 pg PhIP/g of hair, when 50 mg of hair was assayed (607). The analytical method was employed to measure HAA adducts in a pilot study of 12 human volunteers. PhIP was detected in the hair of all six omnivores (non-hair dye users) at levels ranging from 290 to 890 pg/g hair, whereas PhIP was detected in the hair from one out of six vegetarians, and at a level just above the LOQ (65 pg/g hair) (607). These findings demonstrate that PhIP exposure occurs primarily through meat consumption (Figure 12). MeIQx and AαC were below the LOQ (50 pg/g hair) in hair samples from all of the omnivores as well as the six vegetarians.
Figure 12.
Bioaccumulation of PhIP in hair of omnivores and vegetarians. The melanin in the hair follicle has high affinity for PhIP, and sequesters the carcinogen from the blood stream, following first-pass metabolism (24). The LC-ESI/MS/MS trace shows the presence of PhIP in hair of an omnivore but not in the hair sample of a vegetarian. The identity of PhIP was confirmed by its product ion spectrum (607).
The levels of PhIP measured in hair of subjects in the United States (290 to 890 pg/g) (607) are within the same range of the levels of PhIP detected in hair of subjects in Norway (60-7500 pg/g hair) (608) and in subjects of Japan (180-3600 pg/g hair) on unrestricted diets (606). The levels of PhIP in hair samples from two omnivores in the United States were found to vary by less than 24% over a 6 month interval (607). A study on twenty Japanese volunteers reported a reasonably good correlation after adjustment for hair melanin content between intakes of PhIP, MeIQx, Trp-P-1 estimated with a FFQ and the mean PhIP content of hair samples collected 1 - 3 months apart (609). These findings signify that the exposure to PhIP and its accumulation in hair are relatively constant over time.
The hair biomarker represents an integrated exposure to PhIP over a time period of weeks to months and may be a superior method to assess exposure to PhIP than the Food Frequency Questionnaires (FFQ), which is often used in molecular epidemiology studies (19). PhIP levels in hair appear to be a good biomarker of long-term exposure to HAAs; however, this hair biomarker is not a predictor of DNA damage (607). Moreover, levels of PhIP accumulated in hair of individuals are highly variable. This large variation in the levels of PhIP in hair reflect in part the different concentrations of PhIP in the diet (10). The pharmacokinetics and metabolism of PhIP are also likely to influence the levels of PhIP that accumulate in hair. Because of the large interindividual differences in the hepatic P450 1A2 protein content (222,242), the amount of unmetabolized PhIP in the bloodstream that reaches the hair follicle, following first-pass metabolism, is expected to widely range and may affect the levels of PhIP accrued in hair. The pigmentation of hair also may affect the amount of PhIP incorporated into hair. Eumelanin (610), a pigment that is more predominant in black hair than in lighter-colored hair (611) has a high affinity for PhIP. Thus, dark-haired individuals may sequester larger amounts of PhIP than light-haired individuals, on the basis of findings from a study that reported mice with dark pigmented fur accrued more PhIP in their fur than mice with light pigmented fur (610). Approximately 25% of the male population and 42% of the female population have been reported to use hair dyes in the United States, Europe, and Japan (612) with permanent hair dye being the most commonly used hair dye product. The oxidizing conditions used to develop the desired hair dye colors are likely to produce oxidation products of PhIP (120,612,613) during the dye development process and would escape detection by current analytical methods. A robust analytical method to measure PhIP in users of hair dyes still requires development and validation.
Epidemiology of Cooked Meats, Potential Role of HAAs in Human Cancer
Many epidemiological investigations have examined the interrelationships among consumption of cooked red meat, its effect on human cancer risk of the digestive tract, prostate gland, the mammary gland, and the potential causal role of HAAs in the etiology of these cancers (19,70,124). The 2007 WCRF/AICR report on nutrition and cancer concluded that red meat and processed meat are “convincing causes” of colorectal cancer and that there is “limited evidence” that they also cause esophagus, stomach, pancreas, lung, endometrial and prostate cancers (614). Although several classes of carcinogens are present in red meat and processed meat and multiple mechanisms of carcinogenesis are likely to be at play (615-617), the exposure and causal role of HAAs in cancer development through eating meat cooked well-done has been an area of great research interest. The majority of epidemiologic studies that investigated dietary consumption of well-done meat in relation to various tumor sites reported a positive association between cancer risk and well-done meat consumption (see reviews (124,618) and references therein). However, some studies have shown no associations between well-done meat and cancer risk (124,618). Fewer studies have attempted to estimate intake of specific HAAs. A number of them have shown associations with cancer or colorectal adenoma risk (615,619-621), whereas others have not (622) (reviewed in (618)). Thus, overall, the dietary data have been suggestive but inconsistent.
A similarly large number of studies have explored the associations of polymorphisms in genes or pathways involved in the metabolism of HAAs (e.g., NAT2, P450 1A2, P450 1B1) with cancer risk and their results have been quite inconsistent (324,623-625). However, whole-genome association studies have recently demonstrated that common genetic variants only have small effects on risk. In particular, it can be assumed that an effect of a genetic polymorphism in a xenobiotic metabolism enzyme involved in carcinogen bioactivation (or detoxication) would be unlikely to be manifested when there is a low, biologically insufficient level of exposure to the carcinogen. Thus, it is probably important to consider both the exposure and the genetic variants to be able to detect an association with disease risk.
A smaller number of studies have examined the combined effects of dose (e.g., well-done meat or HAA intake, smoking) and metabolic genotypes or phenotypes, and these studies were mainly investigating colorectal cancer or adenoma. Interactions were suggested between intake of red meat, well-done meat or HAA and variants in NAT2 (626-630); NAT1 (631), AHR (632), CYP1B1 (632,633) and SULT1A1 (633), as well as in a combination of metabolic genes (CYP1A2, CYP2E1, CYP1B1, CYP2C9) (634), in relation to colorectal cancer or adenoma risk. However, other studies failed to replicate these associations (627,635-637). Similar interactions were found with NAT2 and meat intake for bladder cancer (638). Again, these data are suggestive but they do not show a high level of consistency across studies.
Because of its high interindividual variation, P450 1A2 activity may be relevant for cancers associated with exposure to HAAs. This enzyme, which is prominently expressed in liver (224) and other enzymes catalyzing the activation and/or detoxification of HAAs (and aromatic amines) that are inducible by lifestyle factors or modulated by genetic polymorphisms may account for interindividual differences in susceptibility to these carcinogens (256). Two case-control studies support the concept that rapid P450 1A2 activity in combination with rapid NAT2 activity is a risk factor for colorectal cancer in individuals eating well-done cooked meat, which is a rich source of HAAs (327,328,639). In one of the two studies, this association was limited to smokers (Figure 13) (328), which makes biological sense since smoking induces P450 1A2. These findings, if confirmed, would support the hypothesis that individuals with high metabolic phenotypes in P450 1A2 and NAT2 activities will have elevated levels of some types of HAA-DNA adducts, which may lead to the development of cancer. However, a third study failed to find any modifying effect of NAT2 or P450 1A2 activity, also measured by urinary caffeine metabolites, or an association of HAA with adenoma (631).
Figure 13.
Three-way interaction of red meat preference, NAT2 genotype and P450 1A2 phenotype on the risk of colorectal cancer among ever-smokers (149 cases and 216 controls), p interaction = 0.01 (639).
A critical limiting factor in most epidemiological studies is the uncertainty in quantitative estimates of chronic exposure to HAAs (or other carcinogens). For most molecular epidemiology studies, the extent of HAA exposure is difficult to assess and, thus, the association of HAAs formed in cooked meat and cancer risk has been difficult to establish. The extent of exposure to HAAs from meat in molecular epidemiology studies is often inferred by FFQ often combined with pictures of meat cooked at different levels of doneness (19,134,190). Intake estimates for meats cooked at a specified level of doneness and with various methods of high-temperature cooking (pan-frying, broiling, and barbecuing/grilling) are based on usual frequency and portion size. HAA intake estimates are then derived using corresponding HAA meat content values (615). There are clear difficulties in quantifying cooking doneness by such methods, the day-to-day variation in diet can be large, and the conventional FFQ (640), can be especially problematic when exposure to the compound of interest spreads over a range of food items at varying levels of concentrations. Moreover, the accuracy of the FFQ is particularly challenging in the assessment of levels of HAA formation, because the levels of HAAs formed are highly dependent on the type of meat cooked and, especially, the method, temperature, and duration of cooking. These variable parameters can lead to differences of HAA concentrations by more than 100-fold (10,125,126,134,189,641). Furthermore, a number of cooked meat samples assayed for HAAs across all levels of doneness categories were reported to have no detectable HAAs of any kind (641). Clearly, the uncertainties in HAA concentrations in daily staples can result in poor estimation of chronic exposure to these compounds. The limitations of the questionnaire-based exposure assessment methods are likely to be a major reason for the inconsistency in the epidemiologic data. Thus, the required data are not currently available to fully characterize the relationship between HAAs and human cancer risk. Stable, long-lived biomarkers of HAAs are required for any reliable assessment of HAA exposure for use in population studies.
Conclusion
The demonstration of exposure and chemical-specific adducts in target tissues, combined with the correlation of specific DNA adducts with mutation spectra in tumor related genes provide a mechanistic understanding of the causal role for a chemical in the development of cancer (20,26,642). The laboratory research conducted on AFB1, a fungal toxicant and a potent animal carcinogen that is found as a contaminant in various crops (15), is the prime example of where biomarkers of carcinogen exposure have been employed to identify and refine cancer risk estimates (642,643). The positive associations observed between dietary AFB1 exposure and the incidence of hepatocellular carcinoma in Asia and Africa were greatly strengthened by the application of validated biomarkers, which included DNA and SA adducts, and a characteristic mutation spectrum in the p53 tumor suppressor gene that is linked to a DNA adduct of AFB1 (571,572,642,644,645). There is also promising biomarker data that support a role of aristolochic acid, a carcinogen present in the plant species of the genus Aristolochia, as a causal agent of urothelial cancer in subjects of the Balkans (564,646) and Taiwan (647).
With regard to aromatic amines, the causal role of some these compounds in human bladder cancer was revealed through epidemiological studies conducted world-wide on factory workers occupationally exposed to high levels of the procarcinogens (1-3,105,106). Later, laboratory studies elucidated the biochemical mechanisms of aromatic amine metabolism and adduction products with protein and DNA (45,76), which set the stage for the employment of arylamine-Hb and DNA adducts as biomarkers of exposure. The implementation of these biomarkers in human epidemiology studies has strengthened the association of arylamines with cancer risk and has also implicated aromatic amines in tobacco-associated bladder cancer (28,31,50,52,536,581,583).
Such extensive biomarker data have yet to be established in molecular epidemiology studies examining the potential cancer causal role of HAAs or other genotoxicants that are formed at the ppb concentrations in cooked foods. The daily exposure to numerous genotoxicants in cooked foods makes dietary hazard assessment a challenging task, particularly when carcinogenesis involves numerous steps (71), and when dietary, environmental, and genetic factors can impact the biological potency of the procarcinogens (9,22,157-159,413). Ultimately, the incorporation of biomarkers in molecular epidemiology studies may help to disentangle the uncertainty about the relative contributions of various dietary genotoxicants to cancer risk (24,185). Although a causal link with cancer has not been established for HAAs, many epidemiology studies have associated frequent consumption of well-done cooked meat products to colorectal cancer, and less consistently, prostate and breast cancer (124,618). Our current knowledge about the biochemistry of HAAs indicates that the underlying biochemical mechanisms of HAA metabolism, DNA adduct formation, DNA repair, and mutations are comparable to those involved in aromatic amine carcinogenicity.
Even though aromatic amines and HAAs are structurally related classes of chemicals and share some common pathways of metabolism, the strategies employed for human bimonitoring of these procarcinogens are different. The assessment of chronic exposure to many arylamines has been successfully done through measurement of Hb sulfinamide adducts formed by reaction of the arylnitroso intermediates with the Hb-Cys93β residue. This adductome approach has been employed to measure 15 aromatic amine Hb sulfinamide adducts (581). HAAs also undergo extensive N-oxidation in humans, as demonstrated by the urinary metabolite profiles of MeIQx and PhIP (98,101,103,176,222,288,289,292), but the HAA-Hb sulfinamide adducts appear to be formed at insufficient levels to exploit this protein adduct for human biomonitoring. An alternative potential adductome approach that can be applied to measure reactive carcinogenic and toxic electrophiles is through their adduction products formed at the Cys34 residue of SA (596). Human SA-Cys34 may be screened for the sulfenamide or sulfinamide adducts of PhIP (90,591,592), or adducts formed with N-oxidized intermediates of other HAAs; however, the structures of these human SA-Cys34 adducts have not yet been fully characterized by spectroscopic techniques and the chemical stability of the adduct(s) is unknown. Further studies on the characterization of PhIP-SA adducts are required to validate this biomarker prior to its application in population-based studies.
The N-glucuronide conjugates of many arylamines and arylhydroxylamines undergo facile hydrolysis in the urinary bladder and as a result are not readily measured (76), whereas the N-glucuronide conjugates of AIAs and HONH-AIAs are stable in the mildly acidic pH conditions of urine (103,293). Therefore, the direct monitoring of urinary N-glucuronide metabolites of MeIQx and PhIP and their N-hydroxylated metabolites may be used to examine metabolic phenotypes such as P450 1A2 or UGTs (176,459); however, the short half-life of these metabolites in urine reflects recent dietary intake only, making it unsuitable for assessment of chronic but intermittent exposures.
The biomonitoring of HAA levels in hair or macromolecular HAA adducts has the potential to assess long-term exposure to these carcinogens. On the basis of the current state of knowledge of HAA exposure and the literature on HAA biomarkers, several biomarkers of PhIP seem to be most promising for employment in molecular epidemiology studies. A very high percentage of humans in different parts of the world contain PhIP in their hair (604,607,609). The implementation of this hair biomarker in epidemiology studies can confirm chronic exposure to PhIP, but it does not represent DNA damage or necessarily represent cancer risk (607).
Putative PhIP-DNA adducts have been detected with very high frequency in human pancreas, prostate and female mammary gland, or in exfoliated epithelial cells from human milk samples of women in the United States, by IHC or 32P-postlabeling methods (30,173,174,177). Surprisingly, the levels of PhIP-DNA adducts formed in these tissues are comparable to the adduct levels reported in the corresponding tissues of rodents given a single acute dose or chronic carcinogenic doses of PhIP, which exceeded human dietary levels by a million-fold or more (155,404,550). The frequent detection of PhIP-DNA adducts at such high levels in human tissues is alarming. However, the proof of identity of PhIP-DNA adducts by IHC and 32P-postlabeling detection methods is equivocal. If the high levels of PhIP-DNA adducts in human tissues are confirmed by selective and quantitative mass spectrometry methods, PhIP would be recognized as a major dietary DNA-damaging agent. Furthermore, the interpretation of the rodent biochemical and carcinogenicity data (9,155,191) extrapolated across species to assess the human health risk of PhIP and possibly other HAAs would require re-examination (96,164,179,181,183,184). Currently available mass spectrometry instruments have the requisite sensitivity to measure PhIP-DNA adducts in human tissues and biological fluids (178,480). Thus, there is an urgent need to corroborate the DNA adduct binding data obtained by 32P-postlabeling and IHC methods with quantitative mass spectrometry techniques.
In conclusion, rapid through-put methods are still needed to be developed for analyses of HAA biomarkers by mass spectrometry methods in large scale human epidemiology studies. The employment of novel analytical approaches and mass spectrometry techniques to measure HAA biomarkers in large prospective studies with appropriate biospecimens presents the most potential to characterize better the health risks of dietary and tobacco-associated HAAs in human cancers.
Acknowledgement
This manuscript is dedicated to the memory of Dr. Fred Kadlubar: a mentor, collaborator, and dear friend, who greatly contributed to the fields of chemical carcinogenesis and molecular epidemiology.
The authors apologize for omissions made necessary by the space requirements for this review. Many of the omitted studies are of undoubted importance to the field of chemical carcinogenesis and molecular epidemiology. We are also greatly appreciative for the critical comments of this manuscript provided by the reviewers.
Funding Support
A portion of the data reported in this manuscript was supported by grant R01 CA122320 (RJT), and by R01 CA60987 and CA72520 (LLM) from the National Cancer Institute, by R21 ES014438 (RJT) from the National Institute of Environmental Health Sciences, and by grants number 2007/58 (RJT) and RFA09/149 (LLM) from the World Cancer Research Fund International.
Abbreviations
- AMS
accelerator mass spectrometry
- AIAs
aminoimidazoarenes
- AAs
aromatic amines
- FFQ
Food Frequency Questionnaire
- GC-NICI-MS
gas chromatography with negative ion chemical ionization-mass spectrometry
- GST
glutathione S-transferase
- HAAs
heterocyclic aromatic amines
- Hb
hemoglobin
- IHC
immunohistochemistry
- LC-ESI/MS/MS
liquid chromatography-electrospray ionization/tandem mass spectrometry
- MOE
margin of exposure
- MR
metabolic ratio
- NATs
N-acetyltransferases
- OAT
O-acetyltransferase
- PBPH/PD
physiologically based pharmacokinetic/pharmacodynamic
- PHS
prostaglandin H synthase
- SA
serum albumin
- SULTs
sulfotransferases
- UGTs
Uridine diphosphate-Glucuronosyltransferases
- 4-ABP
4-aminobiphenyl
- HONH-4-ABP
N-hydroxy-4-aminobiphenyl
- IFP
2-amino-1,6-dimethylfuro[3,2-e]imidazo[4,5-b]pyridine
- AAF
N-acetyl-2-aminofluorene
- AF
2-aminofluorene
- HONH-AF
N-hydroxy-2-aminofluorene
- Trp-P-1
2-amino-1,4-dimethyl-5H-pyrido[4,3-b]indole
- Trp-P-2
2-amino-1-methyl-5H-pyrido[4,3-b]indole
- Glu-P-2
2-aminodiprido[1,2-a:3′,2′-d]imidazole
- Glu-P-1
2-amino-6-methyldiprido[1,2-a:3′,2′-d]imidazole
- APNH
9-(4′-aminophenyl)-9H-pyrido[3,4-b]indole
- AMPNH
9-(4′-amino-3-methylphenyl)-9H-pyrido[3,4-b]indole
- IQx
2-amino-3-methylimidazo[4,5-f]quinoxaline
- IgQx
2-amino-3-methylimidazo[4,5-g]quinoxaline
- 7-MeIgQx
2-amino-3,7-dimethylimidazo[4,5-g]quinoxaline
- 7,9-DiMeIgQx
2-amino-3,7,9-trimethylimidazo[4,5-g]quinoxaline
- MeIQx
2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline
- HONH-MeIQx
N-hydroxy-2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline
- IQx-8-COOH
2-amino-3-methylimidazo[4,5-f]quinoxaline-8-carboxylic acid
- 8-CH2OH-IQx
2-amino-8-(hydroxymethyl)-3-methylimidazo[4,5-f]quinoxaline
- MeIQx-N2-Gl
N2-(ß-1-glucosiduronyl)-2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline
- HON-MeIQx-N2-Gl
N2-(ß-1-glucosiduronyl)-2-(hydroxyamino)-3,8-dimethylimidazo[4,5-f]quinoxaline
- MeIQx-N2-SO3H
N2-(3,8-dimethylimidazo[4,5-f]quinoxalin-2-yl-sulfamic acid
- DMIP
2-amino-1,7-dimethylimidazo[4,5-b]pyridine
- TMIP
2-amino-1,5,6-trimethylimidazo[4,5-b]pyridine
- 4,8-DiMeIQx
2-amino-3,4,8-trimethylimidazo[4,5-f]quinoxaline
- 7,8-DiMeIQx
2-amino-3,7,8-trimethylimidazo[4,5-f]quinoxaline
- MeIQ
2-amino-3,4-dimethylimidazo[4,5-f]quinoline
- IQ
2-amino-3-methylimidazo[4,5-f]quinoline
- HONH-IQ
N-hydroxy-2-amino-3-methylimidazo[4,5-f]quinoline
- PhIP
2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine
- HONH-PhIP
N-hydroxy-2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine
- PhIP
2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine
- HONH-PhIP
HON-PhIP-N2-Gl, N2-(ß-1-glucosiduronyl-2-(hydroxyamino)-1-methyl-6-phenylimidazo[4,5-b]pyridine
- HON-PhIP-N3-Gl
N3-(ß-1-glucosiduronyl-2-(hydroxyamino)-1-methyl-6-phenylimidazo[4,5-b]pyridine
- PhIP-N2-Gl
N2-(ß-1-glucosiduronyl-2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine
- PhIP-N3-Gl
N3-(ß-1-glucosiduronyl-2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine
- MeAαC
2-amino-3-methyl-9H-pyrido[2,3-b]indole
- AαC
2-amino-9H-pyrido[2,3-b]indole
- HONH-AαC
2-hydroxyamino-9H-pyrido[2,3-b]indole
- Bz
benzidine
- N-acetylBz
N-acetylbenzidine
- HONH-N′-acetylBz
N-4-hydroxyamino-N′-acetylbenzidine
- 2-CA
2-chloroaniline
- 4-CA
4-chloroaniline
- 3-EA
3-ethylaniline
- 2-NA
2-naphthylamine
- HONH-2-NA
N-hydroxy-2-aminonaphthalene
- 3,5-DMA
3,5-dimethyaniline
- dG-C8-ABP
N-(deoxyguanosin-8-yl)-ABP
- dG-N2-N4-4-ABP
N-(deoxyguanosin-N2-yl)-ABP
- dG-N2-ABP
3-(deoxyguanosin-N2-yl)-4-ABP
- dA-C8-4-ABP
N-(deoxyadenosin-8-yl)-4-ABP
- N′-acetyl-dG-C8-Bz
N-(deoxyguanosin-8-yl)-N′-acetylbenzidine
- dG-N2-2-NA
1-(deoxyguanosin-N2-yl)-2-NA
- dG-C8-2-NA
N-(deoxyguanosin-8-yl)-2-NA
- ring-opened-dG-C8-NA
ring-opened-N-(deoxyguanosin-8-yl)-2-NA
- dA-N6-2-NA
1-(deoxyadenosin-N6-yl)-2-NA
- dG-N2-NAQI
N4-(deoxyguanosin-N2-yl)-2-amino-1,4-naphthoquinoneimine
- dG-C8-MeIQx
N-(deoxyguanosin-8-yl)-MeIQx
- dG-C8-4,8-DiMeIQx
N-(deoxyguanosin-8-yl)-4,8-MeIQx
- dG-N2-MeIQx
5-(deoxyguanosin-N2-yl)-MeIQx
- dG-C8-IQ
N-(deoxyguanosin-8-yl)-IQ
- dG-C8-MeIQ
N-(deoxyguanosin-8-yl)-MeIQ
- dA-N6-IQ
5-(deoxyadenosin-N6-yl)-IQ
- dG-N2-IQ
5-(deoxyguanosin-N2-yl)-IQ
- dG-C8-AαC
N-(deoxyguanosin-8-yl)-AαC
- dG-C8-MeAαC
N-(deoxyguanosin-8-yl)-MeAαC
- dG-C8-PhIP
N-(deoxyguanosin-8-yl)-PhIP
Reference List
- 1.International Agency for Resarch on Cancer . Overall evaluation of carcinogenicity: An updating of IARC Monographs 1-42. International Agency for Research on Cancer; Lyon, France: 1987. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans 7 (suppl) [PubMed] [Google Scholar]
- 2.International Agency for Research on Cancer . Some aromatic amines, organic dyes, and related exposures. Vol. 99. International Agency for Research on Cancer; Lyon, France: 2010. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. [PMC free article] [PubMed] [Google Scholar]
- 3.International Agency for Research on Cancer . Tobacco smoking. Vol. 38. International Agency for Research on Cancer; Lyon, France: 1986. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. [PMC free article] [PubMed] [Google Scholar]
- 4.Patrianakos C, Hoffmann D. Chemical studies on tobacco smoke LXIV. On the analysis of aromatic amines in cigarette smoke. J. Assoc. Off Anal. Chem. 1979;3:150–154. [Google Scholar]
- 5.Chiang TA, Pei-Fen W, Ying LS, Wang LF, Ko YC. Mutagenicity and aromatic amine content of fumes from heated cooking oils produced in Taiwan. Food Chem. Toxicol. 1999;37:125–134. doi: 10.1016/s0278-6915(98)00081-7. [DOI] [PubMed] [Google Scholar]
- 6.Matsumoto T, Yoshida D, Tomita H. Determination of mutagens, amino-alpha-carbolines in grilled foods and cigarette smoke condensate. Cancer Lett. 1981;12:105–110. doi: 10.1016/0304-3835(81)90045-8. [DOI] [PubMed] [Google Scholar]
- 7.Manabe S, Tohyama K, Wada O, Aramaki T. Detection of a carcinogen, 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine, in cigarette smoke condensate. Carcinogenesis. 1991;12:1945–1947. doi: 10.1093/carcin/12.10.1945. [DOI] [PubMed] [Google Scholar]
- 8.Sugimura T, Nagao N, Kawachi T, Honda M, Yahagi T, Seino Y, Stao S, Matsukura N, Matsushima T, Shirai A, Sawamura M, Matsumoto H. Mutagen-carcinogens in food, with special reference to highly mutagenic pyrolytic products in broiled foods. In: Hiatt HH, Watson JD, Winstein JA, editors. Origins of Human Cancer, Book C. Cold Spring Harbor Laboratory; Cold Spring Harbor, NY: 1977. pp. 1561–1577. [Google Scholar]
- 9.Sugimura T, Wakabayashi K, Nakagama H, Nagao M. Heterocyclic amines: Mutagens/carcinogens produced during cooking of meat and fish. Cancer Sci. 2004;95:290–299. doi: 10.1111/j.1349-7006.2004.tb03205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Felton JS, Jagerstad M, Knize MG, Skog K, Wakabayashi K. Contents in foods, beverages and tobacco. In: Nagao M, Sugimura T, editors. Food Borne Carcinogens Heterocyclic Amines. John Wiley & Sons Ltd.; Chichester, England: 2000. pp. 31–71. [Google Scholar]
- 11.Laser Reutersward A, Skog K, Jagerstad M. Effects of creatine and creatinine content on the mutagenic activity of meat extracts, bouillons and gravies from different sources. Food Chem. Toxicol. 1987;25:747–754. doi: 10.1016/0278-6915(87)90229-8. [DOI] [PubMed] [Google Scholar]
- 12.Gross GA, Turesky RJ, Fay LB, Stillwell WG, Skipper PL, Tannenbaum SR. Heterocyclic aromatic amine formation in grilled bacon, beef and fish and in grill scrapings. Carcinogenesis. 1993;14:2313–2318. doi: 10.1093/carcin/14.11.2313. [DOI] [PubMed] [Google Scholar]
- 13.Yang CC, Jenq SN, Lee H. Characterization of the carcinogen 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline in cooking aerosols under domestic conditions. Carcinogenesis. 1998;19:359–363. doi: 10.1093/carcin/19.2.359. [DOI] [PubMed] [Google Scholar]
- 14.Thiebaud HP, Knize MG, Kuzmicky PA, Hsieh DP, Felton JS. Airborne mutagens produced by frying beef, pork and a soy-based food. Food Chem. Toxicol. 1995;33:821–828. doi: 10.1016/0278-6915(95)00057-9. [DOI] [PubMed] [Google Scholar]
- 15.International Agency for Research on Cancer . Some naturally occurring substances: food items and constituents, heterocyclic aromatic amines and mycotoxins. Vol. 56. International Agency for Research on Cancer; Lyon, France: 1993. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. [Google Scholar]
- 16.National Toxicology Program . Report on Carcinogenesis. Eleventh Edition. U.S. Department of Health and Human Services, Public Health Service, Research Triangle Park; N.C.: 2005. National Toxicology Program. 2005. [Google Scholar]
- 17.Scribner JD, Fisk SR, Scribner NK. Mechanisms of action of carcinogenic aromatic amines: an investigation using mutagenesis in bacteria. Chem. - Biol. Interact. 1979;26:11–25. doi: 10.1016/0009-2797(79)90090-5. [DOI] [PubMed] [Google Scholar]
- 18.Hatch FT, Knize MG, Colvin ME. Extended quantitative structure-activity relationships for 80 aromatic and heterocyclic amines: structural, electronic, and hydropathic factors affecting mutagenic potency. Environ. Mol. Mutagen. 2001;38:268–291. doi: 10.1002/em.10028. [DOI] [PubMed] [Google Scholar]
- 19.Sinha R. An epidemiologic approach to studying heterocyclic amines. Mutat. Res. 2002;506-507:197–204. doi: 10.1016/s0027-5107(02)00166-5. [DOI] [PubMed] [Google Scholar]
- 20.Jarabek AM, Pottenger LH, Andrews LS, Casciano D, Embry MR, Kim JH, Preston RJ, Reddy MV, Schoeny R, Shuker D, Skare J, Swenberg J, Williams GM, Zeiger E. Creating context for the use of DNA adduct data in cancer risk assessment: I. Data organization. Crit. Rev. Toxicol. 2009;39:659–678. doi: 10.1080/10408440903164155. [DOI] [PubMed] [Google Scholar]
- 21.Hecht SS. Human urinary carcinogen metabolites: biomarkers for investigating tobacco and cancer. Carcinogenesis. 2002;23:907–922. doi: 10.1093/carcin/23.6.907. [DOI] [PubMed] [Google Scholar]
- 22.Kadlubar FF, Butler MS, Kaderlik KR, Chou H-C, Lang NP. Polymorphisms for aromatic amines metabolism in humans: relevance for human carcinogenesis. Environ. Health Perspect. 1992;98:69–74. doi: 10.1289/ehp.929869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakahara Y, Takahashi K, Kikura R. Hair analysis for drugs of abuse. X. Effect of physicochemical properties of drugs on the incorporation rates into hair. Biol. Pharm. Bull. 1995;18:1223–1227. doi: 10.1248/bpb.18.1223. [DOI] [PubMed] [Google Scholar]
- 24.Alexander J, Reistad R, Hegstad S, Frandsen H, Ingebrigtsen K, Paulsen JE, Becher G. Biomarkers of exposure to heterocyclic amines: approaches to improve the exposure assessment. Food Chem. Toxicol. 2002;40:1131–1137. doi: 10.1016/s0278-6915(02)00053-4. [DOI] [PubMed] [Google Scholar]
- 25.Gratacos-Cubarsi M, Castellari M, Valero A, Garcia-Regueiro JA. Hair analysis for veterinary drug monitoring in livestock production. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2006;834:14–25. doi: 10.1016/j.jchromb.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 26.Himmelstein MW, Boogaard PJ, Cadet J, Farmer PB, Kim JH, Martin EA, Persaud R, Shuker DE. Creating context for the use of DNA adduct data in cancer risk assessment: II. Overview of methods of identification and quantitation of DNA damage. Crit. Rev. Toxicol. 2009;39:679–694. doi: 10.1080/10408440903164163. [DOI] [PubMed] [Google Scholar]
- 27.Phillips DH. DNA adducts as markers of exposure and risk. Mutat. Res. 2005;577:284–292. doi: 10.1016/j.mrfmmm.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 28.Talaska G, al Juburi AZ, Kadlubar FF. Smoking related carcinogen-DNA adducts in biopsy samples of human urinary bladder: identification of N-(deoxyguanosin-8-yl)-4-aminobiphenyl as a major adduct. Proc. Natl. Acad. Sci. U. S. A. 1991;88:5350–5354. doi: 10.1073/pnas.88.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thompson PA, DeMarini DM, Kadlubar FF, McClure GY, Brooks LR, Green BL, Fares MY, Stone A, Josephy PD, Ambrosone CB. Evidence for the presence of mutagenic arylamines in human breast milk and DNA adducts in exfoliated breast ductal epithelial cells. Environ. Mol. Mutagen. 2002;39:134–142. doi: 10.1002/em.10067. [DOI] [PubMed] [Google Scholar]
- 30.Gorlewska-Roberts K, Green B, Fares M, Ambrosone CB, Kadlubar FF. Carcinogen-DNA adducts in human breast epithelial cells. Environ. Mol. Mutagen. 2002;39:184–192. doi: 10.1002/em.10060. [DOI] [PubMed] [Google Scholar]
- 31.Skipper PL, Tannenbaum SR. Protein adducts in the molecular dosimetry of chemical carcinogens. Carcinogenesis. 1990;11:507–518. doi: 10.1093/carcin/11.4.507. [DOI] [PubMed] [Google Scholar]
- 32.Rundle A. Carcinogen-DNA adducts as a biomarker for cancer risk. Mutat. Res. 2006;600:23–36. doi: 10.1016/j.mrfmmm.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 33.Kriek E. Fifty years of research on N-acetyl-2-aminofluorene, one of the most versatile compounds in experimental cancer research. J. Cancer Res. Clin. Oncol. 1992;118:481–489. doi: 10.1007/BF01225261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clayson DB. Specific aromatic amines as occupational bladder carcinogens. Natl. Cancer Inst. Monogr. 1981:15–19. [PubMed] [Google Scholar]
- 35.Miller JA. Carcinogenesis by chemicals: an overview--G. H. A. Clowes memorial lecture. Cancer Res. 1970;30:559–576. [PubMed] [Google Scholar]
- 36.Miller EC. Some current perspectives on chemical carcinogenesis in humans and experimental animals: Presidential address. Cancer Res. 1978;38:1479–1496. [PubMed] [Google Scholar]
- 37.Beland FA, Kadlubar FF. Formation and persistence of arylamine DNA adducts in vivo. Environ. Health Perspect. 1985;62:19–30. doi: 10.1289/ehp.856219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neumann HG. Biomonitoring of aromatic amines and alkylating agents by measuring hemoglobin adducts. Int. Arch. Occup. Environ. Health. 1988;60:151–155. doi: 10.1007/BF00378690. [DOI] [PubMed] [Google Scholar]
- 39.Kiese M. The biochemical production of ferrihemoglobin-forming derivatives from aromatic amines, and mechanisms of ferrihemoglobin formation. Pharmacol. Rev. 1966;18:1091–1161. [PubMed] [Google Scholar]
- 40.Irving CC. Conjugates of N-Hydroxy Compounds. In: Fishman WH, editor. Metabolic Conjugation and Metabolic Hydrolysis. Academic Press, Inc.; New York: 1973. pp. 53–119. [Google Scholar]
- 41.Miller JA, Miller EC. The metabolic activation of carcinogenic aromatic amines and amides. Prog. Exp. Tumor Res. 1969;11:273–301. doi: 10.1159/000391399. [DOI] [PubMed] [Google Scholar]
- 42.Hoffmann GR, Fuchs RP. Mechanisms of frameshift mutations: insight from aromatic amines. Chem. Res. Toxicol. 1997;10:347–359. doi: 10.1021/tx960128n. [DOI] [PubMed] [Google Scholar]
- 43.Neumann HG. Aromatic amines: mechanisms of carcinogenesis and implications for risk assessment. Front. Biosci. 2010;15:1119–1130. doi: 10.2741/3665. [DOI] [PubMed] [Google Scholar]
- 44.Gorrod JW, Manson D. The metabolism of aromatic amines. Xenobiotica. 1986;16:933–955. doi: 10.3109/00498258609038975. [DOI] [PubMed] [Google Scholar]
- 45.Kadlubar FF, Beland FA. Chemical properties of ultimate carcinogenic metabolites of arylamines and arylamides. In: Harvey RG, editor. Polycyclic Hydrocarbons and Carcinogenesis. American Chemical Society; Washington, D.C.: 1985. pp. 332–370. [Google Scholar]
- 46.Weisburger JH, Weisburger EK. Chemicals as causes of cancer. Chem. Eng. News. 1966;44:124–142. [Google Scholar]
- 47.International Agency for Research on Cancer . Tobacco smoke and involuntary smoking. Vol. 83. International Agency for Research on Cancer; Lyon, France: 2004. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. [PMC free article] [PubMed] [Google Scholar]
- 48.Neumann HG. Analysis of hemoglobin as a dose monitor for alkylating and arylating agents. Arch. Toxicol. 1984;56:1–6. doi: 10.1007/BF00316343. [DOI] [PubMed] [Google Scholar]
- 49.Skipper PL, Peng X, SooHoo CK, Tannenbaum SR. Protein adducts as biomarkers of human carcinogen exposure. Drug Metab. Rev. 1994;26:111–124. doi: 10.3109/03602539409029787. [DOI] [PubMed] [Google Scholar]
- 50.Yu MC, Skipper PL, Tannenbaum SR, Chan KK, Ross RK. Arylamine exposures and bladder cancer risk. Mutat. Res. 2002;506-507:21–28. doi: 10.1016/s0027-5107(02)00148-3. [DOI] [PubMed] [Google Scholar]
- 51.Sabbioni G, Jones CR. Biomonitoring of arylamines and nitroarenes. Biomarkers. 2002;7:347–421. doi: 10.1080/13547500210147253. [DOI] [PubMed] [Google Scholar]
- 52.Talaska G, Al-Zoughool M. Aromatic amines and biomarkers of human exposure. J. Environ. Sci. Health C. Environ. Carcinog. Ecotoxicol. Rev. 2003;21:133–164. doi: 10.1081/GNC-120026234. [DOI] [PubMed] [Google Scholar]
- 53.Richter E, Branner B. Biomonitoring of exposure to aromatic amines: haemoglobin adducts in humans. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2002;778:49–62. doi: 10.1016/s0378-4347(01)00466-2. [DOI] [PubMed] [Google Scholar]
- 54.Kataoka H. Methods for the determination of mutagenic heterocyclic amines and their applications in environmental analysis. J. Chromatogr. A. 1997;774:121–142. doi: 10.1016/s0021-9673(97)00246-x. [DOI] [PubMed] [Google Scholar]
- 55.Pais P, Knize MG. Chromatographic and related techniques for the determination of aromatic heterocyclic amines in foods. J. Chromatogr. B. 2000;747:139–169. doi: 10.1016/s0378-4347(00)00118-3. [DOI] [PubMed] [Google Scholar]
- 56.Busquets R, Mitjans D, Puignou L, Galceran MT. Quantification of heterocyclic amines from thermally processed meats selected from a small-scale population-based study. Mol. Nutr. Food Res. 2008;52:1408–1420. doi: 10.1002/mnfr.200800048. [DOI] [PubMed] [Google Scholar]
- 57.Alaejos MS, Afonso AM. Factors that affect the content of heterocyclic aromatic amines in foods. Comprehensive Reviews in Food Science and Food Safety. 2011;10:52–108. [Google Scholar]
- 58.Jagerstad M, Skog K, Grivas S, Olsson K. Formation of heterocyclic amines using model systems. Mutat. Res. 1991;259:219–233. doi: 10.1016/0165-1218(91)90119-7. [DOI] [PubMed] [Google Scholar]
- 59.Messner C, Murkovic M. Evaluation of a new model system for studying the formation of heterocyclic amines. J. Chromatogr. B. 2004;802:19–26. doi: 10.1016/j.jchromb.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 60.Kato R. Metabolic activation of mutagenic heterocyclic aromatic amines from protein pyrolysates. CRC Crit. Rev. Toxicol. 1986;16:307–348. doi: 10.3109/10408448609037466. [DOI] [PubMed] [Google Scholar]
- 61.Wakabayashi K, Nagao M, Esumi H, Sugimura T. Food-derived mutagens and carcinogens. Cancer Res. 1992;52:2092s–2098s. [PubMed] [Google Scholar]
- 62.Eisenbrand G, Tang W. Food-borne heterocyclic amines. Chemistry, formation, occurrence and biological activities. A literature review. Toxicology. 1993;84:1–82. doi: 10.1016/0300-483x(93)90109-6. [DOI] [PubMed] [Google Scholar]
- 63.Schut HA, Snyderwine EG. DNA adducts of heterocyclic amine food mutagens: implications for mutagenesis and carcinogenesis. Carcinogenesis. 1999;20:353–368. doi: 10.1093/carcin/20.3.353. [DOI] [PubMed] [Google Scholar]
- 64.Gooderham NJ, Murray S, Lynch AM, Yadollahi-Farsani M, Zhao K, Boobis AR, Davies DS. Food-derived heterocyclic amine mutagens: variable metabolism and significance to humans. Drug Metab. Dispos. 2001;29:529–534. [PubMed] [Google Scholar]
- 65.Felton JS, Knize MG, Bennett LM, Malfatti MA, Colvin ME, Kulp KS. Impact of environmental exposures on the mutagenicity/carcinogenicity of heterocyclic amines. Toxicology. 2004;198:135–145. doi: 10.1016/j.tox.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 66.Kadlubar FF, Kaderlik KR, Mulder GJ, Lin D-X, Butler MA, Teitel CH, Minchin RF, Ilett KF, Friesen MD, Bartsch H, Nagao M, Esumi E, Sugimura T, Lang NP. Metabolic activation and DNA adduct detection of PhIP in dogs, rats, and humans in relation to urinary bladder and colon carcinogenesis. In: Adamson RH, Gustafsson J-A, Ito N, Nagao M, Sugimura T, Wakabayashi K, Yamazoe Y, editors. Heterocyclic Amines in Cooked Foods: Possible Human Carcinogens. 23rd Proceedings of the Princess Takamatusu Cancer Society. Princeton Scientific Publishing Co., Inc.; Princeton, NJ: 1995. pp. 207–213. [PubMed] [Google Scholar]
- 67.Airoldi L, Magagnotti C, Pastorelli R, Fanelli R. Enzyme polymorphisms influencing the metabolism of heterocyclic aromatic amines. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2004;802:175–181. doi: 10.1016/j.jchromb.2003.10.055. [DOI] [PubMed] [Google Scholar]
- 68.Glatt H. Metabolic factors affecting the mutagenicity of heteroyclic amines. In: Skog K, Alexander J, editors. Acrylamide and Other Hazardous Compounds in Heat-Treated Foods. Woodhead Publishing Ltd.; Cambridge, England: 2006. pp. 358–404. [Google Scholar]
- 69.Turesky RJ. Formation and biochemistry of carcinogenic heterocyclic aromatic amines in cooked meats. Toxicol. Lett. 2007;168:219–227. doi: 10.1016/j.toxlet.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 70.Alaejos MS, Gonzalez V, Afonso AM. Exposure to heterocyclic aromatic amines from the consumption of cooked red meat and its effect on human cancer risk: a review. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2008;25:2–24. doi: 10.1080/02652030701474235. [DOI] [PubMed] [Google Scholar]
- 71.Sugimura T. Multistep carcinogenesis: A 1992 perspective. Science. 1992;258:603–607. doi: 10.1126/science.1411570. [DOI] [PubMed] [Google Scholar]
- 72.Nagao M, Ushijima T, Toyota M, Inoue R, Sugimura T. Genetic changes induced by heterocyclic amines. Mutat. Res. 1997;376:161–167. doi: 10.1016/s0027-5107(97)00039-0. [DOI] [PubMed] [Google Scholar]
- 73.Nagao M. Mutagenicity. In: Nagao M, Sugimura T, editors. Food Borne Carcinogens Heterocyclic Amines. John Wiley & Sons Ltd.; Chichester, England: 2000. pp. 163–195. [Google Scholar]
- 74.Dashwood RH. Use of transgenic and mutant animal models in the study of heterocyclic amine-induced mutagenesis and carcinogenesis. J. Biochem. Mol. Biol. 2003;36:35–42. doi: 10.5483/bmbrep.2003.36.1.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Turesky RJ, Fay LB, Welti DH. Metabolism of heterocyclic aromatic amines and strategies of human biomonitoring. In: Adamson RH, Gustafsson J-A, Ito N, Nagao M, Sugimura T, Wakabayashi K, Yamazoe Y, editors. Heterocyclic Amines in Cooked Foods: Possible Human Carcinogens. 23rd Proceedings of the Princess Takamatusu Cancer Society. Princeton Scientific Publishing Co., Inc.; Princeton, NJ: 1995. pp. 59–68. [Google Scholar]
- 76.Beland FA, Beranek DT, Dooley KL, Heflich RH, Kadlubar FF. Arylamine-DNA adducts in vitro and in vivo: their role in bacterial mutagenesis and urinary bladder carcinogenesis. Environ. Health Perspect. 1983;49:125–134. doi: 10.1289/ehp.8349125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Castelao JE, Yuan JM, Skipper PL, Tannenbaum SR, Gago-Dominguez M, Crowder JS, Ross RK, Yu MC. Gender- and smoking-related bladder cancer risk. J. Natl. Cancer Inst. 2001;93:538–545. doi: 10.1093/jnci/93.7.538. [DOI] [PubMed] [Google Scholar]
- 78.Skipper PL, Tannenbaum SR, Ross RK, Yu MC. Nonsmoking-related arylamine exposure and bladder cancer risk. Cancer Epidemiol. Biomarkers Prev. 2003;12:503–507. [PubMed] [Google Scholar]
- 79.Kiese M, Taeger K. The fate of phenylhydroxylamine in human red cells. Naunyn Schmiedebergs Arch. Pharmacol. 1976;292:59–66. doi: 10.1007/BF00506490. [DOI] [PubMed] [Google Scholar]
- 80.Green LC, Skipper PL, Turesky RJ, Bryant MS, Tannenbaum SR. In vivo dosimetry of 4-aminobiphenyl in rats via a cysteine adduct in hemoglobin. Cancer Res. 1984;44:4254–4259. [PubMed] [Google Scholar]
- 81.Ringe D, Turesky RJ, Skipper PL, Tannenbaum SR. Structure of the single stable hemoglobin adduct formed by 4-aminobiphenyl in vivo. Chem. Res. Toxicol. 1988;1:22–24. doi: 10.1021/tx00001a003. [DOI] [PubMed] [Google Scholar]
- 82.Skipper PL, Stillwell WG. Aromatic amine-hemoglobin adducts. Methods Enzymol. 1994;231:643–649. doi: 10.1016/0076-6879(94)31044-0. [DOI] [PubMed] [Google Scholar]
- 83.Bryant MS, Skipper PL, Tannenbaum SR, Maclure M. Hemoglobin adducts of 4-aminobiphenyl in smokers and nonsmokers. Cancer Res. 1987;47:602–608. [PubMed] [Google Scholar]
- 84.Turesky RJ, Markovic J, Bracco-Hammer I, Fay LB. The effect of dose and cytochrome P450 induction on the metabolism and disposition of the food-borne carcinogen 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline. Carcinogenesis. 1991;12:1847–1855. doi: 10.1093/carcin/12.10.1847. [DOI] [PubMed] [Google Scholar]
- 85.Sjödin P, Wallin H, Alexander J, Jagerstad M. Disposition and metabolism of the food mutagen 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx) in rats. Carcinogenesis. 1989;10:1269–1275. doi: 10.1093/carcin/10.7.1269. [DOI] [PubMed] [Google Scholar]
- 86.Dragsted LO, Frandsen H, Reistad R, Alexander J, Larsen JC. DNA-binding and disposition of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in the rat. Carcinogenesis. 1995;16:2785–2793. doi: 10.1093/carcin/16.11.2785. [DOI] [PubMed] [Google Scholar]
- 87.Watkins BE, Suzuki M, Wallin H, Wakabayashi K, Alexander J, Vanderlaan M, Sugimura T, Esumi H. The effect of dose and enzyme inducers on the metabolism of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in rats. Carcinogenesis. 1991;12:2291–2295. doi: 10.1093/carcin/12.12.2291. [DOI] [PubMed] [Google Scholar]
- 88.Lynch AM, Murray S, Boobis AR, Davies DS, Gooderham NJ. The measurement of MeIQx adducts with mouse haemoglobin in vitro and in vivo: implications for human dosimetry. Carcinogenesis. 1991;12:1067–1072. doi: 10.1093/carcin/12.6.1067. [DOI] [PubMed] [Google Scholar]
- 89.Turteltaub KW, Dingley KH, Curtis KD, Malfatti MA, Turesky RJ, Garner RC, Felton JS, Lang NP. Macromolecular adduct formation and metabolism of heterocyclic amines in humans and rodents at low doses. Cancer Lett. 1999;143:149–155. doi: 10.1016/s0304-3835(99)00116-0. [DOI] [PubMed] [Google Scholar]
- 90.Dingley KH, Curtis KD, Nowell S, Felton JS, Lang NP, Turteltaub KW. DNA and protein adduct formation in the colon and blood of humans after exposure to a dietary-relevant dose of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine. Cancer Epidemiol. Biomarkers Prev. 1999;8:507–512. [PubMed] [Google Scholar]
- 91.Mauthe RJ, Dingley KH, Leveson SH, Freeman SP, Turesky RJ, Garner RC, Turteltaub KW. Comparison of DNA-adduct and tissue-available dose levels of MeIQx in human and rodent colon following administration of a very low dose. Int. J. Cancer. 1999;80:539–545. doi: 10.1002/(sici)1097-0215(19990209)80:4<539::aid-ijc10>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 92.Garner RC, Lightfoot TJ, Cupid BC, Russell D, Coxhead JM, Kutschera W, Priller A, Rom W, Steier P, Alexander DJ, Leveson SH, Dingley KH, Mauthe RJ, Turteltaub KW. Comparative biotransformation studies of MeIQx and PhIP in animal models and humans. Cancer Lett. 1999;143:161–165. doi: 10.1016/s0304-3835(99)00118-4. [DOI] [PubMed] [Google Scholar]
- 93.Boobis AR, Gooderham N, Edwards RJ, Murray S, Lynch AM, Yadollahi-Farsani M, Davies DS. Enzymic and interindividual differences in the human metabolism of heterocyclic amines. Archives of Toxicol. (Supplement) 1996;18:286–302. doi: 10.1007/978-3-642-61105-6_28. [DOI] [PubMed] [Google Scholar]
- 94.Turesky RJ. Genotoxicity, metabolism, and biomarkers of heterocyclic aromatic amines. In: Skog K, Alexander J, editors. Acrylamide and Other Hazardous Compounds in Heat-Treated Foods. Woodhead Publishing Limited; Cambridge, England: 2006. pp. 247–274. [Google Scholar]
- 95.Teunissen SF, Rosing H, Schinkel AH, Schellens JH, Beijnen JH. Review on the analysis of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine and its phase I and phase II metabolites in biological matrices, foodstuff and beverages. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2010;878:3199–3216. doi: 10.1016/j.jchromb.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 96.Keating GA, Bogen KT. Estimates of heterocyclic amine intake in the US population. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2004;802:127–133. doi: 10.1016/j.jchromb.2003.10.047. [DOI] [PubMed] [Google Scholar]
- 97.Fenn JB, Mann M, Meng CK, Wong SF, Whitehouse CM. Electrospray ionization for mass spectrometry of large biomolecules. Science. 1989;246:64–71. doi: 10.1126/science.2675315. [DOI] [PubMed] [Google Scholar]
- 98.Kulp KS, Knize MG, Fowler ND, Salmon CP, Felton JS. PhIP metabolites in human urine after consumption of well-cooked chicken. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2004;802:143–153. doi: 10.1016/j.jchromb.2003.09.032. [DOI] [PubMed] [Google Scholar]
- 99.Koc H, Swenberg JA. Applications of mass spectrometry for quantitation of DNA adducts. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2002;778:323–343. doi: 10.1016/s1570-0232(02)00135-6. [DOI] [PubMed] [Google Scholar]
- 100.Turesky RJ, Vouros P. Formation and analysis of heterocyclic aromatic amine-DNA adducts in vitro and in vivo. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2004;802:155–166. doi: 10.1016/j.jchromb.2003.10.053. [DOI] [PubMed] [Google Scholar]
- 101.Walters DG, Young PJ, Agus C, Knize MG, Boobis AR, Gooderham NJ, Lake BG. Cruciferous vegetable consumption alters the metabolism of the dietary carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in humans. Carcinogenesis. 2004;25:1659–1669. doi: 10.1093/carcin/bgh164. [DOI] [PubMed] [Google Scholar]
- 102.Singh R, Farmer PB. Liquid chromatography-electrospray ionization-mass spectrometry: the future of DNA adduct detection. Carcinogenesis. 2006;27:178–196. doi: 10.1093/carcin/bgi260. [DOI] [PubMed] [Google Scholar]
- 103.Gu D, McNaughton L, LeMaster D, Lake BG, Gooderham NJ, Kadlubar FF, Turesky RJ. A comprehensive approach to the profiling of the cooked meat carcinogens 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline, 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine, and their metabolites in human urine. Chem. Res. Toxicol. 2010;23:788–801. doi: 10.1021/tx900436m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rehn L. Blasengeschwulste bei Fuchsinarbeitern. Arch. Klin. Chir. 1895;50:588–600. [Google Scholar]
- 105.Case RA, Hosker ME, McDonald DB, Pearson JT. Tumours of the urinary bladder in workmen engaged in the manufacture and use of certain dyestuff intermediates in the British chemical industry. I. The role of aniline, benzidine, alpha-naphthylamine, and beta-naphthylamine. Br. J. Ind. Med. 1954;11:75–104. doi: 10.1136/oem.11.2.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Melick WF, Naryka JJ, Kelly RE. Bladder cancer due to exposure to para aminobiphenyl: a 17-year followup. J. Urol. 1971;106:220–226. doi: 10.1016/s0022-5347(17)61263-1. [DOI] [PubMed] [Google Scholar]
- 107.Hueper WC, Wiley FH, Wolfe HD. Experimental production of bladder tumors in dogs by administration of beta-naphthylamine. J. Industrial Hygiene. 1938;20:46–84. [Google Scholar]
- 108.Radomski JL, Brill E. Bladder cancer induction by aromatic amines: role of N-hydroxy metabolites. Science. 1970;167:992–993. doi: 10.1126/science.167.3920.992. [DOI] [PubMed] [Google Scholar]
- 109.Poirier MC, Beland FA. DNA adduct measurements and tumor incidence during chronic carcinogen exposure in animal models: Implications for DNA adduct-based human cancer risk assessment. Chem. Res. Toxicol. 1992;5:749–755. doi: 10.1021/tx00030a003. [DOI] [PubMed] [Google Scholar]
- 110.Zavon MR, Hoegg U, Bingham E. Benzidine exposure as a cause of bladder tumors. Arch. Environ. Health. 1973;27:1–7. doi: 10.1080/00039896.1973.10666297. [DOI] [PubMed] [Google Scholar]
- 111.Ward EM, Sabbioni G, DeBord DG, Teass AW, Brown KK, Talaska GG, Roberts DR, Ruder AM, Streicher RP. Monitoring of aromatic amine exposures in workers at a chemical plant with a known bladder cancer excess. J. Natl. Cancer Inst. 1996;88:1046–1052. doi: 10.1093/jnci/88.15.1046. [DOI] [PubMed] [Google Scholar]
- 112.Stavric B, Klassen R, Miles W. Gas-liquid chromatographic-mass spectrometric determination of alpha- and beta-naphthylamines in FD&C Red No. 2 (amaranth). J. Assoc. Off. Anal. Chem. 1979;62:1020–1026. [PubMed] [Google Scholar]
- 113.Davis VM, Bailey JE., Jr. Chemical reduction of FD&C yellow No. 5 to determine combined benzidine. J. Chromatogr. 1993;635:160–164. doi: 10.1016/0021-9673(93)83128-f. [DOI] [PubMed] [Google Scholar]
- 114.Garrigos MC, Reche F, Marin ML, Jimenez A. Determination of aromatic amines formed from azo colorants in toy products. J. Chromatogr. A. 2002;976:309–317. doi: 10.1016/s0021-9673(02)01162-7. [DOI] [PubMed] [Google Scholar]
- 115.Lancaster FE, Lawrence JF. Determination of benzidine in the food colours tartrazine and sunset yellow FCF, by reduction and derivatization followed by high-performance liquid chromatography. Food Addit. Contam. 1999;16:381–390. doi: 10.1080/026520399283867. [DOI] [PubMed] [Google Scholar]
- 116.Oh SW, Kang MN, Cho CW, Lee MW. Detection of carcinogenic amines from dyestuffs or dyed substrates. Dyes and Pigments. 1997;33:119–135. [Google Scholar]
- 117.Cioni F, Bartolucci G, Pieraccini G, Meloni S, Moneti G. Development of a solid phase microextraction method for detection of the use of banned azo dyes in coloured textiles and leather. Rapid Commun. Mass Spectrom. 1999;13:1833–1837. doi: 10.1002/(SICI)1097-0231(19990930)13:18<1833::AID-RCM725>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 118.Tokiwa H, Nakagawa R, Horikawa K. Mutagenic/carcinogenic agents in indoor pollutants; the dinitropyrenes generated by kerosene heaters and fuel gas and liquefied petroleum gas burners. Mutat. Res. 1985;157:39–47. doi: 10.1016/0165-1218(85)90047-3. [DOI] [PubMed] [Google Scholar]
- 119.Stabbert R, Schafer KH, Biefel C, Rustemeier K. Analysis of aromatic amines in cigarette smoke. Rapid Commun. Mass Spectrom. 2003;17:2125–2132. doi: 10.1002/rcm.1161. [DOI] [PubMed] [Google Scholar]
- 120.Turesky RJ, Freeman JP, Holland RD, Nestorick DM, Miller DW, Ratnasinghe DL, Kadlubar FF. Identification of aminobiphenyl derivatives in commercial hair dyes. Chem. Res. Toxicol. 2003;16:1162–1173. doi: 10.1021/tx030029r. [DOI] [PubMed] [Google Scholar]
- 121.Akyuz M, Ata S. Determination of aromatic amines in hair dye and henna samples by ion-pair extraction and gas chromatography-mass spectrometry. J. Pharm. Biomed. Anal. 2008;47:68–80. doi: 10.1016/j.jpba.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 122.Neumann HG. Health risk of combustion products: toxicological considerations. Chemosphere. 2001;42:473–479. doi: 10.1016/s0045-6535(00)00219-8. [DOI] [PubMed] [Google Scholar]
- 123.Skipper PL, Kim MY, Sun HL, Wogan GN, Tannenbaum SR. Monocyclic aromatic amines as potential human carcinogens: old is new again. Carcinogenesis. 2010;31:50–58. doi: 10.1093/carcin/bgp267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Knize MG, Felton JS. Formation and human risk of carcinogenic heterocyclic amines formed from natural precursors in meat. Nutr. Rev. 2005;63:158–165. doi: 10.1111/j.1753-4887.2005.tb00133.x. [DOI] [PubMed] [Google Scholar]
- 125.Sinha R, Rothman N, Brown ED, Salmon CP, Knize MG, Swanson CS, Rossi SC, Mark SD, Levander OA, Felton JS. High concentrations of the carcinogen 2-amino-1- methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) occur in chicken but are dependent on the cooking method. Cancer Res. 1995;55:4516–4519. [PubMed] [Google Scholar]
- 126.Ni W, McNaughton L, LeMaster DM, Sinha R, Turesky RJ. Quantitation of 13 heterocyclic aromatic amines in cooked beef, pork, and chicken by liquid chromatography-electrospray ionization/tandem mass spectrometry. J. Agric. Food Chem. 2008;56:68–78. doi: 10.1021/jf072461a. [DOI] [PubMed] [Google Scholar]
- 127.Knize MG, Dolbeare FA, Carroll KL, Moore DH, Felton JS. Effect of cooking time and temperature on the heterocyclic amine content of fried beef patties. Food Chem. Toxicol. 1994;32:595–603. doi: 10.1016/0278-6915(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 128.Yoshida D, Matsumoto T, Yoshimura R, Matsuzaki T. Mutagenicity of amino-alpha-carbolines in pyrolysis products of soybean globulin. Biochem. Biophys. Res. Commun. 1978;83:915–920. doi: 10.1016/0006-291x(78)91482-1. [DOI] [PubMed] [Google Scholar]
- 129.Skog K, Solyakov A, Arvidsson P, Jagerstad M. Analysis of nonpolar heterocyclic amines in cooked foods and meat extracts using gas chromatography-mass spectrometry. J. Chromatogr. A. 1998;803:227–233. doi: 10.1016/s0021-9673(97)01266-1. [DOI] [PubMed] [Google Scholar]
- 130.Skog KI, Johansson MA, Jagerstad MI. Carcinogenic heterocyclic amines in model systems and cooked foods: a review on formation, occurrence and intake. Food Chem. Toxicol. 1998;36:879–896. doi: 10.1016/s0278-6915(98)00061-1. [DOI] [PubMed] [Google Scholar]
- 131.Milic BL, Djilas SM, Candadanoic-Brunet JM. Synthesis of some heterocyclic aminoimidazoarenes. Food Chemistry. 1993;46:273–276. [Google Scholar]
- 132.Murkovic M. Formation of heterocyclic aromatic amines in model systems. J. Chromatogr. B. 2004;802:3–10. doi: 10.1016/j.jchromb.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 133.Shioya M, Wakabayashi K, Sato S, Nagao M, Sugimura T. Formation of a mutagen, 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in cooked beef, by heating a mixture containing creatinine, phenylalanine and glucose. Mutat. Res. 1987;191:133–138. doi: 10.1016/0165-7992(87)90143-6. [DOI] [PubMed] [Google Scholar]
- 134.Sinha R, Rothman N, Salmon CP, Knize MG, Brown ED, Swanson CA, Rhodes D, Rossi S, Felton JS, Levander OA. Heterocyclic amine content in beef cooked by different methods to varying degrees of doneness and gravy made from meat drippings. Food Chem. Toxicol. 1998;36:279–287. doi: 10.1016/s0278-6915(97)00162-2. [DOI] [PubMed] [Google Scholar]
- 135.Okonogi H, Ushijima T, Shimizu H, Sugimura T, Nagao M. Induction of aberrant crypt foci in C57BL/6N mice by 2-amino-9H-pyrido[2,3-b]indole (AaC) and 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx). Cancer Lett. 1997;111:105–109. doi: 10.1016/s0304-3835(96)04505-3. [DOI] [PubMed] [Google Scholar]
- 136.Zhang XB, Felton JS, Tucker JD, Urlando C, Heddle JA. Intestinal mutagenicity of two carcinogenic food mutagens in transgenic mice: 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine and amino(alpha)carboline. Carcinogenesis. 1996;17:2259–2265. doi: 10.1093/carcin/17.10.2259. [DOI] [PubMed] [Google Scholar]
- 137.Fujita H, Nagano K, Ochiai M, Ushijima T, Sugimura T, Nagao M, Matsushima T. Difference in target organs in carcinogenesis with a heterocyclic amine, 2-amino-3,4-dimethylimidazo[4,5-f]quinoline, in different strains of mice. Jpn. J. Cancer Res. 1999;90:1203–1206. doi: 10.1111/j.1349-7006.1999.tb00696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nakagama H, Ochiai M, Ubagai T, Tajima R, Fujiwara K, Sugimura T, Nagao M. A rat colon cancer model induced by 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine, PhIP. Mutat. Res. 2002;506-507:137–144. doi: 10.1016/s0027-5107(02)00160-4. [DOI] [PubMed] [Google Scholar]
- 139.Ochiai M, Imai H, Sugimura T, Nagao M, Nakagama H. Induction of intestinal tumors and lymphomas in C57BL/6N mice by a food-borne carcinogen, 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine. Jpn. J. Cancer Res. 2002;93:478–483. doi: 10.1111/j.1349-7006.2002.tb01281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yoshida D, Matsumoto T. Amino-alpha-carbolines as mutagenic agents in cigarette smoke condensate. Cancer Lett. 1980;10:141–149. doi: 10.1016/0304-3835(80)90037-3. [DOI] [PubMed] [Google Scholar]
- 141.Smith CJ, Qian X, Zha Q, Moldoveanu SC. Analysis of alpha- and beta-carbolines in mainstream smoke of reference cigarettes by gas chromatography-mass spectrometry. J. Chromatogr. A. 2004;1046:211–216. doi: 10.1016/j.chroma.2004.06.082. [DOI] [PubMed] [Google Scholar]
- 142.Zhang L, Ashley DL, Watson CH. Quantitative analysis of six heterocyclic aromatic amines in mainstream cigarette smoke condensate using isotope dilution liquid chromatography-electrospray ionization tandem mass spectrometry. Nicotine. Tob. Res. 2011;13:120–126. doi: 10.1093/ntr/ntq219. [DOI] [PubMed] [Google Scholar]
- 143.Zha Q, Qian NX, Moldoveanu SC. Analysis of polycyclic aromatic hydrocarbons in the particulate phase of cigarette smoke using a gas chromatographic-high-resolution mass spectrometric technique. J. Chromatogr. Sci. 2002;40:403–408. doi: 10.1093/chromsci/40.7.403. [DOI] [PubMed] [Google Scholar]
- 144.Hoffmann D. Letters to the editor: Tobacco smoke components. Beiträge zur Tabakforschung Int. 1998;18:49–52. [Google Scholar]
- 145.Hecht SS. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat. Rev. Cancer. 2003;3:733–744. doi: 10.1038/nrc1190. [DOI] [PubMed] [Google Scholar]
- 146.Kanai Y, Wada O, Manabe S. Detection of carcinogenic glutamic acid pyrolysis products in cigarette smoke condensate. Carcinogenesis. 1990;11:1001–1003. doi: 10.1093/carcin/11.6.1001. [DOI] [PubMed] [Google Scholar]
- 147.Manabe S, Wada O, Kanai Y. Simultaneous determination of amino-alpha-carbolines and amino-gamma-carbolines in cigarette smoke condensate by high-performance liquid chromatography. J. Chromatogr. 1990;529:125–133. doi: 10.1016/s0378-4347(00)83813-x. [DOI] [PubMed] [Google Scholar]
- 148.Yamashita M, Wakabayashi K, Nagao M, Sato S, Yamaizumi Z, Takahashi M, Kinae N, Tomita I, Sugimura T. Detection of 2-amino-3-methylimidazo[4,5-f]quinoline in cigarette smoke condensate. Jpn. J. Cancer Res. 1986;77:419–422. [PubMed] [Google Scholar]
- 149.Sullivan MX. The origin of creatinine in soils. J. Am. Chem. Soc. 1911;33:2035–2042. [Google Scholar]
- 150.Manabe S, Kurihara N, Wada O, Izumikawa S, Asakuno K, Morita M. Detection of a carcinogen, 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine, in airborne particles and diesel-exhaust particles. Environ. Pollut. 1993;80:281–286. doi: 10.1016/0269-7491(93)90049-t. [DOI] [PubMed] [Google Scholar]
- 151.Totsuka Y, Ushiyama H, Ishihara J, Sinha R, Goto S, Sugimura T, Wakabayashi K. Quantification of the co-mutagenic beta-carbolines, norharman and harman, in cigarette smoke condensates and cooked foods. Cancer Lett. 1999;143:139–143. doi: 10.1016/s0304-3835(99)00143-3. [DOI] [PubMed] [Google Scholar]
- 152.Totsuka T, Nishigaki R, Sugimura T, Wakabayashi K. The possible involvement of mutagenic and carcinogenic heteroyclic amines in human cancer. In: Skog K, Alexander J, editors. Acrylamide and Other Hazardous Compounds in Heat-Treated Foods. Woodhead Publisher; Boca Raton, Fl: 2006. pp. 296–515. [Google Scholar]
- 153.Hada N, Totsuka Y, Enya T, Tsurumaki K, Nakazawa M, Kawahara N, Murakami Y, Yokoyama Y, Sugimura T, Wakabayashi K. Structures of mutagens produced by the co-mutagen norharman with o- and m-toluidine isomers. Mutat. Res. 2001;493:115–126. doi: 10.1016/s1383-5718(01)00168-1. [DOI] [PubMed] [Google Scholar]
- 154.Kawamori T, Totsuka Y, Uchiya N, Kitamura T, Shibata H, Sugimura T, Wakabayashi K. Carcinogenicity of aminophenylnorharman, a possible novel endogenous mutagen, formed from norharman and aniline, in F344 rats. Carcinogenesis. 2004;25:1967–1972. doi: 10.1093/carcin/bgh189. [DOI] [PubMed] [Google Scholar]
- 155.Shirai T, Sano M, Tamano S, Takahashi S, Hirose M, Futakuchi M, Hasegawa R, Imaida K, Matsumoto K, Wakabayashi K, Sugimura T, Ito N. The prostate: A target for carcinogenicity of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) derived from cooked foods. Cancer Res. 1997;57:195–198. [PubMed] [Google Scholar]
- 156.Ohgaki H, Hasegawa H, Kato T, Suenaga M, Ubukata M, Sato S, Takayama S, Sugimura T. Carcinogenicity in mice and rats of heterocyclic amines in cooked foods. Environ. Health Perspect. 1986;67:129–134. doi: 10.1289/ehp.8667129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ghoshal A, Preisegger KH, Takayama S, Thorgeirsson SS, Snyderwine EG. Induction of mammary tumors in female Sprague-Dawley rats by the food-derived carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine and effect of dietary fat. Carcinogenesis. 1994;15:2429–2433. doi: 10.1093/carcin/15.11.2429. [DOI] [PubMed] [Google Scholar]
- 158.Ubagai T, Ochiai M, Kawamori T, Imai H, Sugimura T, Nagao M, Nakagama H. Efficient induction of rat large intestinal tumors with a new spectrum of mutations by intermittent administration of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine in combination with a high fat diet. Carcinogenesis. 2002;23:197–200. doi: 10.1093/carcin/23.1.197. [DOI] [PubMed] [Google Scholar]
- 159.Sugimura T. Mutagens, carcinogens, and tumor promoters in our daily food. Cancer. 1982;49:1970–1984. doi: 10.1002/1097-0142(19820515)49:10<1970::aid-cncr2820491005>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 160.Nakagama H, Nakanishi M, Ochiai M. Modeling human colon cancer in rodents using a food-borne carcinogen, PhIP. Cancer Sci. 2005;96:627–636. doi: 10.1111/j.1349-7006.2005.00107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ochiai M, Nakagama H, Watanabe M, Ishiguro Y, Sugimura T, Nagao M. Efficient method for rapid induction of aberrant crypt foci in rats with 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine. Jpn. J. Cancer Res. 1996;87:1029–1033. doi: 10.1111/j.1349-7006.1996.tb03105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Snyderwine EG. Mammary gland carcinogenesis by food-derived heterocyclic amines: metabolism and additional factors influencing carcinogenesis by 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP). Environ. Mol. Mutagen. 2002;39:165–170. doi: 10.1002/em.10053. [DOI] [PubMed] [Google Scholar]
- 163.Adamson RH, Thorgeirsson UP, Snyderwine EG, Thorgeirsson SS, Reeves J, Dalgard DW, Takayama S, Sugimura T. Carcinogenicity of 2-amino-3-methylimidazo[4,5-f]quinoline in nonhuman primates: induction of tumors in three macaques. Jpn. J. Cancer Res. 1990;81:10–14. doi: 10.1111/j.1349-7006.1990.tb02500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bogen KT, Keating GA. U.S. dietary exposures to heterocyclic amines. J. Expo. Anal. Environ. Epidemiol. 2001;11:155–168. doi: 10.1038/sj.jea.7500158. [DOI] [PubMed] [Google Scholar]
- 165.Hasegawa R, Tanaka H, Tamano S, Shirai T, Nagao M, Sugimura T, Ito N. Synergistic enhancement of small and large intestinal carcinogenesis by combined treatment of rats with five heterocyclic amines in a medium-term mutli-organ bioassay. Carcinogenesis. 1994;15:2567–2573. doi: 10.1093/carcin/15.11.2567. [DOI] [PubMed] [Google Scholar]
- 166.Turteltaub KW, Felton JS, Gledhill BL, Vogel JS, Southon JR, Caffee MW, Finkel RC, Nelson DE, Proctor ID, Davis JC. Accelerator mass spectrometry in biomedical dosimetry: relationship between low-level exposure and covalent binding of heterocyclic amine carcinogens to DNA. Proc. Natl. Acad. Sci U. S. A. 1990;87:5288–5292. doi: 10.1073/pnas.87.14.5288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Turesky RJ, Box RM, Markovic J, Gremaud E, Snyderwine EG. Formation and persistence of DNA adducts of 2-amino-3-methylimidazo[4,5-f]quinoline in the rat and nonhuman primates. Mutat. Res. 1997;376:235–241. doi: 10.1016/s0027-5107(97)00048-1. [DOI] [PubMed] [Google Scholar]
- 168.Fukushima S, Wanibuchi H, Morimura K, Iwai S, Nakae D, Kishida H, Tsuda H, Uehara N, Imaida K, Shirai T, Tatematsu M, Tsukamoto T, Hirose M, Furukawa F. Existence of a threshold for induction of aberrant crypt foci in the rat colon with low doses of 2-amino-1-methyl-6-phenolimidazo[4,5-b]pyridine. Toxicol. Sci. 2004;80:109–114. doi: 10.1093/toxsci/kfh104. [DOI] [PubMed] [Google Scholar]
- 169.Friesen MD, Kaderlik K, Lin D, Garren L, Bartsch H, Lang NP, Kadlubar FF. Analysis of DNA adducts of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine in rat and human tissues by alkaline hydrolysis and gas chromatography/electron capture mass spectrometry: validation by comparison with 32P-postlabeling. Chem. Res. Toxicol. 1994;7:733–739. doi: 10.1021/tx00042a004. [DOI] [PubMed] [Google Scholar]
- 170.Tada A, Ochiai M, Wakabayashi K, Nukaya H, Sugimura T, Nagao M. Identification of N-(deoxyguanosin-8-yl)-2-amino-3,4-dimethylimidazo[4,5-f]quinoline (dG-C8-MeIQ) as a major adduct formed by MeIQ with nucleotides in vitro with DNA in vivo. Carcinogenesis. 1994;15:1275–1278. doi: 10.1093/carcin/15.6.1275. [DOI] [PubMed] [Google Scholar]
- 171.Turteltaub KW, Mauthe RJ, Dingley KH, Vogel JS, Frantz CE, Garner RC, Shen N. MeIQx-DNA adduct formation in rodent and human tissues at low doses. Mutat. Res. 1997;376:243–252. doi: 10.1016/s0027-5107(97)00049-3. [DOI] [PubMed] [Google Scholar]
- 172.Lightfoot TJ, Coxhead JM, Cupid BC, Nicholson S, Garner RC. Analysis of DNA adducts by accelerator mass spectrometry in human breast tissue after administration of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine and benzo[a]pyrene. Mutat. Res. 2000;472:119–127. doi: 10.1016/s1383-5718(00)00134-0. [DOI] [PubMed] [Google Scholar]
- 173.Zhu J, Chang P, Bondy ML, Sahin AA, Singletary SE, Takahashi S, Shirai T, Li D. Detection of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine-DNA adducts in normal breast tissues and risk of breast cancer. Cancer Epidemiol. Biomarkers Prev. 2003;12:830–837. [PubMed] [Google Scholar]
- 174.Zhu J, Rashid A, Cleary K, Abbruzzese JL, Friess H, Takahashi S, Shirai T, Li D. Detection of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP)-DNA adducts in human pancreatic tissues. Biomarkers. 2006;11:319–328. doi: 10.1080/13547500600667911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Magagnotti C, Pastorelli R, Pozzi S, Andreoni B, Fanelli R, Airoldi L. Genetic polymorphisms and modulation of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP)-DNA adducts in human lymphocytes. Int. J. Cancer. 2003;107:878–884. doi: 10.1002/ijc.11492. [DOI] [PubMed] [Google Scholar]
- 176.Malfatti MA, Dingley KH, Nowell-Kadlubar S, Ubick EA, Mulakken N, Nelson D, Lang NP, Felton JS, Turteltaub KW. The urinary metabolite profile of the dietary carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine is predictive of colon DNA adducts after a low-dose exposure in humans. Cancer Res. 2006;66:10541–10547. doi: 10.1158/0008-5472.CAN-06-1573. [DOI] [PubMed] [Google Scholar]
- 177.Tang D, Liu JJ, Rundle A, Neslund-Dudas C, Savera AT, Bock CH, Nock NL, Yang JJ, Rybicki BA. Grilled meat consumption and PhIP-DNA adducts in prostate carcinogenesis. Cancer Epidemiol. Biomarkers Prev. 2007;16:803–808. doi: 10.1158/1055-9965.EPI-06-0973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bessette EE, Spivack SD, Goodenough AK, Wang T, Pinto S, Kadlubar FF, Turesky RJ. Identification of carcinogen DNA adducts in human saliva by linear quadrupole ion trap/multistage tandem mass spectrometry. Chem. Res. Toxicol. 2010;23:1234–1244. doi: 10.1021/tx100098f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Layton DW, Bogen KT, Knize MG, Hatch FT, Johnson VM, Felton JS. Cancer risk of heterocyclic amines in cooked foods: an analysis and implications for research. Carcinogenesis. 1995;16:39–52. doi: 10.1093/carcin/16.1.39. [DOI] [PubMed] [Google Scholar]
- 180.Felton JS, Knize MG. A meat and potato war: implications for cancer etiology. Carcinogenesis. 2006;27:2367–2370. doi: 10.1093/carcin/bgl165. [DOI] [PubMed] [Google Scholar]
- 181.Gaylor DW, Kadlubar FF. Quantitative risk assessments of heterocyclic amines in cooked foods. In: Hayatsu H, editor. Mutagens in Foods: Detection and prevention. CRC Press; Boca Raton, Fl: 1991. pp. 229–236. [Google Scholar]
- 182.Lutz WK, Schlatter J. Chemical carcinogens and overnutrition in diet-related cancer. Carcinogenesis. 1992;13:2211–2216. doi: 10.1093/carcin/13.12.2211. [DOI] [PubMed] [Google Scholar]
- 183.Adamson RH, Thorgeirsson UP, Sugimura T. Extrapolation of heterocyclic amine carcinogenesis data from rodents and nonhuman primates to humans. Arch. Toxicol. Suppl. 1996;18:303–318. doi: 10.1007/978-3-642-61105-6_29. [DOI] [PubMed] [Google Scholar]
- 184.Dybing E, O'Brien J, Renwick AG, Sanner T. Risk assessment of dietary exposures to compounds that are genotoxic and carcinogenic--an overview. Toxicol. Lett. 2008;180:110–117. doi: 10.1016/j.toxlet.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 185.Doerge DR, Young JF, Chen JJ, Dinovi MJ, Henry SH. Using dietary exposure and physiologically based pharmacokinetic/pharmacodynamic modeling in human risk extrapolations for acrylamide toxicity. J. Agric. Food Chem. 2008;56:6031–6038. doi: 10.1021/jf073042g. [DOI] [PubMed] [Google Scholar]
- 186.Barlow S, Renwick AG, Kleiner J, Bridges JW, Busk L, Dybing E, Edler L, Eisenbrand G, Fink-Gremmels J, Knaap A, Kroes R, Liem D, Muller DJ, Page S, Rolland V, Schlatter J, Tritscher A, Tueting W, Wurtzen G. Risk assessment of substances that are both genotoxic and carcinogenic report of an International Conference organized by EFSA and WHO with support of ILSI Europe. Food Chem. Toxicol. 2006;44:1636–1650. doi: 10.1016/j.fct.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 187.JEFCA Internet communication. 2011 http://www.who.int/foodsafety/chem/jecfa/summaries/summary_report_64_final.pdf.
- 188.Skog K, Steineck G, Augustsson K, Jagerstad M. Effect of cooking temperature on the formation of heterocyclic amines in fried meat products and pan residues. Carcinogenesis. 1995;16:861–867. doi: 10.1093/carcin/16.4.861. [DOI] [PubMed] [Google Scholar]
- 189.Skog K, Solyakov A. Heterocyclic amines in poultry products: a literature review. Food Chem Toxicol. 2002;40:1213–1221. doi: 10.1016/s0278-6915(02)00062-5. [DOI] [PubMed] [Google Scholar]
- 190.Sinha R, Knize MG, Salmon CP, Brown ED, Rhodes D, Felton JS, Levander OA, Rothman N. Heterocyclic amine content of pork products cooked by different methods and to varying degrees of doneness. Food Chem. Toxicol. 1998;36:289–297. doi: 10.1016/s0278-6915(97)00159-2. [DOI] [PubMed] [Google Scholar]
- 191.Dooley KL, Von Tungelin LS, Bucci T, Kadlubar FF. Comparative carcinogenicity of 4-aminobiphenyl and the food pyrolysates, Glu-P-1, IQ, PhIP, and MeIQx in the neonatal B6C3F1 male mouse. Cancer Lett. 1992;62:205–209. doi: 10.1016/0304-3835(92)90097-f. [DOI] [PubMed] [Google Scholar]
- 192.Schwab CE, Huber WW, Parzefall W, Hietsch G, Kassie F, Schulte-Hermann R, Knasmuller S. Search for compounds that inhibit the genotoxic and carcinogenic effects of heterocyclic aromatic amines. Crit Rev. Toxicol. 2000;30:1–69. doi: 10.1080/10408440091159167. [DOI] [PubMed] [Google Scholar]
- 193.Guengerich FP, Shimada T. Oxidation of toxic and carcinogenic chemicals by human cytochrome P-450 enzymes. Chem. Res. Toxicol. 1991;4:391–407. doi: 10.1021/tx00022a001. [DOI] [PubMed] [Google Scholar]
- 194.Kato R, Yamazoe Y. Metabolic activation and covalent binding to nucleic acids of carcinogenic heterocyclic amines from cooked foods and amino acid pyrolysates. Jpn. J. Cancer Res. 1987;78:297–311. [PubMed] [Google Scholar]
- 195.Alexander J, Heidenreich B, Reistad R, Holme JA. Metabolism of the food carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in the rat and other rodents. In: Adamson RH, Gustafsson J-A, Ito N, Nagao M, Sugimura T, Wakabayashi K, Yamazoe Y, editors. Heterocyclic Amines in Cooked Foods: Possible Human Carcinogens. 23rd Proceedings of the Princess Takamatusu Cancer Society. Princeton Scientific Publishing Co., Inc.; New Jersey: 1995. pp. 59–68. [Google Scholar]
- 196.Snyderwine EG, Turesky RJ, Turteltaub KW, Davis CD, Sadrieh N, Schut HA, Nagao M, Sugimura T, Thorgeirsson UP, Adamson RH, Thorgeirsson SS. Metabolism of food-derived heterocyclic amines in nonhuman primates. Mutat. Res. 1997;376:203–210. doi: 10.1016/s0027-5107(97)00044-4. [DOI] [PubMed] [Google Scholar]
- 197.Yamazoe Y, Nagata K. In vitro metabolism. In: Sugimura T, Nagao M, editors. Food Borne Carcinogens Heterocyclic Amines. John Wiley & Sons Ltd.; Chichester, England: 2000. pp. 74–89. [Google Scholar]
- 198.Turesky RJ. Interspecies metabolism of heterocyclic aromatic amines and the uncertainties in extrapolation of animal toxicity data for human risk assessment. Mol. Nutr. Food Res. 2005;49:101–117. doi: 10.1002/mnfr.200400076. [DOI] [PubMed] [Google Scholar]
- 199.CRAMER JW, Miller JA, Miller EC. N-Hydroxylation: A new metabolic reaction observed in the rat with the carcinogen 2-acetylaminofluorene. J. Biol. Chem. 1960;235:885–888. [PubMed] [Google Scholar]
- 200.Irving CC. Metabolic activation of N-hydroxy compounds by conjugation. Xenobiotica. 1971;1:387–398. doi: 10.3109/00498257109041505. [DOI] [PubMed] [Google Scholar]
- 201.Yamazoe Y, Shimada M, Shinohara A, Saito K, Kamataki T, Kato R. Catalysis of the covalent binding of 3-hydroxyamino-1-methyl-5H-pyrido[4,3-b]indole to DNA by a L-proline- and adenosine triphosphate-dependent enzyme in rat hepatic cytosol. Cancer Res. 1985;45:2495–2500. [PubMed] [Google Scholar]
- 202.Ozawa S, Chou HC, Kadlubar FF, Nagata K, Yamazoe Y, Kato R. Activation of 2-hydroxyamino-1-methyl-6-phenylimidazo[4,5-b]pyridine by cDNA-expressed human and rat arylsulfotransferases. Jpn. J. Cancer Res. 1994;85:1220–1228. doi: 10.1111/j.1349-7006.1994.tb02933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Hein DW, Doll MA, Rustan TD, Gray K, Feng Y, Ferguson RJ, Grant DM. Metabolic activation and deactivation of arylamine carcinogens by recombinant human NAT1 and polymorphic NAT2 acetyltransferases. Carcinogenesis. 1993;14:1633–1638. doi: 10.1093/carcin/14.8.1633. [DOI] [PubMed] [Google Scholar]
- 204.Hein DW. N-Acetyltransferase genetics and their role in predisposition to aromatic and heterocyclic amine-induced carcinogenesis. Toxicol. Lett. 2000;112-113:349–356. doi: 10.1016/s0378-4274(99)00226-x. [DOI] [PubMed] [Google Scholar]
- 205.Nowell S, Ambrosone CB, Ozawa S, MacLeod SL, Mrackova G, Williams S, Plaxco J, Kadlubar FF, Lang NP. Relationship of phenol sulfotransferase activity (SULT1A1) genotype to sulfotransferase phenotype in platelet cytosol. Pharmacogenetics. 2000;10:789–797. doi: 10.1097/00008571-200012000-00004. [DOI] [PubMed] [Google Scholar]
- 206.Agus C, Ilett KF, Kadlubar FF, Minchin RF. Characterization of an ATP-dependent pathway of activation for the heterocyclic amine carcinogen N-hydroxy-2-amino-3-methylimidazo[4,5-f]quinoline. Carcinogenesis. 2000;21:1213–1219. [PubMed] [Google Scholar]
- 207.Lakshmi VM, Schut HA, Zenser TV. 2-Nitrosoamino-3-methylimidazo[4,5-f]quinoline activated by the inflammatory response forms nucleotide adducts. Food Chem. Toxicol. 2005;43:1607–1617. doi: 10.1016/j.fct.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 208.Lakshmi VM, Clapper ML, Chang WC, Zenser TV. Hemin potentiates nitric oxide-mediated nitrosation of 2-amino-3-methylimidazo[4,5-f]quinoline (IQ) to 2-nitrosoamino-3-methylimidazo[4,5-f]quinoline. Chem. Res. Toxicol. 2005;18:528–535. doi: 10.1021/tx049792r. [DOI] [PubMed] [Google Scholar]
- 209.Zenser TV, Lakshmi VM, Schut HA, Zhou HJ, Josephy PD. Activation of aminoimidazole carcinogens by nitrosation: mutagenicity and nucleotide adducts. Mutat. Res. 2009;673:109–115. doi: 10.1016/j.mrgentox.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Whitlock JP, Jr., Chichester CH, Bedgood RM, Okino ST, Ko HP, Ma Q, Dong L, Li H, Clarke-Katzenberg R. Induction of drug-metabolizing enzymes by dioxin. Drug Metab. Rev. 1997;29:1107–1127. doi: 10.3109/03602539709002245. [DOI] [PubMed] [Google Scholar]
- 211.Nebert DW, Dalton TP, Okey AB, Gonzalez FJ. Role of aryl hydrocarbon receptor-mediated induction of the CYP1 enzymes in environmental toxicity and cancer. J. Biol. Chem. 2004;279:23847–23850. doi: 10.1074/jbc.R400004200. [DOI] [PubMed] [Google Scholar]
- 212.Butler MA, Guengerich FP, Kadlubar FF. Metabolic oxidation of the carcinogens 4-aminobiphenyl and 4,4'-methylenebis(2-chloroaniline) by human hepatic microsomes and by purified rat hepatic cytochrome P-450 monooxygenases. Cancer Res. 1989;49:25–31. [PubMed] [Google Scholar]
- 213.Shimada T, Iwasaki M, Martin MV, Guengerich FP. Human liver microsomal cytochrome P-450 enzymes involved in the bioactivation of procarcinogens detected by umu gene response in Salmonella typhimurium TA 1535/pSK1002. Cancer Res. 1989;49:3218–3228. [PubMed] [Google Scholar]
- 214.Wallin H, Mikalsen A, Guengerich FP, Ingelman-Sundberg I, Solberg KE, Rossland OJ, Alexander J. Differential rates of metabolic activation and detoxification of the food mutagen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine by different cytochrome P450 enzymes. Carcinogenesis. 1990;11:489–492. doi: 10.1093/carcin/11.3.489. [DOI] [PubMed] [Google Scholar]
- 215.Rich KJ, Murray BP, Lewis I, Rendell NB, Davies DS, Gooderham NJ, Boobis AR. N-Hydroxy-MeIQx is the major microsomal oxidation product of the dietary carcinogen MeIQx with human liver. Carcinogenesis. 1992;13:2221–2226. doi: 10.1093/carcin/13.12.2221. [DOI] [PubMed] [Google Scholar]
- 216.Zhao K, Murray S, Davies DS, Boobis AR, Gooderham NJ. Metabolism of the food derived mutagen and carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) by human liver microsomes. Carcinogenesis. 1994;15:1285–1288. doi: 10.1093/carcin/15.6.1285. [DOI] [PubMed] [Google Scholar]
- 217.Shimada T, Guengerich FP. Activation of amino-α-carboline, 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine, and a copper phthalocyanine cellulose extract of cigarette smoke condensate by cytochrome P-450 enzymes in rat and human liver microsomes. Cancer Res. 1991;51:5284–5291. [PubMed] [Google Scholar]
- 218.Shimada T, Hayes CL, Yamazaki H, Amin S, Hecht SS, Guengerich FP, Sutter TR. Activation of chemically diverse procarcinogens by human cytochrome P-450 1B1. Cancer Res. 1996;56:2979–2984. [PubMed] [Google Scholar]
- 219.Frandsen H, Nielsen PA, Grivas S, Larsen JC. Microsomal metabolism of the food mutagen 2-amino-3,4,8-trimethyl-3H-imidazo[4,5-f]quinoxaline to mutagenic metabolites. Mutagenesis. 1994;9:59–65. doi: 10.1093/mutage/9.1.59. [DOI] [PubMed] [Google Scholar]
- 220.Hammons GJ, Milton D, Stepps K, Guengerich FP, Kadlubar FF. Metabolism of carcinogenic heterocyclic and aromatic amines by recombinant human cytochrome P450 enzymes. Carcinogenesis. 1997;18:851–854. doi: 10.1093/carcin/18.4.851. [DOI] [PubMed] [Google Scholar]
- 221.Crofts FG, Sutter TR, Strickland PT. Metabolism of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine by human cytochrome P4501A1, P4501A2 and P4501B1. Carcinogenesis. 1998;19:1969–1973. doi: 10.1093/carcin/19.11.1969. [DOI] [PubMed] [Google Scholar]
- 222.Turesky RJ, Constable A, Richoz J, Varga N, Markovic J, Martin MV, Guengerich FP. Activation of heterocyclic aromatic amines by rat and human liver microsomes and by purified rat and human cytochrome P450 1A2. Chem. Res. Toxicol. 1998;11:925–936. doi: 10.1021/tx980022n. [DOI] [PubMed] [Google Scholar]
- 223.Frederiksen H, Frandsen H. Impact of five cytochrome P450 enzymes on the metabolism of two heterocyclic aromatic amines, 2-amino-9H-pyrido[2,3-b]indole (AαC) and 2-amino-3-methyl-9H-pyrido[2,3-b]indole (MeAαC). Pharmacol. Toxicol. 2003;92:246–248. doi: 10.1034/j.1600-0773.2003.920508.x. [DOI] [PubMed] [Google Scholar]
- 224.Guengerich FP, Turvy CG. Comparison of levels of several human microsomal cytochrome P-450 enzymes and epoxide hydrolase in normal and disease states using immunochemical analysis of surgical liver samples. J. Pharmacol. Exp. Ther. 1991;256:1189–1194. [PubMed] [Google Scholar]
- 225.Sutter TR, Tang YM, Hayes CL, Wo Y-YP, Jabs EW, Li X, Yin H, Cody CW, Greenlee WF. Complete cDNA sequence of a human dioxin-inducible mRNA identifies a new gene subfamily of cytochrome P450 that maps to chromosome 2*. J. Biol. Chem. 1994;269:13092–13099. [PubMed] [Google Scholar]
- 226.Ding X, Kaminsky LS. Human extrahepatic cytochromes P450: function in xenobiotic metabolism and tissue-selective chemical toxicity in the respiratory and gastrointestinal tracts. Annu. Rev. Pharmacol. Toxicol. 2003;43:149–173. doi: 10.1146/annurev.pharmtox.43.100901.140251. [DOI] [PubMed] [Google Scholar]
- 227.Kim JH, Sherman ME, Curriero FC, Guengerich FP, Strickland PT, Sutter TR. Expression of cytochromes P450 1A1 and 1B1 in human lung from smokers, non-smokers, and ex-smokers. Toxicol. Appl. Pharmacol. 2004;199:210–219. doi: 10.1016/j.taap.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 228.Martin FL, Patel II, Sozeri O, Singh PB, Ragavan N, Nicholson CM, Frei E, Meinl W, Glatt H, Phillips DH, Arlt VM. Constitutive expression of bioactivating enzymes in normal human prostate suggests a capability to activate pro-carcinogens to DNA-damaging metabolites. Prostate. 2010;70:1586–1599. doi: 10.1002/pros.21194. [DOI] [PubMed] [Google Scholar]
- 229.Williams JA, Stone EM, Millar BC, Gusterson BA, Grover PL, Phillips DH. Determination of the enzymes responsible for activation of the heterocyclic amine 2-amino-3-methylimidazo[4,5-f]quinoline in the human breast. Pharmacogenetics. 1998;8:519–528. doi: 10.1097/00008571-199812000-00009. [DOI] [PubMed] [Google Scholar]
- 230.Bertz RJ, Granneman GR. Use of in vitro and in vivo data to estimate the likelihood of metabolic pharmacokinetic interactions. Clin. Pharmacokinet. 1997;32:210–258. doi: 10.2165/00003088-199732030-00004. [DOI] [PubMed] [Google Scholar]
- 231.Butler MA, Lang NP, Young JF, Caporaso NE, Vineis P, Hayes RB, Teitel CH, Massengill JP, Lawsen MF, Kadlubar FF. Determination of CYP1A2 and NAT2 phenotypes in human populations by analysis of caffeine urinary metabolites. Pharmacogenetics. 1992;2:116–127. doi: 10.1097/00008571-199206000-00003. [DOI] [PubMed] [Google Scholar]
- 232.Kalow W, Tang BK. Use of caffeine metabolite ratios to explore CYP1A2 and xanthine oxidase ratios. Clin. Pharmacol. Ther. 1991;50:508–519. doi: 10.1038/clpt.1991.176. [DOI] [PubMed] [Google Scholar]
- 233.Butler MA, Iwasaki M, Guengerich FP, Kadlubar FF. Human cytochrome P-450PA (P450IA2), the phenacetin O-deethylase, is primarily responsible for the hepatic 3-demethylation of caffeine and N-oxidation of carcinogenic arylamines. Proc. Natl. Acad. Sci. U. S. A. 1989;86:7696–7700. doi: 10.1073/pnas.86.20.7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Yun CH, Shimada T, Guengerich FP. Contributions of human liver cytochrome P450 enzymes to the N-oxidation of 4,4'-methylene-bis(2-chloroaniline). Carcinogenesis. 1992;13:217–222. doi: 10.1093/carcin/13.2.217. [DOI] [PubMed] [Google Scholar]
- 235.Yamazaki H, Inui Y, Wrighton SA, Guengerich FP, Shimada T. Procarcinogen activation by cytochrome P450 3A4 and 3A5 expressed in Escherichia coli and by human liver microsomes. Carcinogenesis. 1995;16:2167–2170. doi: 10.1093/carcin/16.9.2167. [DOI] [PubMed] [Google Scholar]
- 236.Gan J, Skipper PL, Tannenbaum SR. Oxidation of 2,6-dimethylaniline by recombinant human cytochrome P450s and human liver microsomes. Chem. Res. Toxicol. 2001;14:672–677. doi: 10.1021/tx000181i. [DOI] [PubMed] [Google Scholar]
- 237.Crofts FG, Strickland PT, Hayes CL, Sutter TR. Metabolism of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) by human cytochrome P4501B1. Carcinogenesis. 1997;18:1793–1798. doi: 10.1093/carcin/18.9.1793. [DOI] [PubMed] [Google Scholar]
- 238.Nakajima M, Itoh M, Sakai H, Fukami T, Katoh M, Yamazaki H, Kadlubar FF, Imaoka S, Funae Y, Yokoi T. CYP2A13 expressed in human bladder metabolically activates 4-aminobiphenyl. Int. J. Cancer. 2006;119:2520–2526. doi: 10.1002/ijc.22136. [DOI] [PubMed] [Google Scholar]
- 239.King RS, Kadlubar FF, Turesky RJ. In vivo metabolism of heterocyclic amines. In: Nagao M, Sugimura T, editors. Food Borne Carcinogens: Heterocyclic Amines. John Wiley & Sons, Ltd.; Chichester, England: 2000. pp. 90–111. [Google Scholar]
- 240.Schweikl H, Taylor JA, Kitareewan S, Linko P, Nagorney D, Goldstein JA. Expression of CYP1A1 and CYP1A2 genes in human liver. Pharmacogenetics. 1993;3:239–249. doi: 10.1097/00008571-199310000-00003. [DOI] [PubMed] [Google Scholar]
- 241.Eaton DL, Gallagher EP, Bammler TK, Kunze KL. Role of cytochrome P450 1A2 in chemical carcinogenesis: implications for human variability in expression and enzyme activity. Pharmacogenetics. 1995;5:259–274. doi: 10.1097/00008571-199510000-00001. [DOI] [PubMed] [Google Scholar]
- 242.Belloc C, Baird S, Cosme J, Lecoeur S, Gautier J-C, Challine D, de Waziers I, Flinois J-P, Beaune PH. Human cytochrome P450 expressed in Escherichia coli: production of specific antibodies. Toxicology. 1996;106:207–219. doi: 10.1016/0300-483x(95)03178-i. [DOI] [PubMed] [Google Scholar]
- 243.Hammons GJ, Yan-Sanders Y, Jin B, Blann E, Kadlubar FF, Lyn-Cook BD. Specific site methylation in the 5'-flanking region of CYP1A2 interindividual differences in human livers. Life Sci. 2001;69:839–845. doi: 10.1016/s0024-3205(01)01175-4. [DOI] [PubMed] [Google Scholar]
- 244.Nakajima M, Yokoi T, Mizutani M, Kinoshita M, Funayama M, Kamataki T. Genetic polymorphism in the 5'-flanking region of human CYP1A2 gene: effect on the CYP1A2 inducibility in humans. J. Biochem. (Tokyo) 1999;125:803–808. doi: 10.1093/oxfordjournals.jbchem.a022352. [DOI] [PubMed] [Google Scholar]
- 245.Sachse C, Bhambra U, Smith G, Lightfoot TJ, Barrett JH, Scollay J, Garner RC, Boobis AR, Wolf CR, Gooderham NJ. Polymorphisms in the cytochrome P450 CYP1A2 gene (CYP1A2) in colorectal cancer patients and controls: allele frequencies, linkage disequilibrium and influence on caffeine metabolism. Br. J. Clin. Pharmacol. 2003;55:68–76. doi: 10.1046/j.1365-2125.2003.01733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Conney AH. Induction of microsomal enzymes by foreign chemicals and carcinogenesis by polycyclic aromatic hydrocarbons: G. H. A. Clowes Memorial Lecture. Cancer Res. 1982;42:4875–4917. [PubMed] [Google Scholar]
- 247.Conney AH, Pantuck EJ, Hsiao KC, Kuntzman R, Alvares AP, Kappas A. Regulation of drug metabolism in man by environmental chemicals and diet. Fed. Proc. 1977;36:1647–1652. [PubMed] [Google Scholar]
- 248.Vistisen K, Poulsen HE, Loft S. Foreign compound metabolism capacity in man measured from metabolites of dietary caffeine. Carcinogenesis. 1992;13:1561–1568. doi: 10.1093/carcin/13.9.1561. [DOI] [PubMed] [Google Scholar]
- 249.Pantuck EJ, Pantuck CB, Garland WA, Min BH, Wattenberg LW, Anderson KE, Kappas A, Conney AH. Stimulatory effect of brussels sprouts and cabbage on human drug metabolism. Clin. Pharmacol. Ther. 1979;25:88–95. doi: 10.1002/cpt197925188. [DOI] [PubMed] [Google Scholar]
- 250.Bjeldanes LF, Kim JY, Grose KR, Bartholomew JC, Bradfield CA. Aromatic hydrocarbon responsiveness-receptor agonists generated from indole-3-carbinol in vitro and in vivo: comparisons with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Proc. Natl. Acad. Sci. U. S. A. 1991;88:9543–9547. doi: 10.1073/pnas.88.21.9543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Pantuck EJ, Hsiao KC, Conney AH, Garland WA, Kappas A, Anderson KE, Alvares AP. Effect of charcoal-broiled beef on phenacetin metabolism in man. Science. 1976;194:1055–1057. doi: 10.1126/science.982059. [DOI] [PubMed] [Google Scholar]
- 252.Sinha R, Rothman N, Brown ED, Mark SD, Hoover RN, Caporaso NE, Levander OA, Knize MG, Lang NP, Kadlubar FF. Pan-fried meat containing high levels of heterocyclic aromatic amines but low levels of polycyclic aromatic hydrocarbons induces cytochrome P4501A2 activity in humans. Cancer Res. 1994;54:6154–6159. [PubMed] [Google Scholar]
- 253.Diaz D, Fabre I, Daujat M, Saint AB, Bories P, Michel H, Maurel P. Omeprazole is an aryl hydrocarbon-like inducer of human hepatic cytochrome P450. Gastroenterology. 1990;99:737–747. doi: 10.1016/0016-5085(90)90963-2. [DOI] [PubMed] [Google Scholar]
- 254.Le Marchand L, Franke AA, Custer L, Wilkens LR, Cooney RV. Lifestyle and nutritional correlates of cytochrome CYP1A2 activity: inverse associations with plasma lutein and alpha-tocopherol. Pharmacogenetics. 1997;7:11–19. doi: 10.1097/00008571-199702000-00002. [DOI] [PubMed] [Google Scholar]
- 255.Jiang Z, Dragin N, Jorge-Nebert LF, Martin MV, Guengerich FP, Aklillu E, Ingelman-Sundberg M, Hammons GJ, Lyn-Cook BD, Kadlubar FF, Saldana SN, Sorter M, Vinks AA, Nassr N, von RO, Jin L, Nebert DW. Search for an association between the human CYP1A2 genotype and CYP1A2 metabolic phenotype. Pharmacogenet. Genomics. 2006;16:359–367. doi: 10.1097/01.fpc.0000204994.99429.46. [DOI] [PubMed] [Google Scholar]
- 256.Turesky RJ. The role of genetic polymorphisms in metabolism of carcinogenic heterocyclic aromatic amines. Curr. Drug Metab. 2004;5:169–180. doi: 10.2174/1389200043489036. [DOI] [PubMed] [Google Scholar]
- 257.Lin D-X, Lang NP, Kadlubar FF. Species differences in the biotransformation of the food-borne carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine by hepatic microsomes and cytosols from humans, rats, and mice. Drug Metab. Disp. 1995;23:518–524. [PubMed] [Google Scholar]
- 258.Kimura S, Kawabe M, Yu A, Morishima H, Fernandez-Salguero P, Hammons GJ, Ward JM, Kadlubar FF, Gonzalez FJ. Carcinogenesis of the food mutagen PhIP in mice is independent of CYP1A2. Carcinogenesis. 2003;24:583–587. doi: 10.1093/carcin/24.3.583. [DOI] [PubMed] [Google Scholar]
- 259.Kimura S, Kawabe M, Ward JM, Morishima H, Kadlubar FF, Hammons GJ, Fernandez-Salguero P, Gonzalez FJ. CYP1A2 is not the primary enzyme responsible for 4-aminobiphenyl-induced hepatocarcinogenesis in mice. Carcinogenesis. 1999;20:1825–1830. doi: 10.1093/carcin/20.9.1825. [DOI] [PubMed] [Google Scholar]
- 260.Guengerich FP. Comparisons of catalytic selectivity of cytochrome P450 subfamily members from different species. Chem. -Biol. Interact. 1997;106:161–182. doi: 10.1016/s0009-2797(97)00068-9. [DOI] [PubMed] [Google Scholar]
- 261.Turesky RJ, Parisod V, Huynh-Ba T, Langouët S, Guengerich FP. Regioselective differences in C(8)- and N-oxidation of 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline by human and rat liver microsomes and cytochromes P450 1A2. Chem. Res. Toxicol. 2001;14:901–911. doi: 10.1021/tx010035s. [DOI] [PubMed] [Google Scholar]
- 262.Langouët S, Welti DH, Kerriguy N, Fay LB, Huynh-Ba T, Markovic J, Guengerich FP, Guillouzo A, Turesky RJ. Metabolism of 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline in human hepatocytes: 2-amino-3-methylimidazo[4,5-f]quinoxaline-8-carboxylic acid is a major detoxification pathway catalyzed by cytochrome P450 1A2. Chem. Res. Toxicol. 2001;14:211–221. doi: 10.1021/tx000176e. [DOI] [PubMed] [Google Scholar]
- 263.Turesky RJ, Aeschbacher HU, Würzner HP, Skipper PL, Tannenbaum SR. Major routes of metabolism of the food-borne carcinogen 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline in the rat. Carcinogenesis. 1988;9:1043–1048. doi: 10.1093/carcin/9.6.1043. [DOI] [PubMed] [Google Scholar]
- 264.Turesky RJ, Garner RC, Welti DH, Richoz J, Leveson SH, Dingley KH, Turteltaub KW, Fay LB. Metabolism of the food-borne mutagen 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline in humans. Chem. Res. Toxicol. 1998;11:217–225. doi: 10.1021/tx9701891. [DOI] [PubMed] [Google Scholar]
- 265.Wallin H, Holme JA, Becher G, Alexander J. Metabolism of the food carcinogen 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline in isolated rat liver cells. Carcinogenesis. 1989;10:1277–1283. doi: 10.1093/carcin/10.7.1277. [DOI] [PubMed] [Google Scholar]
- 266.Niwa T, Yamazoe Y, Kato R. Metabolic activation of 2-amino-9H-pyrido[2,3-b]indole by rat-liver microsomes. Mutat. Res. 1982;95:159–170. doi: 10.1016/0027-5107(82)90254-8. [DOI] [PubMed] [Google Scholar]
- 267.Yamazoe Y, Kamataki T, Kato R. Species difference in N-hydroxylation of a tryptophan pyrolysis product in relation to mutagenic activation. Cancer Res. 1981;41:4518–4522. [PubMed] [Google Scholar]
- 268.Yamazoe Y, Shimada M, Kamataki T, Kato R. Microsomal activation of 2-amino-3-methylimidazo[4,5-f]quinoline, a pyrolysate of sardine and beef extracts, to a mutagenic intermediate. Cancer Res. 1983;43:5768–5774. [PubMed] [Google Scholar]
- 269.Yamazoe Y, Abu-Zeid M, Manabe S, Toyama S, Kato R. Metabolic activation of a protein pyrolysate promutagen 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline by rat liver microsomes and purified cytochrome P-450. Carcinogenesis. 1988;9:105–109. doi: 10.1093/carcin/9.1.105. [DOI] [PubMed] [Google Scholar]
- 270.Raza H, King RS, Squires RB, Guengerich FP, Miller DW, Freeman JP, Lang NP, Kadlubar FF. Metabolism of 2-amino-alpha-carboline. A food-borne heterocyclic amine mutagen and carcinogen by human and rodent liver microsomes and by human cytochrome P4501A2. Drug Metab. Dispos. 1996;24:395–400. [PubMed] [Google Scholar]
- 271.King RS, Teitel CH, Kadlubar FF. In vitro bioactivation of N-hydroxy-2-amino-alpha-carboline. Carcinogenesis. 2000;21:1347–1354. [PubMed] [Google Scholar]
- 272.Turesky RJ, Guengerich FP, Guillouzo A, Langouet S. Metabolism of heterocyclic aromatic amines by human hepatocytes and cytochrome P4501A2. Mutat. Res. 2002;506-507:187–195. doi: 10.1016/s0027-5107(02)00165-3. [DOI] [PubMed] [Google Scholar]
- 273.Frederiksen H, Frandsen H. In vitro metabolism of two heterocyclic amines, 2-amino-9H-pyrido[2,3-b]indole (A(alpha)C) and 2-amino-3-methyl-9H-pyridol2,3-b]indole (MeA(alpha)C) in human and rat hepatic microsomes. Pharmacol. Toxicol. 2002;90:127–134. doi: 10.1034/j.1600-0773.2002.900303.x. [DOI] [PubMed] [Google Scholar]
- 274.Luks HJ, Spratt TE, Vavrek MT, Roland SF, Weisburger JH. Identification of sulfate and glucuronic acid conjugates of the 5-hydroxy derivative as major metabolites of 2-amino-3-methylimidazo[4,5-f]quinoline in rats. Cancer Res. 1989;49:4407–4411. [PubMed] [Google Scholar]
- 275.Snyderwine EG, Welti DH, Fay LB, Würzner HP, Turesky RJ. Metabolism of the food-mutagen 2-amino-3-methylimidazo[4,5-f]quinoline in nonhuman primates undergoing carcinogen bioassay. Chem. Res. Toxicol. 1992;5:843–851. doi: 10.1021/tx00030a018. [DOI] [PubMed] [Google Scholar]
- 276.Snyderwine EG, Buonarati MH, Felton JS, Turteltaub KW. Metabolism of the food-derived mutagen/carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in nonhuman primates. Carcinogenesis. 1993;13:2517–2522. doi: 10.1093/carcin/14.12.2517. [DOI] [PubMed] [Google Scholar]
- 277.Turesky RJ, Gross GA, Stillwell WG, Skipper PL, Tannenbaum SR. Species differences in metabolism of heterocyclic aromatic amines, human exposure, and biomonitoring. Environ. Health Perspect. 1994;102(Suppl 6):47–51. doi: 10.1289/ehp.94102s647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Malfatti MA, Buonarati MH, Turteltaub KW, Shen NH, Felton JS. The role of sulfation and/or acetylation in the metabolism of the cooked-food mutagen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine in Salmonela typhimurium and isolated rat hepatocytes. Chem. Res. Toxicol. 1994;7:139–147. doi: 10.1021/tx00038a005. [DOI] [PubMed] [Google Scholar]
- 279.Frederiksen H, Frandsen H. Excretion of metabolites in urine and faeces from rats dosed with the heterocyclic amine, 2-amino-9H-pyrido[2,3-b]indole (AαC). Food Chem. Toxicol. 2004;42:879–885. doi: 10.1016/j.fct.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 280.Chen C, Ma X, Malfatti MA, Krausz KW, Kimura S, Felton JS, Idle JR, Gonzalez FJ. A comprehensive investigation of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) metabolism in the mouse using a multivariate data analysis approach. Chem Res. Toxicol. 2007;20:531–542. doi: 10.1021/tx600320w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Lakshmi VM, Hsu FF, Zenser TV. N-Demethylation is a major route of 2-amino-3-methylimidazo[4,5-f]quinoline metabolism in mouse. Drug Metab. Dispos. 2008;36:1143–1152. doi: 10.1124/dmd.107.019166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Turesky RJ, Bracco-Hammer I, Markovic J, Richli U, Kappeler A-M, Welti DH. The contribution of N-oxidation to the metabolism of the food-borne carcinogen 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline in rat hepatocytes. Chem. Res. Toxicol. 1990;3:524–535. doi: 10.1021/tx00018a006. [DOI] [PubMed] [Google Scholar]
- 283.Yuan ZX, Jha G, McGregor MA, King RS. Metabolites of the carcinogen 2-amino-alpha-carboline formed in male Sprague-Dawley rats in vivo and in rat hepatocyte and human HepG2 cell incubates. Chem. Res. Toxicol. 2007;20:497–503. doi: 10.1021/tx600303d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Langouët S, Paehler A, Welti DH, Kerriguy N, Guillouzo A, Turesky RJ. Differential metabolism of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine in rat and human hepatocytes. Carcinogenesis. 2002;23:115–122. doi: 10.1093/carcin/23.1.115. [DOI] [PubMed] [Google Scholar]
- 285.Stillwell WG, Turesky RJ, Gross GA, Skipper PL, Tannenbaum SR. Human urinary excretion of sulfamate and glucuronde conjugates of 2-amino-3,8-dimethylimdazo[4,5-f]quinoxaline (MeIQx). Cancer Epidemiol. Biomarkers Prev. 1994;3:399–405. [PubMed] [Google Scholar]
- 286.Stillwell WG, Kidd L-CKS-B, Wishnok JW, Tannenbaum SR, Sinha R. Urinary excretion of unmetabolized and phase II conjugates of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine and 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline in humans: Relationship to cytochrome P450 1A2 and N-acetyltransferase actvity. Cancer Res. 1997;57:3457–3464. [PubMed] [Google Scholar]
- 287.Malfatti MA, Kulp KS, Knize MG, Davis C, Massengill JP, Williams S, Nowell S, MacLeod S, Dingley KH, Turteltaub KW, Lang NP, Felton JS. The identification of [2-14C]2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine metabolites in humans. Carcinogenesis. 1999;20:705–713. doi: 10.1093/carcin/20.4.705. [DOI] [PubMed] [Google Scholar]
- 288.Stillwell WG, Turesky RJ, Sinha R, Tannenbaum SR. N-oxidative metabolism of 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx) in humans: excretion of the N2-glucuronide conjugate of 2-hydroxyamino-MeIQx in urine. Cancer Res. 1999;59:5154–5159. [PubMed] [Google Scholar]
- 289.Stillwell WG, Sinha R, Tannenbaum SR. Excretion of the N2-glucuronide conjugate of 2-hydroxyamino-1-methyl-6-phenylimidazo[4,5-b]pyridine in urine and its relationship to CYP1A2 and NAT2 activity levels in humans. Carcinogenesis. 2002;23:831–838. doi: 10.1093/carcin/23.5.831. [DOI] [PubMed] [Google Scholar]
- 290.Kulp KS, Knize MG, Malfatti MA, Salmon CP, Felton JS. Identification of urine metabolites of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine following consumption of a single cooked chicken meal in humans. Carcinogenesis. 2000;21:2065–2072. doi: 10.1093/carcin/21.11.2065. [DOI] [PubMed] [Google Scholar]
- 291.Strickland PT, Qian Z, Friesen MD, Rothman N, Sinha R. Metabolites of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in human urine after consumption of charbroiled or fried beef. Mutat. Res. 2002;506-507:163–173. doi: 10.1016/s0027-5107(02)00163-x. [DOI] [PubMed] [Google Scholar]
- 292.Frandsen H. Biomonitoring of urinary metabolites of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) following human consumption of cooked chicken. Food Chem. Toxicol. 2008;46:3200–3205. doi: 10.1016/j.fct.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 293.Fede JM, Thakur AP, Gooderham NJ, Turesky RJ. Biomonitoring of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) and its carcinogenic metabolites in urine. Chem. Res. Toxicol. 2009;22:1096–1105. doi: 10.1021/tx900052c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Edwards RJ, Murray BP, Murray S, Schulz T, Neubert D, Gant TW, Thorgeirsson SS, Boobis AR, Davies DS. Contribution of CYP1A1 and CYP1A2 to the activation of heterocyclic amines in monkeys and humans. Carcinogenesis. 1994;15:829–836. doi: 10.1093/carcin/15.5.829. [DOI] [PubMed] [Google Scholar]
- 295.Barnes WS, Lovelette CA, Tong C, Williams GM, Weisburger JH. Genotoxicity of the food mutagen 2-amino-3-methylimidazo[4,5-f]quinoline (IQ) and analogs. Carcinogenesis. 1985;6:441–444. doi: 10.1093/carcin/6.3.441. [DOI] [PubMed] [Google Scholar]
- 296.Van Tassell RL, Kingston DG, Wilkins TD. Metabolism of dietary genotoxins by the human colonic microflora; the fecapentaenes and heterocyclic amines. Mutat. Res. 1990;238:209–221. doi: 10.1016/0165-1110(90)90013-2. [DOI] [PubMed] [Google Scholar]
- 297.Weisburger JH, Rivenson A, Kingston DG, Wilkins TD, Van Tassell RL, Nagao M, Sugimura T, Hara Y. Dietary modulation of the carcinogenicity of the heterocyclic amines. Princess Takamatsu Symp. 1995;23:240–250. [PubMed] [Google Scholar]
- 298.Hammons GJ, Kadlubar FF. The role of cytochrome P-450 in the metabolism of carcinogens. In: Guengerich FP, editor. Mammalian Cytochromes P-450. CRC Press; Boca Raton, Fl: 1987. pp. 81–130. [Google Scholar]
- 299.Hammons GJ, Dooley KL, Kadlubar FF. 4-Aminobiphenyl-hemoglobin adduct formation as an index of in vivo N-oxidation by hepatic cytochrome P-450IA2. Chem. Res. Toxicol. 1991;4:144–147. doi: 10.1021/tx00020a003. [DOI] [PubMed] [Google Scholar]
- 300.Kunze KL, Trager WF. Isoform-selective mechanism-based inhibition of human cytochrome P450 1A2 by furafylline. Chem. Res. Toxicol. 1993;6:649–656. doi: 10.1021/tx00035a009. [DOI] [PubMed] [Google Scholar]
- 301.Boobis AR, Lynch AM, Murray S, de la Torre R, Solans A, Farré M, Segura J, Gooderham NJ, Davies DS. CYP1A2-catalyzed conversion of dietary heterocyclic amines to their proximate carcinogens is their major route of metabolism in humans. Cancer Res. 1994;54:89–94. [PubMed] [Google Scholar]
- 302.Snyderwine EG, Yu M, Schut HA, Knight-Jones L, Kimura S. Effect of CYP1A2 deficiency on heterocyclic amine DNA adduct levels in mice. Food Chem. Toxicol. 2002;40:1529–1533. doi: 10.1016/s0278-6915(02)00110-2. [DOI] [PubMed] [Google Scholar]
- 303.Shertzer HG, Dalton TP, Talaska G, Nebert DW. Decrease in 4-aminobiphenyl-induced methemoglobinemia in Cyp1a2(-/-) knockout mice. Toxicol. Appl. Pharmacol. 2002;181:32–37. doi: 10.1006/taap.2002.9398. [DOI] [PubMed] [Google Scholar]
- 304.Tsuneoka Y, Dalton TP, Miller ML, Clay CD, Shertzer HG, Talaska G, Medvedovic M, Nebert DW. 4-aminobiphenyl-induced liver and urinary bladder DNA adduct formation in Cyp1a2(-/-) and Cyp1a2(+/+) mice. J. Natl. Cancer Inst. 2003;95:1227–1237. doi: 10.1093/jnci/djg025. [DOI] [PubMed] [Google Scholar]
- 305.Ma Q, Lu AY. CYP1A induction and human risk assessment: an evolving tale of in vitro and in vivo studies. Drug Metab Dispos. 2007;35:1009–1016. doi: 10.1124/dmd.107.015826. [DOI] [PubMed] [Google Scholar]
- 306.Cheung C, Ma X, Krausz KW, Kimura S, Feigenbaum L, Dalton TP, Nebert DW, Idle JR, Gonzalez FJ. Differential metabolism of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in mice humanized for CYP1A1 and CYP1A2. Chem. Res. Toxicol. 2005;18:1471–1478. doi: 10.1021/tx050136g. [DOI] [PubMed] [Google Scholar]
- 307.Zenser TV, Mattammal MB, Armbrecht HJ, Davis BB. Benzidine binding to nucleic acids mediated by the peroxidative activity of prostaglandin endoperoxide synthetase. Cancer Res. 1980;40:2839–2845. [PubMed] [Google Scholar]
- 308.Kadlubar FF, Frederick CB, Weis CC, Zenser TV. Prostaglandin endoperoxide synthetase-mediated metabolism of carcinogenic aromatic amines and their binding to DNA and protein. Biochem. Biophys. Res. Commun. 1982;108:253–258. doi: 10.1016/0006-291x(82)91859-9. [DOI] [PubMed] [Google Scholar]
- 309.Wise RW, Zenser TV, Kadlubar FF, Davis BB. Metabolic activation of carcinogenic aromatic amines by dog bladder and kidney prostaglandin H synthase. Cancer Res. 1984;44:1893–1897. [PubMed] [Google Scholar]
- 310.Boyd JA, Eling TE. Metabolism of aromatic amines by prostaglandin H synthase. Environ. Health Perspect. 1985;64:45–51. doi: 10.1289/ehp.856445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Boyd JA, Eling TE. Prostaglandin H synthase-catalyzed metabolism and DNA binding of 2-naphthylamine. Cancer Res. 1987;47:4007–4014. [PubMed] [Google Scholar]
- 312.Flammang TJ, Yamazoe Y, Benson RW, Roberts DW, Potter DW, Chu DZ, Lang NP, Kadlubar FF. Arachidonic acid-dependent peroxidative activation of carcinogenic arylamines by extrahepatic human tissue microsomes. Cancer Res. 1989;49:1977–1982. [PubMed] [Google Scholar]
- 313.Wolz E, Wild D, Degen GH. Prostaglandin-H synthase mediated metabolism and mutagenic activation of 2-amino-3-methylimidazo[4,5-f]quinoline (IQ). Arch. Toxicol. 1995;69:171–179. doi: 10.1007/s002040050154. [DOI] [PubMed] [Google Scholar]
- 314.Josephy PD. The role of peroxidase-catalyzed activation of aromatic amines in breast cancer. Mutagenesis. 1996;11:3–7. doi: 10.1093/mutage/11.1.3. [DOI] [PubMed] [Google Scholar]
- 315.Degen GH, Wolz E, Gerber M, Pfau W. Bioactivation of 2-amino-3-methylimidazo[4,5-f]quinoline (IQ) by prostaglandin H synthase. Arch. Toxicol. 1998;72:183–186. doi: 10.1007/s002040050485. [DOI] [PubMed] [Google Scholar]
- 316.Wolz E, Pfau W, Degen GH. Bioactivation of the food mutagen 2-amino-3-methyl-imidazo[4,5-f]quinoline (IQ) by prostaglandin-H synthase and by monooxygenases: DNA adduct analysis. Food Chem. Toxicol. 2000;38:513–522. doi: 10.1016/s0278-6915(00)00038-7. [DOI] [PubMed] [Google Scholar]
- 317.Wiese FW, Thompson PA, Kadlubar FF. Carcinogen substrate specificity of human COX-1 and COX-2. Carcinogenesis. 2001;22:5–10. doi: 10.1093/carcin/22.1.5. [DOI] [PubMed] [Google Scholar]
- 318.Zenser TV, Lakshmi VM, Hsu FF, Davis BB. Metabolism of N-acetylbenzidine and initiation of bladder cancer. Mutat. Res. 2002;506-507:29–40. doi: 10.1016/s0027-5107(02)00149-5. [DOI] [PubMed] [Google Scholar]
- 319.Yamazoe Y, Miller DW, Weis CC, Dooley KL, Zenser TV, Beland FA, Kadlubar FF. DNA adducts formed by ring-oxidation of the carcinogen 2-naphthylamine with prostaglandin H synthase in vitro and in the dog urothelium in vivo. Carcinogenesis. 1985;6:1379–1387. doi: 10.1093/carcin/6.9.1379. [DOI] [PubMed] [Google Scholar]
- 320.Lakshmi VM, Zenser TV, Davis BB. N'-(3'-monophospho-deoxyguanosin-8-yl)-N-acetylbenzidine formation by peroxidative metabolism. Carcinogenesis. 1998;19:911–917. doi: 10.1093/carcin/19.5.911. [DOI] [PubMed] [Google Scholar]
- 321.Williams JA, Stone EM, Millar BC, Hewer A, Phillips DH. Pathways of heterocyclic amine activation in the breast: DNA adducts of 2-amino-3-methylimidazo[4,5-f]quinoline (IQ) formed by peroxidases and in human mammary epithelial cells and fibroblasts. Mutagenesis. 2000;15:149–154. doi: 10.1093/mutage/15.2.149. [DOI] [PubMed] [Google Scholar]
- 322.Gorlewska-Roberts KM, Teitel CH, Lay JO, Jr., Roberts DW, Kadlubar FF. Lactoperoxidase-catalyzed activation of carcinogenic aromatic and heterocyclic amines. Chem. Res. Toxicol. 2004;17:1659–1666. doi: 10.1021/tx049787n. [DOI] [PubMed] [Google Scholar]
- 323.Hein DW, Doll MA, Fretland AJ, Leff MA, Webb SJ, Xiao GH, Devanaboyina US, Nangju NA, Feng Y. Molecular genetics and epidemiology of the NAT1 and NAT2 acetylation polymorphisms. Cancer Epidemiol. Biomarkers Prev. 2000;9:29–42. [PubMed] [Google Scholar]
- 324.Hein DW. Molecular genetics and function of NAT1 and NAT2: role in aromatic amine metabolism and carcinogenesis. Mutat. Res. 2002;506-507:65–77. doi: 10.1016/s0027-5107(02)00153-7. [DOI] [PubMed] [Google Scholar]
- 325.Blum M, Demierre A, Grant DM, Heim M, Meyer UA. Molecular mechanism of slow acetylation of drugs and carcinogens in humans. Proc. Natl. Acad. Sci. U. S. A. 1991;88:5237–5241. doi: 10.1073/pnas.88.12.5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Hein DW. N-acetyltransferase 2 genetic polymorphism: effects of carcinogen and haplotype on urinary bladder cancer risk. Oncogene. 2006;25:1649–1658. doi: 10.1038/sj.onc.1209374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Lang NP, Butler MA, Massengill JP, Lawson M, Stotts RC, Hauer-Jensen M, Kadlubar FF. Rapid metabolic phenotypes for acetyltransferase and cytochrome P4501A2 and putative exposure to food-borne heterocyclic amines increase the risk for colorectal cancer or polyps. Cancer Epidemiol. Biomarkers Prev. 1994;3:675–682. [PubMed] [Google Scholar]
- 328.Le Marchand L, Hankin JH, Pierce LM, Sinha R, Nerurkar PV, Franke AA, Wilkens LR, Kolonel LN, Donlon T, Seifried A, Custer LJ, Lum-Jones A, Chang W. Well-done red meat, metabolic phenotypes and colorectal cancer in Hawaii. Mutat. Res. 2002;506-507:205–214. doi: 10.1016/s0027-5107(02)00167-7. [DOI] [PubMed] [Google Scholar]
- 329.Cohen SM, Boobis AR, Meek ME, Preston RJ, McGregor DB. 4-Aminobiphenyl and DNA reactivity: case study within the context of the 2006 IPCS Human Relevance Framework for Analysis of a cancer mode of action for humans. Crit Rev. Toxicol. 2006;36:803–819. doi: 10.1080/10408440600977651. [DOI] [PubMed] [Google Scholar]
- 330.Lakshmi VM, Zenser TV, Davis BB. Rat liver cytochrome P450 metabolism of N-acetylbenzidine and N,N'-diacetylbenzidine. Drug Metab. Dispos. 1997;25:481–488. [PubMed] [Google Scholar]
- 331.Zenser TV, Lakshmi VM, Rustan TD, Doll MA, Deitz AC, Davis BB, Hein DW. Human N-acetylation of benzidine: role of NAT1 and NAT2. Cancer Res. 1996;56:3941–3947. [PubMed] [Google Scholar]
- 332.Rothman N, Bhatnagar VK, Hayes RB, Zenser TV, Kashyap SK, Butler MA, Bell DA, Lakshmi V, Jaeger M, Kashyap R, Hirvonen A, Schulte PA, Dosemeci M, Hsu F, Parikh DJ, Davis BB, Talaska G. The impact of interindividual variation in NAT2 activity on benzidine urinary metabolites and urothelial DNA adducts in exposed workers. Proc. Natl. Acad. Sci. U. S. A. 1996;93:5084–5089. doi: 10.1073/pnas.93.10.5084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Flammang TJ, Yamazoe Y, Guengerich FP, Kadlubar FF. The S-acetyl coenzyme A-dependent metabolic activation of the carcinogen N-hydroxy-2-aminofluorene by human liver cytosol and its relationship to the aromatic amine N-acetyltransferase phenotype. Carcinogenesis. 1987;8:1967–1970. doi: 10.1093/carcin/8.12.1967. [DOI] [PubMed] [Google Scholar]
- 334.Hein DW, Rustan TD, Ferguson RJ, Doll MA, Gray K. Metabolic activation of aromatic and heterocylic N-hydroxyarylamines by wild-type and mutant recombinant human NAT1 and NAT2 acetyltransferases. Arch. Toxicol. 1994;68:129–133. doi: 10.1007/s002040050045. [DOI] [PubMed] [Google Scholar]
- 335.Minchin RF, Reeves PT, Teitel CH, McManus ME, Mojarrabi B, Ilett KF, Kadlubar FF. N- and O-acetylation of aromatic and hetercyclic amine carcinogens by human monomorphic and polymorphic acetyltransferases expressed in COS-1 cells. Biochem. Biophys. Res. Commun. 1992;185:839–844. doi: 10.1016/0006-291x(92)91703-s. [DOI] [PubMed] [Google Scholar]
- 336.Sugamori KS, Brenneman D, Grant DM. In vivo and in vitro metabolism of arylamine procarcinogens in acetyltransferase-deficient mice. Drug Metab. Dispos. 2006;34:1697–1702. doi: 10.1124/dmd.106.010819. [DOI] [PubMed] [Google Scholar]
- 337.Metry KJ, Neale JR, Bendaly J, Smith NB, Pierce WM, Jr., Hein DW. Effect of N-acetyltransferase 2 polymorphism on tumor target tissue DNA adduct levels in rapid and slow acetylator congenic rats administered 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine or 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline. Drug Metab Dispos. 2009;37:2123–2126. doi: 10.1124/dmd.109.029512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Steffensen IL, Fretland AJ, Paulsen JE, Feng Y, Eide TJ, Devanaboyina US, Hein DW, Alexander J. DNA adduct levels and intestinal lesions in congenic rapid and slow acetylator Syrian hamsters administered food mutagens 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) or 2-amino-3-methylimidazo[4,5-f]quinoline (IQ). Pharmacol. Toxicol. 2000;86:257–263. doi: 10.1111/j.0901-9928.2000.860603.x. [DOI] [PubMed] [Google Scholar]
- 339.Turesky RJ, Lang NP, Butler MA, Teitel CH, Kadlubar FF. Metabolic activation of carcinogenic heterocyclic aromatic amines by human liver and colon. Carcinogenesis. 1991;12:1839–1845. doi: 10.1093/carcin/12.10.1839. [DOI] [PubMed] [Google Scholar]
- 340.Lin D, Kaderlik KR, Turesky RJ, Miller DW, Lay JO, Jr., Kadlubar FF. Identification of N-(deoxyguanosin-8-yl)-2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine as the major adduct formed by the food-borne carcinogen, 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine, with DNA. Chem. Res. Toxicol. 1992;5:691–697. doi: 10.1021/tx00029a016. [DOI] [PubMed] [Google Scholar]
- 341.Frandsen H, Grivas S, Andersson R, Dragsted L, Larsen JC. Reaction of the N2-acetoxy derivative of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) with 2'-deoxyguanosine and DNA. Synthesis and identification of N2-(2'-deoxyguanosin-8-yl)-PhIP. Carcinogenesis. 1992;13:629–635. doi: 10.1093/carcin/13.4.629. [DOI] [PubMed] [Google Scholar]
- 342.Wu RW, Tucker JD, Sorensen KJ, Thompson LH, Felton JS. Differential effect of acetyltransferase expression on the genotoxicity of heterocyclic amines in CHO cells. Mutat. Res. 1997;390:93–103. doi: 10.1016/s0165-1218(97)00005-0. [DOI] [PubMed] [Google Scholar]
- 343.Metry KJ, Zhao S, Neale JR, Doll MA, States JC, McGregor WG, Pierce WM, Jr., Hein DW. 2-Amino-1-methyl-6-phenylimidazo[4,5-b]pyridine-induced DNA adducts and genotoxicity in Chinese hamster ovary (CHO) cells expressing human CYP1A2 and rapid or slow acetylator N-acetyltransferase 2. Mol. Carcinog. 2007;46:553–563. doi: 10.1002/mc.20302. [DOI] [PubMed] [Google Scholar]
- 344.Wild D, Feser W, Michel S, Lord HL, Josephy PD. Metabolic activation of heterocyclic aromatic amines catalyzed by human arylamine N-acetyltransferase isozymes (NAT1 and NAT2) expressed in Salmonella typhimurium. Carcinogenesis. 1995;16:643–648. doi: 10.1093/carcin/16.3.643. [DOI] [PubMed] [Google Scholar]
- 345.Muckel E, Frandsen H, Glatt HR. Heterologous expression of human N-acetyltransferases 1 and 2 and sulfotransferase 1A1 in Salmonella typhimurium for mutagenicity testing of heterocyclic amines. Food Chem. Toxicol. 2002;40:1063–1068. doi: 10.1016/s0278-6915(02)00032-7. [DOI] [PubMed] [Google Scholar]
- 346.Wu RW, Panteleakos FN, Kadkhodayan S, Bolton-Grob R, McManus ME, Felton JS. Genetically modified Chinese hamster ovary cells for investigating sulfotransferase-mediated cytotoxicity and mutation by 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine. Environ. Mol. Mutagen. 2000;35:57–65. [PubMed] [Google Scholar]
- 347.Bendaly J, Zhao S, Neale JR, Metry KJ, Doll MA, States JC, Pierce WM, Jr., Hein DW. 2-Amino-3,8-dimethylimidazo[4,5-f]quinoxaline-induced DNA adduct formation and mutagenesis in DNA repair-deficient Chinese hamster ovary cells expressing human cytochrome P4501A1 and rapid or slow acetylator N-acetyltransferase 2. Cancer Epidemiol. Biomarkers Prev. 2007;16:1503–1509. doi: 10.1158/1055-9965.EPI-07-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Turesky RJ, Bendaly J, Yasa I, Doll MA, Hein DW. The impact of NAT2 acetylator genotype on mutagenesis and DNA adducts from 2-amino-9H-pyrido[2,3-b]indole. Chem. Res. Toxicol. 2009;22:726–733. doi: 10.1021/tx800473w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Garcia-Closas M, Malats N, Silverman D, Dosemeci M, Kogevinas M, Hein DW, Tardon A, Serra C, Carrato A, Garcia-Closas R, Lloreta J, Castano-Vinyals G, Yeager M, Welch R, Chanock S, Chatterjee N, Wacholder S, Samanic C, Tora M, Fernandez F, Real FX, Rothman N. NAT2 slow acetylation, GSTM1 null genotype, and risk of bladder cancer: results from the Spanish Bladder Cancer Study and meta-analyses. Lancet. 2005;366:649–659. doi: 10.1016/S0140-6736(05)67137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Shinohara A, Yamazoe Y, Saito K, Kamataki T, Kato R. Species differences in the N-acetylation by liver cytosol of mutagenic heterocyclic aromatic amines in protein pyrolysates. Carcinogenesis. 1984;5:683–686. doi: 10.1093/carcin/5.5.683. [DOI] [PubMed] [Google Scholar]
- 351.Shinohara A, Saito K, Yamazoe Y, Kamataki T, Kato R. Acetyl coenzyme A dependent activation of N-hydroxy derivatives of carcinogenic arylamines: Mechanism of activation, species diffrences, tissue distribution, and acetyl donor specificty. Cancer Res. 1986;46:4362–4367. [PubMed] [Google Scholar]
- 352.Blanchard RL, Freimuth RR, Buck J, Weinshilboum RM, Coughtrie MW. A proposed nomenclature system for the cytosolic sulfotransferase (SULT) superfamily. Pharmacogenetics. 2004;14:199–211. doi: 10.1097/00008571-200403000-00009. [DOI] [PubMed] [Google Scholar]
- 353.Nowell S, Falany CN. Pharmacogenetics of human cytosolic sulfotransferases. Oncogene. 2006;25:1673–1678. doi: 10.1038/sj.onc.1209376. [DOI] [PubMed] [Google Scholar]
- 354.Hempel N, Gamage N, Martin JL, McManus ME. Human cytosolic sulfotransferase SULT1A1. Int. J. Biochem. Cell Biol. 2007;39:685–689. doi: 10.1016/j.biocel.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 355.Teubner W, Meinl W, Florian S, Kretzschmar M, Glatt H. Identification and localization of soluble sulfotransferases in the human gastrointestinal tract. Biochem. J. 2007;404:207–215. doi: 10.1042/BJ20061431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Gamage N, Barnett A, Hempel N, Duggleby RG, Windmill KF, Martin JL, McManus ME. Human sulfotransferases and their role in chemical metabolism. Toxicol. Sci. 2006;90:5–22. doi: 10.1093/toxsci/kfj061. [DOI] [PubMed] [Google Scholar]
- 357.Turesky RJ, Skipper PL, Tannenbaum SR, Coles B, Ketterer B. Sulfamate formation is a major route for detoxification of 2-amino-3-methylimidazo[4,5-f]quinoline in the rat. Carcinogenesis. 1986;7:1483–1485. doi: 10.1093/carcin/7.9.1483. [DOI] [PubMed] [Google Scholar]
- 358.Turesky RJ, Aeschbacher HU, Malnoe A, Würzner HP. Metabolism of the food-borne mutagen/carcinogen 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline in the rat: assessment of biliary metabolties for genotoxicity. Food Chem. Toxicol. 1988;26:105–110. doi: 10.1016/0278-6915(88)90106-8. [DOI] [PubMed] [Google Scholar]
- 359.Ozawa S, Nagata K, Yamazoe Y, Kato R. Formation of 2-amino-3-methylimidazo[4,5-f]quinoline- and 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline-sulfamates by cDNA-expressed mammalian phenol sulfotransferases. Jpn. J. Cancer Res. 1995;86:264–269. doi: 10.1111/j.1349-7006.1995.tb03049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Boyland E, Manson D, Orr SF. The biochemistry of aromatic amines. II. The conversion of arylamines into arylsulphamic acids and arylamine-N-glucosiduronic acids. Biochem. J. 1957;65:417–423. doi: 10.1042/bj0650417a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Chou HC, Lang NP, Kadlubar FF. Metabolic activation of N-hydroxy arylamines and N-hydroxy heterocyclic amines by human sulfotransferase(s). Cancer Res. 1995;55:525–529. [PubMed] [Google Scholar]
- 362.Yamazoe Y, Nagata K, Ozawa S, Gong DW, Kato R. Activation and detoxication of carcinogenic arylamines by sulfation. Princess Takamatsu Symp. 1995;23:154–162. [PubMed] [Google Scholar]
- 363.Abu-Zeid M, Yamazoe Y, Kato R. Sulfotransferase-mediated DNA binding of N-hydroxyarylamines(amide) in liver cytosols from human and experimental animals. Carcinogenesis. 1992;13:1307–1314. doi: 10.1093/carcin/13.8.1307. [DOI] [PubMed] [Google Scholar]
- 364.Di Paolo OA, Teitel CH, Nowell S, Coles BF, Kadlubar FF. Expression of cytochromes P450 and glutathione S-transferases in human prostate, and the potential for activation of heterocyclic amine carcinogens via acetyl-coA-, PAPS- and ATP-dependent pathways. Int. J. Cancer. 2005;117:8–13. doi: 10.1002/ijc.21152. [DOI] [PubMed] [Google Scholar]
- 365.Williams JA, Stone EM, Fakis G, Johnson N, Cordell JA, Meinl W, Glatt H, Sim E, Phillips DH. N-Acetyltransferases, sulfotransferases and heterocyclic amine activation in the breast. Pharmacogenetics. 2001;11:373–388. doi: 10.1097/00008571-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 366.Williams JA. Single nucleotide polymorphisms, metabolic activation and environmental carcinogenesis: why molecular epidemiologists should think about enzyme expression. Carcinogenesis. 2001;22:209–214. doi: 10.1093/carcin/22.2.209. [DOI] [PubMed] [Google Scholar]
- 367.Lewis AJ, Walle UK, King RS, Kadlubar FF, Falany CN, Walle T. Bioactivation of the cooked food mutagen N-hydroxy-2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine by estrogen sulfotransferase in cultured human mammary epithelial cells. Carcinogenesis. 1998;19:2049–2053. doi: 10.1093/carcin/19.11.2049. [DOI] [PubMed] [Google Scholar]
- 368.Ozawa S, Shimizu M, Katoh T, Miyajima A, Ohno Y, Matsumoto Y, Fukuoka M, Tang YM, Lang NP, Kadlubar FF. Sulfating-activity and stability of cDNA-expressed allozymes of human phenol sulfotransferase, ST1A3*1 ((213)Arg) and ST1A3*2 ((213)His), both of which exist in Japanese as well as Caucasians. J. Biochem. (Tokyo) 1999;126:271–277. doi: 10.1093/oxfordjournals.jbchem.a022445. [DOI] [PubMed] [Google Scholar]
- 369.Carlini EJ, Raftogianis RB, Wood TC, Jin F, Zheng W, Rebbeck TR, Weinshilboum RM. Sulfation pharmacogenetics: SULT1A1 and SULT1A2 allele frequencies in Caucasian, Chinese and African-American subjects. Pharmacogenetics. 2001;11:57–68. doi: 10.1097/00008571-200102000-00007. [DOI] [PubMed] [Google Scholar]
- 370.Bamber DE, Fryer AA, Strange RC, Elder JB, Deakin M, Rajagopal R, Fawole A, Gilissen RA, Campbell FC, Coughtrie MW. Phenol sulphotransferase SULT1A1*1 genotype is associated with reduced risk of colorectal cancer. Pharmacogenetics. 2001;11:679–685. doi: 10.1097/00008571-200111000-00006. [DOI] [PubMed] [Google Scholar]
- 371.Zheng W, Xie D, Cerhan JR, Sellers TA, Wen W, Folsom AR. Sulfotransferase 1A1 polymorphism, endogenous estrogen exposure, well-done meat intake, and breast cancer risk. Cancer Epidemiol. Biomarkers Prev. 2001;10:89–94. [PubMed] [Google Scholar]
- 372.Moreno V, Glatt H, Guino E, Fisher E, Meinl W, Navarro M, Badosa JM, Boeing H. Polymorphisms in sulfotransferases SULT1A1 and SULT1A2 are not related to colorectal cancer. Int. J. Cancer. 2005;113:683–686. doi: 10.1002/ijc.20613. [DOI] [PubMed] [Google Scholar]
- 373.Steiner M, Bastian M, Schulz WA, Pulte T, Franke KH, Rohring A, Wolff JM, Seiter H, Schuff-Werner P. Phenol sulphotransferase SULT1A1 polymorphism in prostate cancer: lack of association. Arch. Toxicol. 2000;74:222–225. doi: 10.1007/s002040000118. [DOI] [PubMed] [Google Scholar]
- 374.Vineis P, McMichael A. Interplay between heterocyclic amines in cooked meat and metabolic phenotype in the etiology of colon cancer. Cancer Causes Control. 1996;7:479–486. doi: 10.1007/BF00052675. [DOI] [PubMed] [Google Scholar]
- 375.Burchell B. Genetic variation of human UDP-glucuronosyltransferase: implications in disease and drug glucuronidation. Am. J. Pharmacogenomics. 2003;3:37–52. doi: 10.2165/00129785-200303010-00006. [DOI] [PubMed] [Google Scholar]
- 376.Guillemette C. Pharmacogenomics of human UDP-glucuronosyltransferase enzymes. Pharmacogenomics J. 2003;3:136–158. doi: 10.1038/sj.tpj.6500171. [DOI] [PubMed] [Google Scholar]
- 377.Nagar S, Remmel RP. Uridine diphosphoglucuronosyltransferase pharmacogenetics and cancer. Oncogene. 2006;25:1659–1672. doi: 10.1038/sj.onc.1209375. [DOI] [PubMed] [Google Scholar]
- 378.Green MD, Tephly TR. Glucuronidation of amines and hydroxylated xenobiotics and endobiotics catalyzed by expressed human UGT1.4 protein. Drug Metab. Dispos. 1996;24:356–363. [PubMed] [Google Scholar]
- 379.Green MD, Tephly TR. Glucuronidation of amine substrates by purified and expressed UDP-glucuronosyltransferase proteins. Drug Metab. Dispos. 1998;26:860–867. [PubMed] [Google Scholar]
- 380.Ciotti M, Lakshmi VM, Basu N, Davis BB, Owens IS, Zenser TV. Glucuronidation of benzidine and its metabolites by cDNA-expressed human UDP-glucuronosyltransferases and pH stability of glucuronides. Carcinogenesis. 1999;20:1963–1969. doi: 10.1093/carcin/20.10.1963. [DOI] [PubMed] [Google Scholar]
- 381.Al-Zoughool M, Talaska G. 4-Aminobiphenyl N-glucuronidation by liver microsomes: optimization of the reaction conditions and characterization of the UDP-glucuronosyltransferase isoforms. J. Appl. Toxicol. 2006;26:524–532. doi: 10.1002/jat.1172. [DOI] [PubMed] [Google Scholar]
- 382.Orzechowski A, Schrenk D, Bock-Hennig BS, Bock KW. Glucuronidation of carcinogenic arylamines and their N-hydroxy derivatives by rat and human phenol UDP-glucuronosyltransferase of the UGT1 gene complex. Carcinogenesis. 1994;15:1549–1553. doi: 10.1093/carcin/15.8.1549. [DOI] [PubMed] [Google Scholar]
- 383.Kadlubar FF, Miller JA, Miller EC. Hepatic microsomal N-glucuronidation and nucleic acid binding of N-hydroxy arylamines in relation to urinary bladder carcinogenesis. Cancer Res. 1977;37:805–814. [PubMed] [Google Scholar]
- 384.Babu SR, Lakshmi VM, Hsu FF, Zenser TV, Davis BB. Glucuronidation of N-hydroxy metabolites of N-acetylbenzidine. Carcinogenesis. 1995;16:3069–3074. doi: 10.1093/carcin/16.12.3069. [DOI] [PubMed] [Google Scholar]
- 385.Babu SR, Lakshmi VM, Huang GP, Zenser TV, Davis BB. Glucuronide conjugates of 4-aminobiphenyl and its N-hydroxy metabolites. pH stability and synthesis by human and dog liver. Biochem. Pharmacol. 1996;51:1679–1685. doi: 10.1016/0006-2952(96)00165-7. [DOI] [PubMed] [Google Scholar]
- 386.Zenser TV, Lakshmi VM, Davis BB. N-glucuronidation of benzidine and its metabolites. Role in bladder cancer. Drug Metab. Dispos. 1998;26:856–859. [PubMed] [Google Scholar]
- 387.Irving CC. Glucuronide formation in the metabolism of N-substituted aryl compounds. Natl. Cancer Inst. Monogr. 1981;58:109–111. [PubMed] [Google Scholar]
- 388.Radomski JL, Hearn WL, Radomski T, Moreno H, Scott WE. Isolation of the glucuronic acid conjugate of N-hydroxy-4-aminobiphenyl from dog urine and its mutagenic activity. Cancer Res. 1977;37:1757–1762. [PubMed] [Google Scholar]
- 389.Mulder GJ, Meerman JH. Sulfation and glucuronidation as competing pathways in the metabolism of hydroxamic acids: the role of N,O-sulfonation in chemical carcinogenesis of aromatic amines. Environ. Health Perspect. 1983;49:27–32. doi: 10.1289/ehp.834927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Skipper PL, Obiedzinski MW, Tannenbaum SR, Miller DW, Mitchum RK, Kadlubar FF. Identification of the major serum albumin adduct formed by 4-aminobiphenyl in vivo in rats. Cancer Res. 1985;45:5122–5127. [PubMed] [Google Scholar]
- 391.Tannenbaum SR, Skipper PL, Wishnok JS, Stillwell WG, Day BW, Taghizadeh K. Characterization of various classes of protein adducts. Environ. Health Perspect. 1993;99:51–55. doi: 10.1289/ehp.939951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Styczynski PB, Blackmon RC, Groopman JD, Kensler TW. The direct glucuronidation of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) by human and rabbit liver microsomes. Chem. Res. Toxicol. 1993;6:846–851. doi: 10.1021/tx00036a014. [DOI] [PubMed] [Google Scholar]
- 393.Alexander J, Wallin H, Rossland OJ, Solberg KE, Holme JA, Becher G, Andersson R, Grivas S. Formation of a glutathione conjugate and a semistable transportable glucuronide conjugate of N2-oxidized species of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in rat liver. Carcinogenesis. 1991;12:2239–2245. doi: 10.1093/carcin/12.12.2239. [DOI] [PubMed] [Google Scholar]
- 394.Kaderlik KR, Mulder GJ, Turesky RJ, Lang NP, Teitel CH, Chiarelli MP, Kadlubar FF. Glucuronidation of N-hydroxy heterocyclic amines by human and rat liver microsomes. Carcinogenesis. 1994;15:1695–1701. doi: 10.1093/carcin/15.8.1695. [DOI] [PubMed] [Google Scholar]
- 395.Snyderwine EG, Welti DH, Davis CD, Fay LB, Turesky RJ. Metabolism of the food-derived carcinogen 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx) in nonhuman primates. Carcinogenesis. 1995;16:1377–1384. doi: 10.1093/carcin/16.6.1377. [DOI] [PubMed] [Google Scholar]
- 396.Malfatti MA, Felton JS. N-glucuronidation of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) and N-hydroxy-PhIP by specific human UDP-glucuronosyltransferases. Carcinogenesis. 2001;22:1087–1093. doi: 10.1093/carcin/22.7.1087. [DOI] [PubMed] [Google Scholar]
- 397.Malfatti MA, Felton JS. Human UDP-glucuronosyltransferase 1A1 is the primary enzyme responsible for the N-glucuronidation of N-hydroxy-PhIP in vitro. Chem. Res. Toxicol. 2004;17:1137–1144. doi: 10.1021/tx049898m. [DOI] [PubMed] [Google Scholar]
- 398.Girard H, Thibaudeau J, Court MH, Fortier LC, Villeneuve L, Caron P, Hao Q, von Moltke LL, Greenblatt DJ, Guillemette C. UGT1A1 polymorphisms are important determinants of dietary carcinogen detoxification in the liver. Hepatology. 2005;42:448–457. doi: 10.1002/hep.20770. [DOI] [PubMed] [Google Scholar]
- 399.Strassburg CP, Strassburg A, Nguyen N, Li Q, Manns MP, Tukey RH. Regulation and function of family 1 and family 2 UDP-glucuronosyltransferase genes (UGT1A, UGT2B) in human oesophagus. Biochem. J. 1999;338(Pt 2):489–498. [PMC free article] [PubMed] [Google Scholar]
- 400.Yueh MF, Nguyen N, Famourzadeh M, Strassburg CP, Oda Y, Guengerich FP, Tukey RH. The contribution of UDP-glucuronosyltransferase 1A9 on CYP1A2-mediated genotoxicity by aromatic and heterocyclic amines. Carcinogenesis. 2001;22:943–950. doi: 10.1093/carcin/22.6.943. [DOI] [PubMed] [Google Scholar]
- 401.Dellinger RW, Chen G, Blevins-Primeau AS, Krzeminski J, Amin S, Lazarus P. Glucuronidation of PhIP and N-OH-PhIP by UDP-glucuronosyltransferase 1A10. Carcinogenesis. 2007;28:2412–2418. doi: 10.1093/carcin/bgm164. [DOI] [PubMed] [Google Scholar]
- 402.Nowell SA, Massengill JS, Williams S, Radominska-Pandya A, Tephly TR, Cheng Z, Strassburg CP, Tukey RH, MacLeod SL, Lang NP, Kadlubar FF. Glucuronidation of 2-hydroxyamino-1-methyl-6-phenylimidazo[4,5-b]pyridine by human microsomal UDP-glucuronosyltransferases: identification of specific UGT1A family isoforms involved. Carcinogenesis. 1999;20:1107–1114. doi: 10.1093/carcin/20.6.1107. [DOI] [PubMed] [Google Scholar]
- 403.Nussbaum M, Fiala ES, Kulkarni B, el-Bayoumy K, Weisburger JH. In vivo metabolism of 3,2'-dimethyl-4-aminobiphenyl (DMAB) bearing on its organotropism in the Syrian golden hamster and the F344 rat. Environ. Health Perspect. 1983;49:223–231. doi: 10.1289/ehp.8349223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Kaderlik KR, Minchin RF, Mulder GJ, Ilett KF, Daugaard-Jenson M, Teitel CH, Kadlubar FF. Metabolic activation pathway for the formation of DNA adducts of the carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in rat extrahepatic tissues. Carcinogenesis. 1994;15:1703–1709. doi: 10.1093/carcin/15.8.1703. [DOI] [PubMed] [Google Scholar]
- 405.Heller HE, Hughes EE, Ingold CK. A new view of the arylhydroxylamine rearrangement. Nature. 1951;168:909–910. [Google Scholar]
- 406.Rothman N, Talaska G, Hayes RB, Bhatnagar VK, Bell DA, Lakshmi VM, Kashyap SK, Dosemeci M, Kashyap R, Hsu FF, Jaeger M, Hirvonen A, Parikh DJ, Davis BB, Zenser TV. Acidic urine pH is associated with elevated levels of free urinary benzidine and N-acetylbenzidine and urothelial cell DNA adducts in exposed workers. Cancer Epidemiol. Biomarkers Prev. 1997;6:1039–1042. [PubMed] [Google Scholar]
- 407.Alguacil J, Kogevinas M, Silverman D, Malats N, Real FX, Garcia-Closas M, Tardon A, Rivas M, Tora M, Garcia-Closas R, Serra C, Carrato A, Pfeiffer R, Fortuny J, Samanic C, Rothman N. Urinary pH, cigarette smoking and bladder cancer risk. Carcinogenesis. doi: 10.1093/carcin/bgr048. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Snyderwine EG, Roller PP, Adamson RH, Sato S, Thorgeirsson SS. Reaction of the N-hydroxylamine and N-acetoxy derivatives of 2-amino-3-methylimidazo[4,5-f]quinoline with DNA. Synthesis and identification of N-(deoxyguanosin-8-yl)-IQ. Carcinogenesis. 1988;9:1061–1065. doi: 10.1093/carcin/9.6.1061. [DOI] [PubMed] [Google Scholar]
- 409.Walpole AL, Williams MH, Roberts DC. The carcinogenic action of 4-aminodiphenyl and 3:2'-dimethyl-4-amino-diphenyl. Br. J. Ind. Med. 1952;9:255–263. doi: 10.1136/oem.9.4.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Navarrete A, Spjut HJ. Effect of colostomy on experimentally produced neoplasms of the colon of the rat. Cancer. 1967;20:1466–1472. doi: 10.1002/1097-0142(196709)20:9<1466::aid-cncr2820200913>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 411.Cleveland JC, Litvak SF, Cole JW. Identification of the route of action of the carcinogen 3:2-dimethyl-4-aminobiphenyl in the induction of intestinal neoplasia. Cancer Res. 1967;27:708–714. [PubMed] [Google Scholar]
- 412.Zenser TV, Lakshmi VM, Davis BB. Human and Escherichia coli beta-glucuronidase hydrolysis of glucuronide conjugates of benzidine and 4-aminobiphenyl, and their hydroxy metabolites. Drug Metab. Dispos. 1999;27:1064–1067. [PubMed] [Google Scholar]
- 413.Kaderlik KR, Kadlubar FF. Metabolic polymorphisms and carcinogen-DNA adduct formation in human populations. Pharmacogenetics. 1995;5:S108–S117. doi: 10.1097/00008571-199512001-00011. [DOI] [PubMed] [Google Scholar]
- 414.Strassburg CP, Vogel A, Kneip S, Tukey RH, Manns MP. Polymorphisms of the human UDP-glucuronosyltransferase (UGT) 1A7 gene in colorectal cancer. Gut. 2002;50:851–856. doi: 10.1136/gut.50.6.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Butler LM, Duguay Y, Millikan RC, Sinha R, Gagne JF, Sandler RS, Guillemette C. Joint effects between UDP-glucuronosyltransferase 1A7 genotype and dietary carcinogen exposure on risk of colon cancer. Cancer Epidemiol. Biomarkers Prev. 2005;14:1626–1632. doi: 10.1158/1055-9965.EPI-04-0682. [DOI] [PubMed] [Google Scholar]
- 416.Girard H, Butler LM, Villeneuve L, Millikan RC, Sinha R, Sandler RS, Guillemette C. UGT1A1 and UGT1A9 functional variants, meat intake, and colon cancer, among Caucasians and African-Americans. Mutat. Res. 2008;644:56–63. doi: 10.1016/j.mrfmmm.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annu. Rev. Pharmacol. Toxicol. 2005;45:51–88. doi: 10.1146/annurev.pharmtox.45.120403.095857. [DOI] [PubMed] [Google Scholar]
- 418.Mannervik B, Board PG, Hayes JD, Listowsky I, Pearson WR. Nomenclature for mammalian soluble glutathione transferases. Methods Enzymol. 2005;401:1–8. doi: 10.1016/S0076-6879(05)01001-3. [DOI] [PubMed] [Google Scholar]
- 419.Eyer P. Reactions of oxidatively activated arylamines with thiols: reaction mechanisms and biologic implications. An overview. Environ. Health Perspect. 1994;102(Suppl 6):123–132. doi: 10.1289/ehp.94102s6123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Dolle B, Topner W, Neumann HG. Reaction of arylnitroso compounds with mercaptans. Xenobiotica. 1980;10:527–536. doi: 10.3109/00498258009033787. [DOI] [PubMed] [Google Scholar]
- 421.Kazanis S, McClelland RA. Electrophilic intermediate in the reaction of glutathione and nitrosoarenes. J. Am. Chem Soc. 1992;114:3052–3059. [Google Scholar]
- 422.Ellis MK, Hill S, Foster PM. Reactions of nitrosonitrobenzenes with biological thiols: identification and reactivity of glutathion-S-yl conjugates. Chem. -Biol. Interact. 1992;82:151–163. doi: 10.1016/0009-2797(92)90107-v. [DOI] [PubMed] [Google Scholar]
- 423.Kaderlik KR, Mulder GJ, Shaddock JG, Casciano DA, Teitel CH, Kadlubar FF. Effect of glutathione depletion and inhibition of glucuronidation and sulfation on 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) metabolism, PhIP-DNA adduct formation and unscheduled DNA synthesis in primary rat hepatocytes. Carcinogenesis. 1994;15:1711–1716. doi: 10.1093/carcin/15.8.1711. [DOI] [PubMed] [Google Scholar]
- 424.Loretz LJ, Pariza MW. Effect of glutathione levels, sulfate levels, and metabolic inhibitors on covalent binding of 2-amino-3-methylimidazo[4,5-f]quinoline and 2-acetylaminofluorene to cell macromolecules in primary monolayer cultures of adult rat hepatocytes. Carcinogenesis. 1984;5:895–899. doi: 10.1093/carcin/5.7.895. [DOI] [PubMed] [Google Scholar]
- 425.Zenser TV, Lakshmi VM, Hsu FF, Davis BB. Methemoglobin oxidation of N-acetylbenzidine to form a sulfinamide. Drug Metab. Dispos. 2001;29:401–406. [PubMed] [Google Scholar]
- 426.Mulder GJ, Unruh LE, Evans FE, Ketterer B, Kadlubar FF. Formation and identification of glutathione conjugates from 2-nitrosofluorene and N-hydroxy-2-aminofluorene. Chem. -Biol. Interact. 1982;39:111–127. doi: 10.1016/0009-2797(82)90010-2. [DOI] [PubMed] [Google Scholar]
- 427.Meerman JH, Beland FA, Ketterer B, Srai SK, Bruins AP, Mulder GJ. Identification of glutathione conjugates formed from N-hydroxy-2-acetylaminofluorene in the rat. Chem. -Biol. Interact. 1982;39:149–168. doi: 10.1016/0009-2797(82)90118-1. [DOI] [PubMed] [Google Scholar]
- 428.Umemoto A, Grivas S, Yamaizumi Z, Sato S, Sugimura T. Non-enzymatic glutathione conjugation of 2-nitroso-6-methyldipyrido [1,2-a:3',2'-d] imidazole (NO-Glu-P-1) in vitro: N-hydroxy-sulfonamide, a new binding form of arylnitroso compounds and thiols. Chem. -Biol. Interact. 1988;68:57–69. doi: 10.1016/0009-2797(88)90006-3. [DOI] [PubMed] [Google Scholar]
- 429.Saito K, Yamazoe Y, Kamataki T, Kato R. Activation and detoxication of N-hydroxy-Trp-P-2 by glutathione and glutathione transferases. Carcinogenesis. 1983;4:1551–1557. doi: 10.1093/carcin/4.12.1551. [DOI] [PubMed] [Google Scholar]
- 430.Lin D-X, Meyer DJ, Ketterer B, Lang NP, Kadlubar FF. Effects of human and rat glutathione-S-transferase on the covalent binding of the N-acetoxy derivatives of heterocyclic amine carcinogens in vitro: a possible mechanism of organ specificity in their carcinogensis. Cancer Res. 1994;54:4920–4926. [PubMed] [Google Scholar]
- 431.Coles B, Nowell SA, MacLeod SL, Sweeney C, Lang NP, Kadlubar FF. The role of human glutathione S-transferases (hGSTs) in the detoxification of the food-derived carcinogen metabolite N-acetoxy-PhIP, and the effect of a polymorphism in hGSTA1 on colorectal cancer risk. Mutat. Res. 2001;482:3–10. doi: 10.1016/s0027-5107(01)00187-7. [DOI] [PubMed] [Google Scholar]
- 432.Nelson CP, Kidd LC, Sauvageot J, Isaacs WB, De Marzo AM, Groopman JD, Nelson WG, Kensler TW. Protection against 2-hydroxyamino-1-methyl-6-phenylimidazo[4,5-b]pyridine cytotoxicity and DNA adduct formation in human prostate by glutathione S-transferase P1. Cancer Res. 2001;61:103–109. [PubMed] [Google Scholar]
- 433.Howie AF, Forrester LM, Glancey MJ, Schlager JJ, Powis G, Beckett GJ, Hayes JD, Wolf CR. Glutathione S-transferase and glutathione peroxidase expression in normal and tumour human tissues. Carcinogenesis. 1990;11:451–458. doi: 10.1093/carcin/11.3.451. [DOI] [PubMed] [Google Scholar]
- 434.Coles BF, Morel F, Rauch C, Huber WW, Yang M, Teitel CH, Green B, Lang NP, Kadlubar FF. Effect of polymorphism in the human glutathione S-transferase A1 promoter on hepatic GSTA1 and GSTA2 expression. Pharmacogenetics. 2001;11:663–669. doi: 10.1097/00008571-200111000-00004. [DOI] [PubMed] [Google Scholar]
- 435.Sweeney C, Coles BF, Nowell S, Lang NP, Kadlubar FF. Novel markers of susceptibility to carcinogens in diet: associations with colorectal cancer. Toxicology. 2002;181-182:83–87. doi: 10.1016/s0300-483x(02)00259-7. [DOI] [PubMed] [Google Scholar]
- 436.van der Logt EM, Bergevoet SM, Roelofs HM, van HZ, te Morsche RH, Wobbes T, de Kok JB, Nagengast FM, Peters WH. Genetic polymorphisms in UDP-glucuronosyltransferases and glutathione S-transferases and colorectal cancer risk. Carcinogenesis. 2004;25:2407–2415. doi: 10.1093/carcin/bgh251. [DOI] [PubMed] [Google Scholar]
- 437.Weisburger JH, Grantham PH, Steibigel NH, Dall DP, Weisburger EK. Activation and detoxication of N-2-fluorenylacetamide in man. Cancer Res. 1964;24:475–479. [PubMed] [Google Scholar]
- 438.Cocker J, Boobis AR, Wilson HK, Gompertz D. Evidence that a beta-N-glucuronide of 4,4'-methylenebis (2-chloroaniline) (MOCA) is a major urinary metabolite in man: implications for biological monitoring. Br. J. Ind. Med. 1990;47:154–161. doi: 10.1136/oem.47.3.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Cocker J, Boobis AR, Davies DS. Determination of the N-acetyl metabolites of 4,4'-methylene dianiline and 4,4'-methylene-bis(2-chloroaniline) in urine. Biomed. Environ. Mass Spectrom. 1988;17:161–167. doi: 10.1002/bms.1200170303. [DOI] [PubMed] [Google Scholar]
- 440.Seyler TH, Bernert JT. Analysis of 4-aminobiphenyl in smoker's and nonsmoker's urine by tandem mass spectrometry. Biomarkers. 2011 doi: 10.3109/1354750X.2010.544755. [DOI] [PubMed] [Google Scholar]
- 441.Boyland E, MAnson D. The biochemistry of aromatic amines. The metabolism of 2-naphthylamine and 2-naphthylhydroxylamine derivatives. Biochem. J. 1966;101:84–102. doi: 10.1042/bj1010084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.el-Bayoumy K, Donahue JM, Hecht SS, Hoffmann D. Identification and quantitative determination of aniline and toluidines in human urine. Cancer Res. 1986;46:6064–6067. [PubMed] [Google Scholar]
- 443.Weiss T, Angerer J. Simultaneous determination of various aromatic amines and metabolites of aromatic nitro compounds in urine for low level exposure using gas chromatography-mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2002;778:179–192. doi: 10.1016/s0378-4347(01)00542-4. [DOI] [PubMed] [Google Scholar]
- 444.Grimmer G, Dettbarn G, Seidel A, Jacob J. Detection of carcinogenic aromatic amines in the urine of non-smokers. Sci. Total Environ. 2000;247:81–90. doi: 10.1016/s0048-9697(99)00471-4. [DOI] [PubMed] [Google Scholar]
- 445.Riedel K, Scherer G, Engl J, Hagedorn HW, Tricker AR. Determination of three carcinogenic aromatic amines in urine of smokers and nonsmokers. J. Anal. Toxicol. 2006;30:187–195. doi: 10.1093/jat/30.3.187. [DOI] [PubMed] [Google Scholar]
- 446.Riffelmann M, Muller G, Schmieding W, Popp W, Norpoth K. Biomonitoring of urinary aromatic amines and arylamine hemoglobin adducts in exposed workers and nonexposed control persons. Int. Arch. Occup. Environ. Health. 1995;68:36–43. doi: 10.1007/BF01831631. [DOI] [PubMed] [Google Scholar]
- 447.Holland RD, Taylor J, Schoenbachler L, Jones RC, Freeman JP, Miller DW, Lake BG, Gooderham NJ, Turesky RJ. Rapid biomonitoring of heterocyclic aromatic amines in human urine by tandem solvent solid phase extraction liquid chromatography electrospray ionization mass spectrometry. Chem. Res. Toxicol. 2004;17:1121–1136. doi: 10.1021/tx049910a. [DOI] [PubMed] [Google Scholar]
- 448.Ushiyama H, Wakabayashi K, Hirose M, Itoh H, Sugimura T, Nagao M. Presence of carcinogenic heterocyclic amines in urine of healthy volunteers eating normal diet, but not of inpatients receiving parenteral alimentation. Carcinogenesis. 1991;12:1417–1422. doi: 10.1093/carcin/12.8.1417. [DOI] [PubMed] [Google Scholar]
- 449.Stillwell WG, Turesky RJ, Sinha R, Skipper PL, Tannenbaum SR. Biomonitoring of heterocyclic aromatic amine metabolites in human urine. Cancer Lett. 1999;143:145–148. doi: 10.1016/s0304-3835(99)00144-5. [DOI] [PubMed] [Google Scholar]
- 450.Frandsen H. Deconjugation of N-glucuronide conjugated metabolites with hydrazine hydrate--biomarkers for exposure to the food-borne carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP). Food Chem. Toxicol. 2007;45:863–870. doi: 10.1016/j.fct.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 451.Murray S, Gooderham NJ, Boobis AR, Davies DS. Detection and measurement of MeIQx in human urine after ingestion of a cooked meat meal. Carcinogenesis. 1989;10:763–765. doi: 10.1093/carcin/10.4.763. [DOI] [PubMed] [Google Scholar]
- 452.Lynch AM, Knize MG, Boobis AR, Gooderham N, Davies DS, Murray S. Intra- and interindividual variability in systemic exposure in humans to 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline and 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine, carcinogens present in food. Cancer Res. 1992;52:6216–6223. [PubMed] [Google Scholar]
- 453.Ji H, Yu MC, Stillwell WG, Skipper PL, Ross RK, Henderson BE, Tannenbaum SR. Urinary excretion of 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline in white, black, and Asian men in Los Angeles County. Cancer Epidemiol. Biomarkers Prev. 1994;3:407–411. [PubMed] [Google Scholar]
- 454.Reistad R, Rossland OJ, Latva-Kala KJ, Rasmussen T, Vikse R, Becher G, Alexander J. Heterocyclic aromatic amines in human urine following a fried meat meal. Food Chem. Toxicol. 1997;35:945–955. doi: 10.1016/s0278-6915(97)00112-9. [DOI] [PubMed] [Google Scholar]
- 455.Kidd LC, Stillwell WG, Yu MC, Wishnok JS, Skipper PL, Ross RK, Henderson BE, Tannenbaum SR. Urinary excretion of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in White, African-American, and Asian-American men in Los Angeles County. Cancer Epidemiol. Biomarkers Prev. 1999;8:439–445. [PubMed] [Google Scholar]
- 456.Turesky RJ, Yuan JM, Wang R, Peterson S, Yu MC. Tobacco smoking and urinary levels of 2-amino-9H-pyrido[2,3-b]indole in men of Shanghai, China. Cancer Epidemiol. Biomarkers Prev. 2007;16:1554–1560. doi: 10.1158/1055-9965.EPI-07-0132. [DOI] [PubMed] [Google Scholar]
- 457.Nishigaki R, Totsuka Y, Kataoka H, Ushiyama H, Goto S, Akasu T, Watanabe T, Sugimura T, Wakabayashi K. Detection of aminophenylnorharman, a possible endogenous mutagenic and carcinogenic compound, in human urine samples. Cancer Epidemiol. Biomarkers Prev. 2007;16:151–156. doi: 10.1158/1055-9965.EPI-06-0052. [DOI] [PubMed] [Google Scholar]
- 458.Frederiksen H. Two food-borne heterocyclic amines: metabolism and DNA adduct formation of amino-alpha-carbolines. Mol. Nutr. Food Res. 2005;49:263–273. doi: 10.1002/mnfr.200400061. [DOI] [PubMed] [Google Scholar]
- 459.Sinha R, Rothman N, Mark SD, Murray S, Brown ED, Levander OA, Davies DS, Lang NP, Kadlubar FF, Hoover RN. Lower levels of urinary 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx) in humans with higher CYP1A2 activity. Carcinogenesis. 1995;16:2859–2861. doi: 10.1093/carcin/16.11.2859. [DOI] [PubMed] [Google Scholar]
- 460.Oda Y, Totsuka Y, Wakabayashi K, Guengerich FP, Shimada T. Activation of aminophenylnorharman, aminomethylphenylnorharman and aminophenylharman to genotoxic metabolites by human N-acetyltransferases and cytochrome P450 enzymes expressed in Salmonella typhimurium umu tester strains. Mutagenesis. 2006;21:411–416. doi: 10.1093/mutage/gel047. [DOI] [PubMed] [Google Scholar]
- 461.Strickland PT, Qian Z, Friesen MD, Rothman N, Sinha R. Measurement of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in acid-hydrolyzed urine by high-performance liquid chromatograpy with fluorescence detection. Biomarkers. 2001;6:313–325. [Google Scholar]
- 462.King RS, Teitel CH, Shaddock JG, Casciano DA, Kadlubar FF. Detoxification of carcinogenic aromatic and heterocyclic amines by enzymatic reduction of the N-hydroxy derivative. Cancer Lett. 1999;143:167–171. doi: 10.1016/s0304-3835(99)00119-6. [DOI] [PubMed] [Google Scholar]
- 463.Kurian JR, Chin NA, Longlais BJ, Hayes KL, Trepanier LA. Reductive detoxification of arylhydroxylamine carcinogens by human NADH cytochrome b5 reductase and cytochrome b5. Chem. Res. Toxicol. 2006;19:1366–1373. doi: 10.1021/tx060106t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Malfatti MA, Wu RW, Felton JS. The effect of UDP-glucuronosyltransferase 1A1 expression on the mutagenicity and metabolism of the cooked-food carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine in CHO cells. Mutat. Res. 2005;570:205–214. doi: 10.1016/j.mrfmmm.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 465.Miners JO, Osborne NJ, Tonkin AL, Birkett DJ. Perturbation of paracetamol urinary metabolic ratios by urine flow rate. Br. J. Clin. Pharmacol. 1992;34:359–362. doi: 10.1111/j.1365-2125.1992.tb05643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Kriek E. On the interaction of N-2-fluorenylhydroxylamine with nucleic acids in vitro. Biochem. Biophys. Res. Commun. 1965;20:793–799. doi: 10.1016/0006-291x(65)90088-4. [DOI] [PubMed] [Google Scholar]
- 467.King CM, Phillips B. N-hydroxy-2-fluorenylacetamide. Reaction of the carcinogen with guanosine, ribonucleic acid, deoxyribonucleic acid, and protein following enzymatic deacetylation or esterification. J. Biol. Chem. 1969;244:6209–6216. [PubMed] [Google Scholar]
- 468.Beland FA, Allaben WT, Evans FE. Acyltransferase-mediated binding of N-hydroxyarylamides to nucleic acids. Cancer Res. 1980;40:834–840. [PubMed] [Google Scholar]
- 469.Kriek E. Persistent binding of a new reaction product of the carcinogen N-hydroxy-N-2-acetylaminofluorene with guanine in rat liver DNA in vivo. Cancer Res. 1972;32:2042–2048. [PubMed] [Google Scholar]
- 470.Westra JG, Kriek E, Hittenhausen H. Identification of the persistently bound form of the carcinogen N-acetyl-2-aminofluorene to rat liver DNA in vivo. Chem. Biol. Interact. 1976;15:149–164. doi: 10.1016/0009-2797(76)90160-5. [DOI] [PubMed] [Google Scholar]
- 471.Hashimoto Y, Shudo K. Chemical modification of DNA with muta-carcinogens. I. 3-Amino-1-methyl-5H-pyrido[4,3-b]indole and 2-amino-6-methyldipyrido[1,2-a:3',2'-d]imidazole: metabolic activation and structure of the DNA adducts. Environ. Health Perspect. 1985;62:209–214. doi: 10.1289/ehp.8562209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Humphreys WG, Kadlubar FF, Guengerich FP. Mechanism of C8 alkylation of guanine residues by activated arylamines: evidence for initial adduct formation at the N7 position. Proc. Natl. Acad. Sci. U. S. A. 1992;89:8278–8282. doi: 10.1073/pnas.89.17.8278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Kennedy SA, Novak M, Kolb BA. Reactions of ester derivatives of carcinogenic N-(4-biphenylyl)hydroxylamine and the corresponding hydroxamic acid with purine nucleosides. J Am. Chem Soc. 1997;119:7654–7664. [Google Scholar]
- 474.Turesky RJ, Rossi SC, Welti DH, Lay JO, Jr., Kadlubar FF. Characterization of DNA adducts formed in vitro by reaction of N-hydroxy-2-amino-3-methylimidazo[4,5-f]quinoline and N-hydroxy-2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline at the C-8 and N2 atoms of guanine. Chem. Res. Toxicol. 1992;5:479–490. doi: 10.1021/tx00028a005. [DOI] [PubMed] [Google Scholar]
- 475.Jamin EL, Arquier D, Canlet C, Rathahao E, Tulliez J, Debrauwer L. New insights in the formation of deoxynucleoside adducts with the heterocyclic aromatic amines PhIP and IQ by means of ion trap MSn and accurate mass measurement of fragment ions. J. Am. Soc. Mass Spectrom. 2007;18:2107–2118. doi: 10.1016/j.jasms.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 476.Bessette EE, Goodenough AK, Langouet S, Yasa I, Kozekov ID, Spivack SD, Turesky RJ. Screening for DNA adducts by data-dependent constant neutral loss-triple stage mass spectrometry with a linear quadrupole ion trap mass spectrometer. Anal. Chem. 2009;81:809–819. doi: 10.1021/ac802096p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Frandsen H, Grivas S, Turesky RJ, Anndersson R, Dragsted LO, Larsen JC. Formation of DNA adducts by the food mutagen 2-amino-3,4,8-trimethyl-3H-imidazo[4,5-f]quinoxaline in vitro and in vivo. Identification of a N2-(2'-deoxyguanosin-8-yl)-4,8-DiMeIQx adduct. Carcinogenesis. 1994;15:2553–2558. doi: 10.1093/carcin/15.11.2553. [DOI] [PubMed] [Google Scholar]
- 478.Frederiksen H, Frandsen H, Pfau W. Syntheses of DNA-adducts of two heterocyclic amines, 2-amino-3-methyl-9H-pyrido[2,3-b]indole (MeAαC) and 2-amino-9H-pyrido[2,3-b]indole (AαC) and identification of DNA-adducts in organs from rats dosed with MeAαC. Carcinogenesis. 2004;25:1525–1533. doi: 10.1093/carcin/bgh156. [DOI] [PubMed] [Google Scholar]
- 479.Ochiai M, Nakagama H, Turesky RJ, Sugimura T, Nagao M. A new modification of the 32P-post-labeling method to recover IQ-DNA adducts as mononucleotides. Mutagenesis. 1999;14:239–242. doi: 10.1093/mutage/14.2.239. [DOI] [PubMed] [Google Scholar]
- 480.Singh R, Arlt VM, Henderson CJ, Phillips DH, Farmer PB, Gamboa daCosta G. Detection and quantitation of N-(deoxyguanosin-8-yl)-2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine adducts in DNA using online column-switching liquid chromatography tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2010;878:2155–2162. doi: 10.1016/j.jchromb.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Pfau W, Schulze C, Shirai T, Hasegawa R, Brockstedt U. Identification of the major hepatic DNA adduct formed by the food mutagen 2-amino-9H-pyrido[2,3-b]indole (AαC). Chem. Res. Toxicol. 1997;10:1192–1197. doi: 10.1021/tx9701182. [DOI] [PubMed] [Google Scholar]
- 482.Hashimoto Y, Shudo K, Okamoto T. Modification of nucleic acids with muta-carcinogenic heteroaromatic amines in vivo. Identification of modified bases in DNA extracted from rats injected with 3-amino-1-methyl-5H-pyrido[4,3-b]indole and 2-amino-6-methyldipyrido[1,2-a3:3',2'-d]imidazole. Mutat. Res. 1982;105:9–13. doi: 10.1016/0165-7992(82)90200-7. [DOI] [PubMed] [Google Scholar]
- 483.Turesky RJ, Gremaud E, Markovic J, Snyderwine EG. DNA adduct formation of the food-derived mutagen 2-amino-3-methylimidazo[4,5-f]quinoline in nonhuman primates undergoing carcinogen bioassay. Chem. Res. Toxicol. 1996;9:403–408. doi: 10.1021/tx950132j. [DOI] [PubMed] [Google Scholar]
- 484.Turesky RJ, Markovic J. DNA adduct formation of the food carcinogen 2-amino-3-methylimidazo[4,5-f]quinoline (IQ) in liver, kidney and colorectum of rats. Carcinogenesis. 1995;16:2275–2279. doi: 10.1093/carcin/16.9.2275. [DOI] [PubMed] [Google Scholar]
- 485.Paehler A, Richoz J, Soglia J, Vouros P, Turesky RJ. Analysis and quantification of DNA adducts of 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline in liver of rats by liquid chromatography/electrospray tandem mass spectrometry. Chem. Res. Toxicol. 2002;15:551–561. doi: 10.1021/tx010178e. [DOI] [PubMed] [Google Scholar]
- 486.Lakshman MK. Synthesis of biologically important nucleoside analogs by palladium-catalyzed C-N bond formation. Cur. Org. Synth. 2005;2:83–112. [Google Scholar]
- 487.Elmquist CE, Stover JS, Wang Z, Rizzo CJ. Site-specific synthesis and properties of oligonucleotides containing C8-deoxyguanosine adducts of the dietary mutagen IQ. J. Am. Chem. Soc. 2004;126:11189–11201. doi: 10.1021/ja0487022. [DOI] [PubMed] [Google Scholar]
- 488.Stover JS, Rizzo CJ. Synthesis of oligonucleotides containing the N2-deoxyguanosine adduct of the dietary carcinogen 2-amino-3-methylimidazo[4,5-f]quinoline. Chem. Res. Toxicol. 2007;20:1972–1979. doi: 10.1021/tx7002867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Bonala R, Torres MC, Iden CR, Johnson F. Synthesis of the PhIP adduct of 2'-deoxyguanosine and its incorporation into oligomeric DNA. Chem Res. Toxicol. 2006;19:734–738. doi: 10.1021/tx0600191. [DOI] [PubMed] [Google Scholar]
- 490.Takamura-Enya T, Ishikawa S, Mochizuki M, Wakabayashi K. Chemical synthesis of 2'-deoxyguanosine-C8 adducts with heterocyclic amines: an application to synthesis of oligonucleotides site-specifically adducted with 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine. Chem. Res. Toxicol. 2006;19:770–778. doi: 10.1021/tx050296s. [DOI] [PubMed] [Google Scholar]
- 491.Patel DJ, Mao B, Gu Z, Hingerty BE, Gorin A, Basu AK, Broyde S. Nuclear magnetic resonance solution structures of covalent aromatic amine-DNA adducts and their mutagenic relevance. Chem. Res. Toxicol. 1998;11:391–407. doi: 10.1021/tx9702143. [DOI] [PubMed] [Google Scholar]
- 492.Shibutani S, Suzuki N, Grollman AP. Mutagenic specificity of (acetylamino)fluorene-derived DNA adducts in mammalian cells. Biochemistry. 1998;37:12034–12041. doi: 10.1021/bi981059+. [DOI] [PubMed] [Google Scholar]
- 493.Shibutani S, Fernandes A, Suzuki N, Zhou L, Johnson F, Grollman AP. Mutagenesis of the N-(deoxyguanosin-8-yl)-2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine DNA adduct in mammalian cells. Sequence context effects. J. Biol. Chem. 1999;274:27433–27438. doi: 10.1074/jbc.274.39.27433. [DOI] [PubMed] [Google Scholar]
- 494.Brown K, Hingerty BE, Guenther EA, Krishnan VV, Broyde S, Turteltaub KW, Cosman M. Solution structure of the 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine C8-deoxyguanosine adduct in duplex DNA. Proc. Natl. Acad. Sci. U. S. A. 2001;98:8507–8512. doi: 10.1073/pnas.151251898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Cho BP. Dynamic conformational heterogeneities of carcinogen-DNA adducts and their mutagenic relevance. J. Environ. Sci. Health C. Environ. Carcinog. Ecotoxicol. Rev. 2004;22:57–90. doi: 10.1081/LESC-200038217. [DOI] [PubMed] [Google Scholar]
- 496.Choi JY, Stover JS, Angel KC, Chowdhury G, Rizzo CJ, Guengerich FP. Biochemical basis of genotoxicity of heterocyclic arylamine food mutagens: Human DNA polymerase eta selectively produces a two-base deletion in copying the N2-guanyl adduct of 2-amino-3-methylimidazo[4,5-f]quinoline but not the C8 adduct at the NarI G3 site. J. Biol. Chem. 2006;281:25297–25306. doi: 10.1074/jbc.M605699200. [DOI] [PubMed] [Google Scholar]
- 497.Stover JS, Chowdhury G, Zang H, Guengerich FP, Rizzo CJ. Translesion synthesis past the C8- and N2-deoxyguanosine adducts of the dietary mutagen 2-amino-3-methylimidazo[4,5-f]quinoline in the NarI recognition sequence by prokaryotic DNA polymerases. Chem. Res. Toxicol. 2006;19:1506–1517. doi: 10.1021/tx0601455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Wang F, DeMuro NE, Elmquist CE, Stover JS, Rizzo CJ, Stone MP. Base-displaced intercalated structure of the food mutagen 2-amino-3-methylimidazo[4,5-f]quinoline in the recognition sequence of the NarI restriction enzyme, a hotspot for -2 bp deletions. J. Am. Chem. Soc. 2006;128:10085–10095. doi: 10.1021/ja062004v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Broyde S, Wang L, Zhang L, Rechkoblit O, Geacintov NE, Patel DJ. DNA adduct structure-function relationships: comparing solution with polymerase structures. Chem. Res. Toxicol. 2008;21:45–52. doi: 10.1021/tx700193x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500.Delaney JC, Essigmann JM. Biological properties of single chemical-DNA adducts: a twenty year perspective. Chem. Res. Toxicol. 2008;21:232–252. doi: 10.1021/tx700292a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Randerath K, Randerath E, Agrawal HP, Gupta RC, Schurda ME, Reddy MV. Postlabeling methods for carcinogen-DNA adduct analysis. Environ. Health Perspect. 1985;62:57–65. doi: 10.1289/ehp.856257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502.Culp SJ, Roberts DW, Talaska G, Lang NP, Fu PP, Lay JO, Jr., Teitel CH, Snawder JE, Von Tungeln LS, Kadlubar FF. Immunochemical, 32P-postlabeling, and GC/MS detection of 4-aminobiphenyl-DNA adducts in human peripheral lung in relation to metabolic activation pathways involving pulmonary N-oxidation, conjugation, and peroxidation. Mutat. Res. 1997;378:97–112. doi: 10.1016/s0027-5107(97)00101-2. [DOI] [PubMed] [Google Scholar]
- 503.Poirier MC, Santella RM, Weston A. Carcinogen macromolecular adducts and their measurement. Carcinogenesis. 2000;21:353–359. doi: 10.1093/carcin/21.3.353. [DOI] [PubMed] [Google Scholar]
- 504.Faraglia B, Chen SY, Gammon MD, Zhang Y, Teitelbaum SL, Neugut AI, Ahsan H, Garbowski GC, Hibshoosh H, Lin D, Kadlubar FF, Santella RM. Evaluation of 4-aminobiphenyl-DNA adducts in human breast cancer: the influence of tobacco smoke. Carcinogenesis. 2003;24:719–725. doi: 10.1093/carcin/bgg013. [DOI] [PubMed] [Google Scholar]
- 505.Lin D, Lay JO, Jr., Bryant MS, Malaveille C, Friesen M, Bartsch H, Lang NP, Kadlubar FF. Analysis of 4-aminobiphenyl-DNA adducts in human urinary bladder and lung by alkaline hydrolysis and negative ion gas chromatography-mass spectrometry. Environ. Health Perspect. 1994;102(Suppl 6):11–16. doi: 10.1289/ehp.94102s611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.Dingley KH, Roberts ML, Velsko CA, Turteltaub KW. Attomole detection of 3H in biological samples using accelerator mass spectrometry: application in low-dose, dual-isotope tracer studies in conjunction with 14C accelerator mass spectrometry. Chem. Res. Toxicol. 1998;11:1217–1222. doi: 10.1021/tx9801458. [DOI] [PubMed] [Google Scholar]
- 507.Doerge DR, Churchwell MI, Marques MM, Beland FA. Quantitative analysis of 4-aminobiphenyl-C8-deoxyguanosyl DNA adducts produced in vitro and in vivo using HPLC-ES-MS. Carcinogenesis. 1999;20:1055–1061. doi: 10.1093/carcin/20.6.1055. [DOI] [PubMed] [Google Scholar]
- 508.Beland FA, Doerge DR, Churchwell MI, Poirier MC, Schoket B, Marques MM. Synthesis, characterization, and quantitation of a 4-aminobiphenyl-DNA adduct standard. Chem. Res. Toxicol. 1999;12:68–77. doi: 10.1021/tx980172y. [DOI] [PubMed] [Google Scholar]
- 509.Gangl ET, Turesky RJ, Vouros P. Determination of in vitro- and in vivo-formed DNA adducts of 2-amino-3-methylimidazo[4,5-f]quinoline by capillary liquid chromatography/microelectrospray mass spectrometry. Chem. Res. Toxicol. 1999;12:1019–1027. doi: 10.1021/tx990060m. [DOI] [PubMed] [Google Scholar]
- 510.Soglia JR, Turesky RJ, Paehler A, Vouros P. Quantification of the heterocyclic aromatic amine DNA adduct N-(deoxyguanosin-8-yl)-2-amino-3-methylimidazo[4,5-f]quinoline in livers of rats using capillary liquid chromatography/microelectrospray mass spectrometry: a dose-response study. Anal. Chem. 2001;73:2819–2827. doi: 10.1021/ac010218j. [DOI] [PubMed] [Google Scholar]
- 511.Crosbie SJ, Murray S, Boobis AR, Gooderham NJ. Mass spectrometric detection and measurement of N2-(2'-deoxyguanosin-8-yl)PhIP adducts in DNA. J. Chromatogr. B Biomed. Sci. Appl. 2000;744:55–64. doi: 10.1016/s0378-4347(00)00227-9. [DOI] [PubMed] [Google Scholar]
- 512.Ricicki EM, Soglia JR, Teitel C, Kane R, Kadlubar F, Vouros P. Detection and quantification of N-(deoxyguanosin-8-yl)-4-aminobiphenyl adducts in human pancreas tissue using capillary liquid chromatography-microelectrospray mass spectrometry. Chem Res. Toxicol. 2005;18:692–699. doi: 10.1021/tx049692l. [DOI] [PubMed] [Google Scholar]
- 513.Randall KL, Argoti D, Paonessa JD, Ding Y, Oaks Z, Zhang Y, Vouros P. An improved liquid chromatography-tandem mass spectrometry method for the quantification of 4-aminobiphenyl DNA adducts in urinary bladder cells and tissues. J. Chromatogr. A. 2010;1217:4135–4143. doi: 10.1016/j.chroma.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514.Hatcher JF, Swaminathan S. Identification of N-(deoxyguanosin-8-yl)-4-azobiphenyl by (32)P-postlabeling analyses of DNA in human uroepithelial cells exposed to proximate metabolites of the environmental carcinogen 4-aminobiphenyl. Environ. Mol. Mutagen. 2002;39:314–322. doi: 10.1002/em.10079. [DOI] [PubMed] [Google Scholar]
- 515.Swaminathan S, Hatcher JF. Identification of new DNA adducts in human bladder epithelia exposed to the proximate metabolite of 4-aminobiphenyl using 32P-postlabeling method. Chem. -Biol. Interact. 2002;139:199–213. doi: 10.1016/s0009-2797(01)00300-3. [DOI] [PubMed] [Google Scholar]
- 516.Poirier MC, Fullerton NF, Smith BA, Beland FA. DNA adduct formation and tumorigenesis in mice during the chronic administration of 4-aminobiphenyl at multiple dose levels. Carcinogenesis. 1995;16:2917–2921. doi: 10.1093/carcin/16.12.2917. [DOI] [PubMed] [Google Scholar]
- 517.Martin CN, Beland FA, Roth RW, Kadlubar FF. Covalent binding of benzidine and N-acetylbenzidine to DNA at the C-8 atom of deoxyguanosine in vivo and in vitro. Cancer Res. 1982;42:2678–2686. [PubMed] [Google Scholar]
- 518.Kennelly JC, Beland FA, Kadlubar FF, Martin CN. Binding of N-acetylbenzidine and N,N'-diacetylbenzidine to hepatic DNA of rat and hamster in vivo and in vitro. Carcinogenesis. 1984;5:407–412. doi: 10.1093/carcin/5.3.407. [DOI] [PubMed] [Google Scholar]
- 519.Yamazoe Y, Roth RW, Kadlubar FF. Reactivity of benzidine diimine with DNA to form N-(deoxyguanosin-8-yl)-benzidine. Carcinogenesis. 1986;7:179–182. doi: 10.1093/carcin/7.1.179. [DOI] [PubMed] [Google Scholar]
- 520.Segerbäck D, Kadlubar FF. Characterization of 4,4'-methylenebis(2-chloroaniline)--DNA adducts formed in vivo and in vitro. Carcinogenesis. 1992;13:1587–1592. doi: 10.1093/carcin/13.9.1587. [DOI] [PubMed] [Google Scholar]
- 521.Segerbäck D, Kaderlik KR, Talaska G, Dooley KL, Kadlubar FF. 32P-postlabelling analysis of DNA adducts of 4,4'-methylenebis(2-chloroaniline) in target and nontarget tissues in the dog and their implications for human risk assessment. Carcinogenesis. 1993;14:2143–2147. doi: 10.1093/carcin/14.10.2143. [DOI] [PubMed] [Google Scholar]
- 522.Turesky RJ. DNA adducts of heterocyclic aromatic amines, arylazides and 4-nitroquinoline 1-oxide. In: Hemminki K, Dipple A, Shuker DEG, Kadlubar FF, Segerbäck D, Bartsch H, editors. DNA Adducts: Identification and Biological Significance. International Agency for Research on Cancer; Lyon: 1994. pp. 217–228. [PubMed] [Google Scholar]
- 523.Marques MM, Mourato LL, Santos MA, Beland FA. Synthesis, characterization, and conformational analysis of DNA adducts from methylated anilines present in tobacco smoke. Chem. Res. Toxicol. 1996;9:99–108. doi: 10.1021/tx950044z. [DOI] [PubMed] [Google Scholar]
- 524.Jones CR, Sabbioni G. Identification of DNA adducts using HPLC/MS/MS following in vitro and in vivo experiments with arylamines and nitroarenes. Chem. Res. Toxicol. 2003;16:1251–1263. doi: 10.1021/tx020064i. [DOI] [PubMed] [Google Scholar]
- 525.Cui L, Sun HL, Wishnok JS, Tannenbaum SR, Skipper PL. Identification of adducts formed by reaction of N-acetoxy-3,5-dimethylaniline with DNA. Chem. Res. Toxicol. 2007;20:1730–1736. doi: 10.1021/tx700306c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526.Skipper PL, Trudel LJ, Kensler TW, Groopman JD, Egner PA, Liberman RG, Wogan GN, Tannenbaum SR. DNA adduct formation by 2,6-dimethyl-, 3,5-dimethyl-, and 3-ethylaniline in vivo in mice. Chem. Res. Toxicol. 2006;19:1086–1090. doi: 10.1021/tx060082q. [DOI] [PubMed] [Google Scholar]
- 527.Gupta RC, Reddy MV, Randerath K. 32P-postlabeling analysis of non-radioactive aromatic carcinogen--DNA adducts. Carcinogenesis. 1982;3:1081–1092. doi: 10.1093/carcin/3.9.1081. [DOI] [PubMed] [Google Scholar]
- 528.Pfau W, Lecoq S, Hughes NC, Seidel A, Platt KL, Grover PL, Phillips DH. Separation of 32P-labelled nucleoside 3',5'-bisphosphate adducts by HPLC. IARC Sci. Publ. 1993:233–242. [PubMed] [Google Scholar]
- 529.Baranczewski P, Moller L. Relationship between content and activity of cytochrome P450 and induction of heterocyclic amine DNA adducts in human liver samples in vivo and in vitro. Cancer Epidemiol. Biomarkers Prev. 2004;13:1071–1078. [PubMed] [Google Scholar]
- 530.Pfau W, Brockstedt U, Sohren KD, Marquardt H. 32P-post-labelling analysis of DNA adducts formed by food-derived heterocyclic amines: evidence for incomplete hydrolysis and a procedure for adduct pattern simplification. Carcinogenesis. 1994;15:877–882. doi: 10.1093/carcin/15.5.877. [DOI] [PubMed] [Google Scholar]
- 531.Turteltaub KW, Vogel JS, Frantz CE, Shen N. Fate and distribution of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine in mice at a human dietary equivalent dose. Cancer Res. 1992;52:4682–4687. [PubMed] [Google Scholar]
- 532.Ghoshal A, Davis CD, Schut HAJ, Snyderwine EG. Possible mechanisms for PhIP-DNA adduct fomation in the mammary gland of female Sprague-Dawley rats. Carcinogenesis. 1995;16:2725–2731. doi: 10.1093/carcin/16.11.2725. [DOI] [PubMed] [Google Scholar]
- 533.Turesky RJ, Markovic J, Aeschlimann JM. Formation and differential removal of C-8 and N2-guanine adducts of the food carcinogen 2-amino-3-methylimidazo[4,5-f]quinoline in the liver, kidney, and colorectum of the rat. Chem. Res. Toxicol. 1996;9:397–402. doi: 10.1021/tx950131r. [DOI] [PubMed] [Google Scholar]
- 534.Phillips DH. Smoking-related DNA and protein adducts in human tissues. Carcinogenesis. 2002;23:1979–2004. doi: 10.1093/carcin/23.12.1979. [DOI] [PubMed] [Google Scholar]
- 535.Mooney LA, Santella RM, Covey L, Jeffrey AM, Bigbee W, Randall MC, Cooper TB, Ottman R, Tsai WY, Wazneh L. Decline of DNA damage and other biomarkers in peripheral blood following smoking cessation. Cancer Epidemiol. Biomarkers Prev. 1995;4:627–634. [PubMed] [Google Scholar]
- 536.Talaska G, Schamer M, Skipper P, Tannenbaum S, Caporaso N, Unruh L, Kadlubar FF, Bartsch H, Malaveille C, Vineis P. Detection of carcinogen-DNA adducts in exfoliated urothelial cells of cigarette smokers: association with smoking, hemoglobin adducts, and urinary mutagenicity. Cancer Epidemiol. Biomarkers Prev. 1991;1:61–66. [PubMed] [Google Scholar]
- 537.Wang LY, Chen CJ, Zhang YJ, Tsai WY, Lee PH, Feitelson MA, Lee CS, Santella RM. 4-Aminobiphenyl DNA damage in liver tissue of hepatocellular carcinoma patients and controls. Am. J. Epidemiol. 1998;147:315–323. doi: 10.1093/oxfordjournals.aje.a009452. [DOI] [PubMed] [Google Scholar]
- 538.Anderson KE, Hammons GJ, Kadlubar FF, Potter JD, Kaderlik KR, Ilett KF, Minchin RF, Teitel CH, Chou HC, Martin MV, Guengerich FP, Barone GW, Lang NP, Peterson LA. Metabolic activation of aromatic amines by human pancreas. Carcinogenesis. 1997;18:1085–1092. doi: 10.1093/carcin/18.5.1085. [DOI] [PubMed] [Google Scholar]
- 539.Besaratinia A, Van Straaten HW, Kleinjans JC, Van Schooten FJ. Immunoperoxidase detection of 4-aminobiphenyl- and polycyclic aromatic hydrocarbons-DNA adducts in induced sputum of smokers and non-smokers. Mutat. Res. 2000;468:125–135. doi: 10.1016/s1383-5718(00)00049-8. [DOI] [PubMed] [Google Scholar]
- 540.Flamini G, Romano G, Curigliano G, Chiominto A, Capelli G, Boninsegna A, Signorelli C, Ventura L, Santella RM, Sgambato A, Cittadini A. 4-Aminobiphenyl-DNA adducts in laryngeal tissue and smoking habits: an immunohistochemical study. Carcinogenesis. 1998;19:353–357. doi: 10.1093/carcin/19.2.353. [DOI] [PubMed] [Google Scholar]
- 541.Airoldi L, Orsi F, Magagnotti C, Coda R, Randone D, Casetta G, Peluso M, Hautefeuille A, Malaveille C, Vineis P. Determinants of 4-aminobiphenyl-DNA adducts in bladder cancer biopsies. Carcinogenesis. 2002;23:861–866. doi: 10.1093/carcin/23.5.861. [DOI] [PubMed] [Google Scholar]
- 542.Bohm F, Schmid D, Denzinger S, Wieland WF, Richter E. DNA adducts of ortho-toluidine in human bladder. Biomarkers. 2011;16:120–128. doi: 10.3109/1354750X.2010.534556. [DOI] [PubMed] [Google Scholar]
- 543.Zayas B, Stillwell SW, Wishnok JS, Trudel LJ, Skipper P, Yu MC, Tannenbaum SR, Wogan GN. Detection and quantification of 4-ABP adducts in DNA from bladder cancer patients. Carcinogenesis. 2007;28:342–349. doi: 10.1093/carcin/bgl142. [DOI] [PubMed] [Google Scholar]
- 544.Ambrosone CB, Abrams SM, Gorlewska-Roberts K, Kadlubar FF. Hair dye use, meat intake, and tobacco exposure and presence of carcinogen-DNA adducts in exfoliated breast ductal epithelial cells. Arch. Biochem. Biophys. 2007;464:169–175. doi: 10.1016/j.abb.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 545.Zhou Q, Talaska G, Jaeger M, Bhatnagar VK, Hayes RB, Zenzer TV, Kashyap SK, Lakshmi VM, Kashyap R, Dosemeci M, Hsu FF, Parikh DJ, Davis B, Rothman N. Benzidine-DNA adduct levels in human peripheral white blood cells significantly correlate with levels in exfoliated urothelial cells. Mutat. Res. 1997;393:199–205. doi: 10.1016/s1383-5718(97)00097-1. [DOI] [PubMed] [Google Scholar]
- 546.Kaderlik KR, Talaska G, DeBord DG, Osorio AM, Kadlubar FF. 4,4'-Methylene-bis(2-chloroaniline)-DNA adduct analysis in human exfoliated urothelial cells by 32P-postlabeling. Cancer Epidemiol. Biomarkers Prev. 1993;2:63–69. [PubMed] [Google Scholar]
- 547.Totsuka Y, Fukutome K, Takahashi M, Takashi S, Tada A, Sugimura T, Wakabayashi K. Presence of N2-(deoxyguanosin-8-yl)-2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (dG-C8-MeIQx) in human tissues. Carcinogenesis. 1996;17:1029–1034. doi: 10.1093/carcin/17.5.1029. [DOI] [PubMed] [Google Scholar]
- 548.Coles BF, Kadlubar FF. Detoxification of electrophilic compounds by glutathione S-transferase catalysis: Determinants of individual response to chemical carcinogens and chemotherapeutic drugs? Biofactors. 2003;17:115–130. doi: 10.1002/biof.5520170112. [DOI] [PubMed] [Google Scholar]
- 549.Jonsson C, Stal P, Sjoqvist U, Akerlund JE, Lofberg R, Möller L. DNA adducts in normal colonic mucosa from healthy controls and patients with colon polyps and colorectal carcinomas. Mutagenesis. 2010;25:499–504. doi: 10.1093/mutage/geq033. [DOI] [PubMed] [Google Scholar]
- 550.He YH, Friesen MD, Ruch RJ, Schut HA. Indole-3-carbinol as a chemopreventive agent in 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) carcinogenesis: inhibition of PhIP-DNA adduct formation, acceleration of PhIP metabolism, and induction of cytochrome P450 in female F344 rats. Food Chem Toxicol. 2000;38:15–23. doi: 10.1016/s0278-6915(99)00117-9. [DOI] [PubMed] [Google Scholar]
- 551.Phillips DH, Castegnaro M. Standardization and validation of DNA adduct postlabelling methods: report of interlaboratory trials and production of recommended protocols. Mutagenesis. 1999;14:301–315. doi: 10.1093/mutage/14.3.301. [DOI] [PubMed] [Google Scholar]
- 552.Goodenough AK, Schut HA, Turesky RJ. Novel LC-ESI/MS/MSn method for the characterization and quantification of 2'-deoxyguanosine adducts of the dietary carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine by 2-D linear quadrupole ion trap mass spectrometry. Chem. Res. Toxicol. 2007;20:263–276. doi: 10.1021/tx0601713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 553.Vondracek M, Xi Z, Larsson P, Baker V, Mace K, Pfeifer A, Tjalve H, Donato MT, Gomez-Lechon MJ, Grafstrom RC. Cytochrome P450 expression and related metabolism in human buccal mucosa. Carcinogenesis. 2001;22:481–488. doi: 10.1093/carcin/22.3.481. [DOI] [PubMed] [Google Scholar]
- 554.Spivack SD, Hurteau GJ, Jain R, Kumar SV, Aldous KM, Gierthy JF, Kaminsky LS. Gene-environment interaction signatures by quantitative mRNA profiling in exfoliated buccal mucosal cells. Cancer Res. 2004;64:6805–6813. doi: 10.1158/0008-5472.CAN-04-1771. [DOI] [PubMed] [Google Scholar]
- 555.Kragelund C, Hansen C, Torpet LA, Nauntofte B, Brosen K, Pedersen AM, Buchwald C, Therkildsen MH, Reibel J. Expression of two drug-metabolizing cytochrome P450-enzymes in human salivary glands. Oral Dis. 2008;14:533–540. doi: 10.1111/j.1601-0825.2007.01415.x. [DOI] [PubMed] [Google Scholar]
- 556.Ihalin R, Loimaranta V, Tenovuo J. Origin, structure, and biological activities of peroxidases in human saliva. Arch. Biochem. Biophys. 2006;445:261–268. doi: 10.1016/j.abb.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 557.Jones NJ, McGregor AD, Waters R. Detection of DNA adducts in human oral tissue: correlation of adduct levels with tobacco smoking and differential enhancement of adducts using the butanol extraction and nuclease P1 versions of 32P postlabeling. Cancer Res. 1993;53:1522–1528. [PubMed] [Google Scholar]
- 558.Stone JG, Jones NJ, McGregor AD, Waters R. Development of a human biomonitoring assay using buccal mucosa: comparison of smoking-related DNA adducts in mucosa versus biopsies. Cancer Res. 1995;55:1267–1270. [PubMed] [Google Scholar]
- 559.Foiles PG, Miglietta LM, Quart AM, Quart E, Kabat GC, Hecht SS. Evaluation of 32P-postlabeling analysis of DNA from exfoliated oral mucosa cells as a means of monitoring exposure of the oral cavity to genotoxic agents. Carcinogenesis. 1989;10:1429–1434. doi: 10.1093/carcin/10.8.1429. [DOI] [PubMed] [Google Scholar]
- 560.Chacko M, Gupta RC. Evaluation of DNA damage in the oral mucosa of tobacco users and non-users by 32P-adduct assay. Carcinogenesis. 1988;9:2309–2313. doi: 10.1093/carcin/9.12.2309. [DOI] [PubMed] [Google Scholar]
- 561.Zhang YJ, Hsu TM, Santella RM. Immunoperoxidase detection of polycyclic aromatic hydrocarbon-DNA adducts in oral mucosa cells of smokers and nonsmokers. Cancer Epidemiol. Biomarkers Prev. 1995;4:133–138. [PubMed] [Google Scholar]
- 562.Romano G, Mancini R, Fedele P, Curigliano G, Flamini G, Giovagnoli MR, Malara N, Boninsegna A, Vecchione A, Santella RM, Cittadini A. Immunohistochemical analysis of 4-aminobiphenyl-DNA adducts in oral mucosal cells of smokers and nonsmokers. Anticancer Res. 1997;17:2827–2830. [PubMed] [Google Scholar]
- 563.Besarati NA, Van Straaten HW, Godschalk RW, Van Zandwijk N, Balm AJ, Kleinjans JC, Van Schooten FJ. Immunoperoxidase detection of polycyclic aromatic hydrocarbon-DNA adducts in mouth floor and buccal mucosa cells of smokers and nonsmokers. Environ. Mol. Mutagen. 2000;36:127–133. doi: 10.1002/1098-2280(2000)36:2<127::aid-em7>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 564.Grollman AP, Shibutani S, Moriya M, Miller F, Wu L, Moll U, Suzuki N, Fernandes A, Rosenquist T, Medverec Z, Jakovina K, Brdar B, Slade N, Turesky RJ, Goodenough AK, Rieger R, Vukelic M, Jelakovic B. Aristolochic acid and the etiology of endemic (Balkan) nephropathy. Proc. Natl. Acad. Sci. U. S. A. 2007;104:12129–12134. doi: 10.1073/pnas.0701248104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 565.Takiguchi M, Darwish WS, Ikenaka Y, Ohno M, Ishizuka M. Metabolic activation of heterocyclic amines and expression of CYP1A1 in the tongue. Toxicol. Sci. 2010;116:79–91. doi: 10.1093/toxsci/kfq087. [DOI] [PubMed] [Google Scholar]
- 566.Takayama S, Nakatsuru Y, Ohgaki H, Sato S, Sugimura T. Atrophy of salivary glands and pancreas of rats fed on diet with 2-amino-3-methyl-α–carboline. (Ser. B).Proc. Japanese Acad. 1985;61:277–280. [Google Scholar]
- 567.Klinkhamer JM. Human oral leukocytes. Journal of the American Society of Periodontists. 1963;1:109–117. [Google Scholar]
- 568.Osswald K, Mittas A, Glei M, Pool-Zobel BL. New revival of an old biomarker: characterisation of buccal cells and determination of genetic damage in the isolated fraction of viable leucocytes. Mutat. Res. 2003;544:321–329. doi: 10.1016/j.mrrev.2003.06.008. [DOI] [PubMed] [Google Scholar]
- 569.Squier CA, JOhnson NW, Hopps RM. Human Oral Mucosa: Development, Structure and Function. Blackwell; Oxford, UK: 1976. pp. 3–42. [Google Scholar]
- 570.Ashkenazi M, Dennison DK. A new method for isolation of salivary neutrophils and determination of their functional activity. J. Dent. Res. 1989;68:1256–1261. doi: 10.1177/00220345890680080901. [DOI] [PubMed] [Google Scholar]
- 571.Gan LS, Skipper PL, Peng XC, Groopman JD, Chen JS, Wogan GN, Tannenbaum SR. Serum albumin adducts in the molecular epidemiology of aflatoxin carcinogenesis: correlation with aflatoxin B1 intake and urinary excretion of aflatoxin M1. Carcinogenesis. 1988;9:1323–1325. doi: 10.1093/carcin/9.7.1323. [DOI] [PubMed] [Google Scholar]
- 572.Wild CP, Hudson GJ, Sabbioni G, Chapot B, Hall AJ, Wogan GN, Whittle H, Montesano R, Groopman JD. Dietary intake of aflatoxins and the level of albumin-bound aflatoxin in peripheral blood in The Gambia, West Africa. Cancer Epidemiol. Biomarkers Prev. 1992;1:229–234. [PubMed] [Google Scholar]
- 573.Granath F, Ehrenberg L, Tornqvist M. Degree of alkylation of macromolecules in vivo from variable exposure. Mutat. Res. 1992;284:297–306. doi: 10.1016/0027-5107(92)90014-s. [DOI] [PubMed] [Google Scholar]
- 574.Tornqvist M, Fred C, Haglund J, Helleberg H, Paulsson B, Rydberg P. Protein adducts: quantitative and qualitative aspects of their formation, analysis and applications. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2002;778:279–308. doi: 10.1016/s1570-0232(02)00172-1. [DOI] [PubMed] [Google Scholar]
- 575.Miller EC, Miller JA. The presence and significance of bound aminoazo dyes in the livers of rats fed p-dimethylaminoazobenzene. Cancer Res. 1947;7:468–480. [Google Scholar]
- 576.Miller EC, Miller JA, Sapp RW, Weber GM. Studies on the protein-bound aminazo dyes formed in vtro from 4-dimethylaminoazobenze and its C-monomethyl derivatives. Cancer Res. 1949;9:336–343. [PubMed] [Google Scholar]
- 577.Ehrenberg L, Hiesche KD, Osterman-Golkar S, Wenneberg I. Evaluation of genetic risks of alkylating agents: tissue doses in the mouse from air contaminated with ethylene oxide. Mutat. Res. 1974;24:83–103. doi: 10.1016/0027-5107(74)90123-7. [DOI] [PubMed] [Google Scholar]
- 578.Osterman-Golkar S, Ehrenberg L, Segerbäck D, Hallstrom I. Evaluation of genetic risks of alkylating agents. II. Haemoglobin as a dose monitor. Mutat. Res. 1976;34:1–10. doi: 10.1016/0027-5107(76)90256-6. [DOI] [PubMed] [Google Scholar]
- 579.Sabbioni G, Skipper PL, Buchi G, Tannenbaum SR. Isolation and characterization of the major serum albumin adduct formed by aflatoxin B1 in vivo in rats. Carcinogenesis. 1987;8:819–824. doi: 10.1093/carcin/8.6.819. [DOI] [PubMed] [Google Scholar]
- 580.Peters T, Jr., Peters JC. The biosynthesis of rat serum albumin. VI. Intracellular transport of albumin and rates of albumin and liver protein synthesis in vivo under various physiological conditions. J. Biol. Chem. 1972;247:3858–3863. [PubMed] [Google Scholar]
- 581.Bryant MS, Vineis P, Skipper PL, Tannenbaum SR. Hemoglobin adducts of aromatic amines: associations with smoking status and type of tobacco. Proc. Natl. Acad. Sci. U. S. A. 1988;85:9788–9791. doi: 10.1073/pnas.85.24.9788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 582.Beyerbach A, Rothman N, Bhatnagar VK, Kashyap R, Sabbioni G. Hemoglobin adducts in workers exposed to benzidine and azo dyes. Carcinogenesis. 2006;27:1600–1606. doi: 10.1093/carcin/bgi362. [DOI] [PubMed] [Google Scholar]
- 583.Bartsch H, Caporaso N, Coda M, Kadlubar F, Malaveille C, Skipper P, Talaska G, Tannenbaum SR, Vineis P. Carcinogen hemoglobin adducts, urinary mutagenicity, and metabolic phenotype in active and passive cigarette smokers. J. Natl. Cancer Inst. 1990;82:1826–1831. doi: 10.1093/jnci/82.23.1826. [DOI] [PubMed] [Google Scholar]
- 584.Yu MC, Skipper PL, Taghizadeh K, Tannenbaum SR, Chan KK, Henderson BE, Ross RK. Acetylator phenotype, aminobiphenyl-hemoglobin adduct levels, and bladder cancer risk in white, black, and Asian men in Los Angeles, California. J. Natl. Cancer Inst. 1994;86:712–716. doi: 10.1093/jnci/86.9.712. [DOI] [PubMed] [Google Scholar]
- 585.Gan J, Skipper PL, Gago-Dominguez M, Arakawa K, Ross RK, Yu MC, Tannenbaum SR. Alkylaniline-hemoglobin adducts and risk of non-smoking-related bladder cancer. J Natl. Cancer Inst. 2004;96:1425–1431. doi: 10.1093/jnci/djh274. [DOI] [PubMed] [Google Scholar]
- 586.Turesky RJ, Skipper PL, Tannenbaum SR. Binding of 2-amino-3-methylimidazo[4,5-f]quinoline to hemoglobin and albumin in vivo in the rat. Identification of an adduct suitable for dosimetry. Carcinogenesis. 1987;8:1537–1542. doi: 10.1093/carcin/8.10.1537. [DOI] [PubMed] [Google Scholar]
- 587.Lynch AM, Murray S, Zhao K, Gooderham NJ, Boobis AR, Davies DS. Molecular dosimetry of the food-borne carcinogen MeIQx using adducts of serum albumin. Carcinogenesis. 1993;14:191–194. doi: 10.1093/carcin/14.2.191. [DOI] [PubMed] [Google Scholar]
- 588.Dingley KH, Freeman SP, Nelson DO, Garner RC, Turteltaub KW. Covalent binding of 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline to albumin and hemoglobin at environmentally relevant doses. Comparison of human subjects and F344 rats. Drug Metab. Dispos. 1998;26:825–828. [PubMed] [Google Scholar]
- 589.Umemoto A, Monden Y, Tsuda M, Grivas S, Sugimura T. Oxidation of the 2-hydroxyamino derivative of 2-amino-6-methyl-dipyrido[1,2-a: 3',2'-d] imidazole (Glu-P-1) to its 2-nitroso form, an ultimate form reacting with hemoglobin thiol groups. Biochem. Biophys. Res. Commun. 1988;151:1326–1331. doi: 10.1016/s0006-291x(88)80507-2. [DOI] [PubMed] [Google Scholar]
- 590.Peters T., Jr. All about Albumin. Biochemistry, Genetics, and Medical applications. Academic Press; San Diego: 1996. [Google Scholar]
- 591.Magagnotti C, Orsi F, Bagnati R, Celli N, Rotilio D, Fanelli R, Airoldi L. Effect of diet on serum albumin and hemoglobin adducts of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in humans. Int. J. Cancer. 2000;88:1–6. doi: 10.1002/1097-0215(20001001)88:1<1::aid-ijc1>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 592.Chepanoske CL, Brown K, Turteltaub KW, Dingley KH. Characterization of a peptide adduct formed by N-acetoxy-2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP), a reactive intermediate of the food carcinogen PhIP. Food Chem. Toxicol. 2004;42:1367–1372. doi: 10.1016/j.fct.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 593.Funk WE, Li H, Iavarone AT, Williams ER, Riby J, Rappaport SM. Enrichment of cysteinyl adducts of human serum albumin. Anal. Biochem. 2010;400:61–68. doi: 10.1016/j.ab.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 594.Carballal S, Radi R, Kirk MC, Barnes S, Freeman BA, Alvarez B. Sulfenic acid formation in human serum albumin by hydrogen peroxide and peroxynitrite. Biochemistry. 2003;42:9906–9914. doi: 10.1021/bi027434m. [DOI] [PubMed] [Google Scholar]
- 595.Beck JL, Ambahera S, Yong SR, Sheil MM, de JJ, Ralph SF. Direct observation of covalent adducts with Cys34 of human serum albumin using mass spectrometry. Anal. Biochem. 2004;325:326–336. doi: 10.1016/j.ab.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 596.Rappaport SM, Li H, Grigoryan H, Funk WE, Williams ER. Adductomics: Characterizing exposures to reactive electrophiles. Toxicol Lett. doi: 10.1016/j.toxlet.2011.04.002. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 597.Reistad R, Frandsen H, Grivas S, Alexander J. In vitro formation and degradation of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) protein adducts. Carcinogenesis. 1994;15:2547–2552. doi: 10.1093/carcin/15.11.2547. [DOI] [PubMed] [Google Scholar]
- 598.Noort D, Fidder A, Hulst AG. Modification of human serum albumin by acrylamide at cysteine-34: a basis for a rapid biomonitoring procedure. Arch. Toxicol. 2003;77:543–545. doi: 10.1007/s00204-003-0484-5. [DOI] [PubMed] [Google Scholar]
- 599.Noort D, Hulst AG, de Jong LP, Benschop HP. Alkylation of human serum albumin by sulfur mustard in vitro and in vivo: mass spectrometric analysis of a cysteine adduct as a sensitive biomarker of exposure. Chem Res. Toxicol. 1999;12:715–721. doi: 10.1021/tx9900369. [DOI] [PubMed] [Google Scholar]
- 600.Bechtold WE, Willis JK, Sun JD, Griffith WC, Reddy TV. Biological markers of exposure to benzene: S-phenylcysteine in albumin. Carcinogenesis. 1992;13:1217–1220. doi: 10.1093/carcin/13.7.1217. [DOI] [PubMed] [Google Scholar]
- 601.Hoffmann KJ, Streeter AJ, Axworthy DB, Baillie TA. Structural characterization of the major covalent adduct formed in vitro between acetaminophen and bovine serum albumin. Chem. -Biol. Interact. 1985;53:155–172. doi: 10.1016/s0009-2797(85)80093-4. [DOI] [PubMed] [Google Scholar]
- 602.Kim D, Kadlubar FF, Teitel CH, Guengerich FP. Formation and reduction of aryl and heterocyclic nitroso compounds and significance in the flux of hydroxylamines. Chem. Res. Toxicol. 2004;17:529–536. doi: 10.1021/tx034267y. [DOI] [PubMed] [Google Scholar]
- 603.DuPont RL, Baumgartner WA. Drug testing by urine and hair analysis: complementary features and scientific issues. Forensic Sci. Int. 1995;70:63–76. doi: 10.1016/0379-0738(94)01625-f. [DOI] [PubMed] [Google Scholar]
- 604.Hegstad S, Lundanes E, Reistad R, Haug LS, Becher G, Alexander J. Determination of the food carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in human hair by solid-phase extraction and gas chromatography-mass spectrometry. Chromatographia. 2000;52:499–504. [Google Scholar]
- 605.Hashimoto H, Hanaoka T, Kobayashi M, Tsugane S. Analytical method of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine in human hair by column-switching liquid chromatography-mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2004;803:209–213. doi: 10.1016/j.jchromb.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 606.Kobayashi M, Hanaoka T, Hashimoto H, Tsugane S. 2-Amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) level in human hair as biomarkers for dietary grilled/stir-fried meat and fish intake. Mutat. Res. 2005;588:136–142. doi: 10.1016/j.mrgentox.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 607.Bessette EE, Yasa I, Dunbar D, Wilkens LR, Marchand LL, Turesky RJ. Biomonitoring of carcinogenic heterocyclic aromatic amines in hair: A validation study. Chem. Res. Toxicol. 2009;22:1454–1463. doi: 10.1021/tx900155f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 608.Reistad R, Nyholm SH, Huag LS, Becher G, Alexander J. 2-Amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in human hair as biomarker for dietary exposure. Biomarkers. 1999;4:263–271. doi: 10.1080/135475099230796. [DOI] [PubMed] [Google Scholar]
- 609.Kobayashi M, Hanaoka T, Tsugane S. Validity of a self-administered food frequency questionnaire in the assessment of heterocyclic amine intake using 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) levels in hair. Mutat. Res. 2007;630:14–19. doi: 10.1016/j.mrgentox.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 610.Hegstad S, Reistad R, Haug LS, Alexander J. Eumelanin is a major determinant for 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) incorporation into hair of mice. Pharmacol. Toxicol. 2002;90:333–337. doi: 10.1034/j.1600-0773.2002.900607.x. [DOI] [PubMed] [Google Scholar]
- 611.Ozeki H, Ito S, Wakamatsu K, Thody AJ. Spectrophotometric characterization of eumelanin and pheomelanin in hair. Pigment Cell Res. 1996;9:265–270. doi: 10.1111/j.1600-0749.1996.tb00116.x. [DOI] [PubMed] [Google Scholar]
- 612.Reisch MS. Flush with color: Continuing progress in hair dye chemistry satisfies diverse desires of young and old. Chem. Eng. News. 2003;81:25–27. [Google Scholar]
- 613.Corbett JF, Menkart J. Hair Coloring. Cutis. 1973;12:190–197. [Google Scholar]
- 614.Food, Nutrition, Physical Activity and the Prevention of Cancer: a Global Perspective. AICR; Washington, DC: 2007. 2007. [Google Scholar]
- 615.Sinha R, Kulldorff M, Chow WH, Denobile J, Rothman N. Dietary intake of heterocyclic amines, meat-derived mutagenic activity, and risk of colorectal adenomas. Cancer Epidemiol. Biomarkers Prev. 2001;10:559–562. [PubMed] [Google Scholar]
- 616.Cross AJ, Sinha R. Meat-related mutagens/carcinogens in the etiology of colorectal cancer. Environ. Mol. Mutagen. 2004;44:44–55. doi: 10.1002/em.20030. [DOI] [PubMed] [Google Scholar]
- 617.Ferguson LR. Meat and cancer. Meat Sci. 2010;84:308–313. doi: 10.1016/j.meatsci.2009.06.032. [DOI] [PubMed] [Google Scholar]
- 618.Zheng W, Lee SA. Well-done meat intake, heterocyclic amine exposure, and cancer risk. Nutr. Cancer. 2009;61:437–446. doi: 10.1080/01635580802710741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 619.Nowell S, Coles B, Sinha R, MacLeod S, Luke RD, Stotts C, Kadlubar FF, Ambrosone CB, Lang NP. Analysis of total meat intake and exposure to individual heterocyclic amines in a case-control study of colorectal cancer: contribution of metabolic variation to risk. Mutat. Res. 2002;506-507:175–185. doi: 10.1016/s0027-5107(02)00164-1. [DOI] [PubMed] [Google Scholar]
- 620.Butler LM, Sinha R, Millikan RC, Martin CF, Newman B, Gammon MD, Ammerman AS, Sandler RS. Heterocyclic amines, meat intake, and association with colon cancer in a population-based study. Am. J. Epidemiol. 2003;157:434–445. doi: 10.1093/aje/kwf221. [DOI] [PubMed] [Google Scholar]
- 621.Wu K, Giovannucci E, Byrne C, Platz EA, Fuchs C, Willett WC, Sinha R. Meat mutagens and risk of distal colon adenoma in a cohort of U.S. men. Cancer Epidemiol. Biomarkers Prev. 2006;15:1120–1125. doi: 10.1158/1055-9965.EPI-05-0782. [DOI] [PubMed] [Google Scholar]
- 622.Augustsson K, Skog K, Jagerstad M, Dickman PW, Steineck G. Dietary heterocyclic amines and cancer of the colon, rectum, bladder, and kidney: a population-based study. Lancet. 1999;353:703–707. doi: 10.1016/S0140-6736(98)06099-1. [DOI] [PubMed] [Google Scholar]
- 623.Brockton N, Little J, Sharp L, Cotton SC. N-acetyltransferase polymorphisms and colorectal cancer: a HuGE review. Am. J Epidemiol. 2000;151:846–861. doi: 10.1093/oxfordjournals.aje.a010289. [DOI] [PubMed] [Google Scholar]
- 624.Agundez JA. Polymorphisms of human N-acetyltransferases and cancer risk. Curr. Drug. Metab. 2008;9:520–531. doi: 10.2174/138920008784892083. [DOI] [PubMed] [Google Scholar]
- 625.Dong LM, Potter JD, White E, Ulrich CM, Cardon LR, Peters U. Genetic susceptibility to cancer: the role of polymorphisms in candidate genes. JAMA. 2008;299:2423–2436. doi: 10.1001/jama.299.20.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 626.Welfare MR, Cooper J, Bassendine MF, Daly AK. Relationship between acetylator status, smoking, and diet and colorectal cancer risk in the north-east of England. Carcinogenesis. 1997;18:1351–1354. doi: 10.1093/carcin/18.7.1351. [DOI] [PubMed] [Google Scholar]
- 627.Tiemersma EW, Bunschoten A, Kok FJ, Glatt H, de Boer SY, Kampman E. Effect of SULT1A1 and NAT2 genetic polymorphism on the association between cigarette smoking and colorectal adenomas. Int. J. Cancer. 2004;108:97–103. doi: 10.1002/ijc.11533. [DOI] [PubMed] [Google Scholar]
- 628.Chan AT, Tranah GJ, Giovannucci EL, Willett WC, Hunter DJ, Fuchs CS. Prospective study of N-acetyltransferase-2 genotypes, meat intake, smoking and risk of colorectal cancer. Int. J. Cancer. 2005;115:648–652. doi: 10.1002/ijc.20890. [DOI] [PubMed] [Google Scholar]
- 629.Lilla C, Verla-Tebit E, Risch A, Jager B, Hoffmeister M, Brenner H, Chang-Claude J. Effect of NAT1 and NAT2 genetic polymorphisms on colorectal cancer risk associated with exposure to tobacco smoke and meat consumption. Cancer Epidemiol. Biomarkers Prev. 2006;15:99–107. doi: 10.1158/1055-9965.EPI-05-0618. [DOI] [PubMed] [Google Scholar]
- 630.Nothlings U, Yamamoto JF, Wilkens LR, Murphy SP, Park SY, Henderson BE, Kolonel LN, Le ML. Meat and heterocyclic amine intake, smoking, NAT1 and NAT2 polymorphisms, and colorectal cancer risk in the multiethnic cohort study. Cancer Epidemiol. Biomarkers Prev. 2009;18:2098–2106. doi: 10.1158/1055-9965.EPI-08-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 631.Ishibe N, Sinha R, Hein DW, Kulldorff M, Strickland P, Fretland AJ, Chow WH, Kadlubar FF, Lang NP, Rothman N. Genetic polymorphisms in heterocyclic amine metabolism and risk of colorectal adenomas. Pharmacogenetics. 2002;12:145–150. doi: 10.1097/00008571-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 632.Wang H, Yamamoto JF, Caberto C, Saltzman B, Decker R, Vogt TM, Yokochi L, Chanock S, Wilkens LR, Le ML. Genetic variation in the bioactivation pathway for polycyclic hydrocarbons and heterocyclic amines in relation to risk of colorectal neoplasia. Carcinogenesis. 2011;32:203–209. doi: 10.1093/carcin/bgq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 633.Cotterchio M, Boucher BA, Manno M, Gallinger S, Okey AB, Harper PA. Red meat intake, doneness, polymorphisms in genes that encode carcinogen-metabolizing enzymes, and colorectal cancer risk. Cancer Epidemiol. Biomarkers Prev. 2008;17:3098–3107. doi: 10.1158/1055-9965.EPI-08-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 634.Kury S, Buecher B, Robiou-du-Pont S, Scoul C, Sebille V, Colman H, Le HC, Le NT, Bourdon J, Faroux R, Ollivry J, Lafraise B, Chupin LD, Bezieau S. Combinations of cytochrome P450 gene polymorphisms enhancing the risk for sporadic colorectal cancer related to red meat consumption. Cancer Epidemiol. Biomarkers Prev. 2007;16:1460–1467. doi: 10.1158/1055-9965.EPI-07-0236. [DOI] [PubMed] [Google Scholar]
- 635.Barrett JH, Smith G, Waxman R, Gooderham N, Lightfoot T, Garner RC, Augustsson K, Wolf CR, Bishop DT, Forman D. Investigation of interaction between N-acetyltransferase 2 and heterocyclic amines as potential risk factors for colorectal cancer. Carcinogenesis. 2003;24:275–282. doi: 10.1093/carcin/24.2.275. [DOI] [PubMed] [Google Scholar]
- 636.Murtaugh MA, Ma KN, Sweeney C, Caan BJ, Slattery ML. Meat consumption patterns and preparation, genetic variants of metabolic enzymes, and their association with rectal cancer in men and women. J Nutr. 2004;134:776–784. doi: 10.1093/jn/134.4.776. [DOI] [PubMed] [Google Scholar]
- 637.Kampman E, Slattery ML, Bigler J, Leppert M, Samowitz W, Caan BJ, Potter JD. Meat consumption, genetic susceptibility, and colon cancer risk: a United States multicenter case-control study. Cancer Epidemiol. Biomarkers Prev. 1999;8:15–24. [PubMed] [Google Scholar]
- 638.Lumbreras B, Garte S, Overvad K, Tjonneland A, Clavel-Chapelon F, Linseisen JP, Boeing H, Trichopoulou A, Palli D, Peluso M, Krogh V, Tumino R, Panico S, Bueno-De-Mesquita HB, Peeters PH, Lund E, Martinez C, Dorronsoro M, Barricarte A, Chirlaque MD, Quiros JR, Berglund G, Hallmans G, Day NE, Key TJ, Saracci R, Kaaks R, Malaveille C, Ferrari P, Boffetta P, Norat T, Riboli E, Gonzalez CA, Vineis P. Meat intake and bladder cancer in a prospective study: a role for heterocyclic aromatic amines? Cancer Causes Control. 2008;19:649–656. doi: 10.1007/s10552-008-9121-1. [DOI] [PubMed] [Google Scholar]
- 639.Le Marchand L, Hankin JH, Wilkens LR, Pierce LM, Franke A, Kolonel LN, Seifried A, Custer LJ, Chang W, Lum-Jones A, Donlon T. Combined effects of well-done red meat, smoking, and rapid N-acetyltransferase 2 and CYP1A2 phenotypes in increasing colorectal cancer risk. Cancer Epidemiol. Biomarkers Prev. 2001;10:1259–1266. [PubMed] [Google Scholar]
- 640.Kristal AR, Peters U, Potter JD. Is it time to abandon the food frequency questionnaire? Cancer Epidemiol. Biomarkers Prev. 2005;14:2826–2828. doi: 10.1158/1055-9965.EPI-12-ED1. [DOI] [PubMed] [Google Scholar]
- 641.Knize MG, Kulp KS, Salmon CP, Keating GA, Felton JS. Factors affecting human heterocyclic amine intake and the metabolism of PhIP. Mutat. Res. 2002;506-507:153–162. doi: 10.1016/s0027-5107(02)00162-8. [DOI] [PubMed] [Google Scholar]
- 642.Loeb LA, Harris CC. Advances in chemical carcinogenesis: a historical review and prospective. Cancer Res. 2008;68:6863–6872. doi: 10.1158/0008-5472.CAN-08-2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 643.Wogan GN, Hecht SS, Felton JS, Conney AH, Loeb LA. Environmental and chemical carcinogenesis. Semin. Cancer Biol. 2004;14:473–486. doi: 10.1016/j.semcancer.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 644.Ross RK, Yuan JM, Yu MC, Wogan GN, Qian GS, Tu JT, Groopman JD, Gao YT, Henderson BE. Urinary aflatoxin biomarkers and risk of hepatocellular carcinoma [see comments]. Lancet. 1992;339:943–946. doi: 10.1016/0140-6736(92)91528-g. [DOI] [PubMed] [Google Scholar]
- 645.Greenblatt MS, Bennett WP, Hollstein M, Harris CC. Mutations in the p53 tumor suppressor gene: clues to cancer etiology and molecular pathogenesis. Cancer Res. 1994;54:4855–4878. [PubMed] [Google Scholar]
- 646.Schmeiser HH, Stiborova M, Arlt VM. Chemical and molecular basis of the carcinogenicity of Aristolochia plants. Curr. Opin. Drug Discov. Devel. 2009;12:141–148. [PubMed] [Google Scholar]
- 647.Lai MN, Wang SM, Chen PC, Chen YY, Wang JD. Population-based case-control study of Chinese herbal products containing aristolochic acid and urinary tract cancer risk. J Natl. Cancer Inst. 2010;102:179–186. doi: 10.1093/jnci/djp467. [DOI] [PMC free article] [PubMed] [Google Scholar]